A Novel RP-HPLC Method for Estimating Fulvestrant, Benzoyl Alcohol, and Benzyl Benzoate in Injection Formulation ()
1. Introduction
Fulvestrant (Figure 1) is an oestrogen receptor antagonist used to treat postmenopausal women with hormone receptor metastatic breast cancer who have progressed after antiestrogen therapy. On days 1, 15, 29, and once monthly afterwards, 2 five mL injections, and 1 in each buttock, should be given intramuscularly into the buttocks slowly (one - two minutes per injection). To accept the 500 mg suggested dose from the two 5 mL syringe packages, both syringes’ fillings must be administered (Figure 2).
Estrogen receptors (ER) are found in many breast malignancies, and oestrogen can encourage tumour growth. Fulvestrant is a receptor antagonist that ER protein in humanoid breast cancer cells by binding to the receptor in a modest way with attraction similar to that of estradiol. In immature or ovariectomized mice and rats, fulvestrant had no agonist-like effects in vivo uterotropic experiments. Fulvestrant inhibited estradiol’s uterotrophic effect in immature rats and ovariectomized monkeys in vivo. The absence of increases in FSH and LH plasma concentrations in response to fulvestrant medication (250 mg monthly) in postmenopausal women suggests no peripheral steroidal effects.
Fulvestrant has a molecular weight of 606.77 and is a white powder. The injectable solution is a thick, clear, liquid from colourless to yellow. As co-solvents, each injection contains 10 percent of weight/volume Alcohol, USP, 10 percent weight/volume Benzyl Alcohol, NF, and 15 percent weight/volume Benzyl Benzoate, USP, and is produced up to 100 percent weight/volume with Castor Oil, USP as a co-solvent and rate of release modifier.
Because Benzyl Alcohol and Benzyl Benzoate presented in the Fulvestrant are injectable. Each drug product undergoes a co-solvents assay to be quantified for initial and long-term stability before being released into the market. The measurement of fulvestrant in rabbit plasma using a sensitive and rapid high-performance liquid chromatography tandem mass spectrometry approach [1] was published with a run time of 2.5 minutes, the article unable to detect benzyl alcohol and benzoate. In comparison to the Linear Sweep Voltammetric Method, Stability Indicating HPLC Method for the Determination of Fulvestrant in Pharmaceutical Formulation [2] , method was published with a 20% organic solvent and a 5-minute run duration, but the co-solvents could not be separated.
![]()
Figure 1. Chemical structure of Fulvestrant.
HPLC-UV technique for estimating fulvestrant in bulk drug development and validation [3] , method was explored in relation to bulk drugs and was found to be ineffective for Fulvestrant injection. Development and Validation of a Novel RP-HPLC Analytical Method for Fulvestrant Injection in accordance with ICH Guidelines [4] , method was published with 70 minutes run time and also not discussed about co-solvents. The 70-minute run time approach is not suited for routine testing of quality control samples for assay quantification. The measurement of fulvestrant in oil-based prefilled syringe injection matrix formulations using a new UPLC-PDA isocratic technique [5] , co-solvents were not disclosed in the procedure, which was isocratic and employed 70% organic in the mobile phase. Both co-solvents benzyl alcohol and benzyl benzoate may be eluted in the void volume because the mobile phase includes 70% organic. On Fulvestrant pharmacology and pharmacokinetics several research articles have been published [6] [7] [8] [9] [10] , which are not discussed and reported about the co-solvents of Benzyl alcohol and Benzyl benzoate. Only the assay of Fulvestrant is demonstrated in all the papers using HPLC, UV/PDA, and UPLC, LC-MS assay methods, the assay of Benzyl Alcohol and Benzyl Benzoate is not demonstrated, however present in the Fulvestrant injection. Method development and validation [11] - [17] referred for reference to this work. The measurement of active ingredients and their co-solvents in pharmaceutical products is crucial in the quality control department. For the reasons stated above, a novel HPLC technique for the analysis of Fulvestrant injection was devised and validated.
2. Experimental
2.1. Chemicals and Reagents
The Fulvestrant reference standard was given by Manasa Life Sciences. Fulvestrant injection was bought on the market. Merck provided Benzyl alcohol, Benzyl benzoate, Water, Methanol, and Acetonitrile.
2.2. Instrumentation
For method development and validation, the SHIMADZU i-Prominence HPLC system with auto sampler and UV detector was used. The data was analysed using Empower software.
2.3. Chromatographic Conditions
The drug and co-solvents were separated chromatographically using a Phenomenex Luna C8, 150 × 4.6 mm, 3 microns column. In mobile phase A, deionized water was used, and in mobile phase B, acetonitrile was used. The Gradient method (T/percent B) was finalised as 0.01/40, 2.5/40, 3.5/60, 10.0/60, 11.0/40, 15.0/40, and wavelength detection at 254 nm for Benzyl alcohol & Benzoyl Benzoate and 280 nm for Fulvestrant at 1.5 mL/minute flow rate, injection volume of 10 µL, and column temperature of 35˚C.
2.4. Preparation of Standard, Placebo and Sample Solutions
For Standard, Placebo and Sample solutions Methanol is used as a diluent. The standard solution was made with Fulvestrant reference standard, Benzyl Alcohol, and Benzyl Benzoate at concentrations of 150 µg/mL for Fulvestrant, 300 µg/mL for Benzyl Alcohol, and 450 µg/mL for Benzyl Benzoate. Weighing roughly 150 mg of Fulvestrant injection and Placebo solution into an individual 50 mL volumetric flask yielded a test sample and placebo solution. Vertex for 5 minutes with 35 mL methanol, then dilute with methanol to volume and thoroughly mix of both the solutions.
2.5. Procedure for Method Validation
Following the International Conference on Harmonization (ICH) recommendations, the developed RP-HPLC technique for the measurement of Fulvestrant, Benzyl alcohol, and Benzyl Benzoate was validated.
2.5.1. System Suitability
As part of the analytical test method, a standard solution was produced and injected six times into the chromatographic device. To determine the optimal approach, system appropriateness criteria such as percent RSD, tailing factor and theoretical plates were evaluated.
2.5.2. Specificity
Blank solution, placebo solution, and standard solutions are all tested for potential interferences using specificity. Because Blank and Placebo did not interfere with the standard, the approach was confirmed to be specific. The chromatograms (Figure 3) showed that Benzyl alcohol, Benzyl Benzoate, and Fulvestrant were eluted in standard solution at retention durations of 1.943 ± 0.05 min, 7.728 ± 0.05 min, and 9.334 ± 0.05 min, respectively, with a total runtime of 15 minutes under optimum chromatographic conditions.
2.5.3. Linearity
Linearity was determined by plotting a graph of Fulvestrant, Benzyl alcohol, and Benzyl benzoate concentration (X-axis) vs peak area (Y-axis) and calculating the correlation coefficient. A series of solutions with concentrations ranging from roughly 50% to 150 percent of the target concentration were created. Linearity solutions for Fulvestrant of 75, 120, 150, 180, and 225 µg/mL, Benzyl alcohol of 150, 240, 300, 360, and 450 µg/mL, Benzyl benzoate of 225, 360, 450, 540, and 675 µg/mL were prepared and injected 10 µL into the HPLC system.
2.5.4. Precision
To assess the precision of the test procedures, Fulvestrant injection 250 mg/5mL were injected at target concentrations and assessed using analytical test techniques. Six samples were prepared according to the analytical test procedure, inserted into a chromatographic system, and evaluated. Fulvestrant, Benzyl alcohol, and Benzyl benzoate had their mean percent assay and relative standard deviation calculated and test chromatogram was shown in Figure 4.
>
 (a)
(a)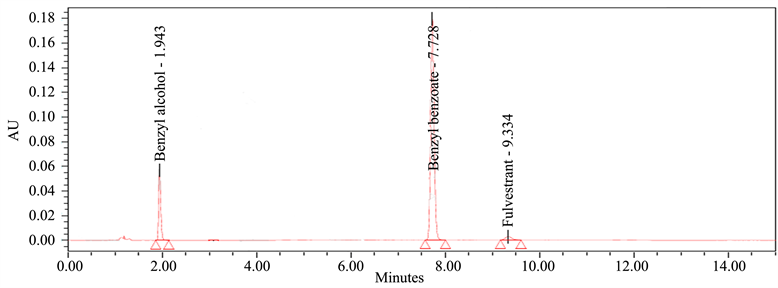 (b)
(b)
Figure 3. Reference chromatograms: (a) Placebo chromatogram, and (b) Standard chromatogram.
2.5.5. Limit of Detection (LOD) and Limit of Quantification (LOQ)
A study was undertaken to determine the limits of detection and quantification for Fulvestrant, Benzyl alcohol, and Benzyl benzoate. The signal to noise ratio was used to determine the detection and quantification limits. As per the analytical test procedure, a series of solutions containing Fulvestrant, Benzyl alcohol, and Benzyl benzoate were produced and injected into the chromatographic apparatus. The limit of detection was determined by determining the concentration that produces a signal-to-noise ratio of around 3. The quantification limit was determined by determining the quantity that produces a signal-to-noise ratio of roughly 10.
2.5.6. Accuracy
A research of Fulvestrant injection recovery was carried out on drug product by altering sample volumes in relation to the spike level. Test samples were generated in triplicate at each level of the target assay concentration of Fulvestrant, Benzyl alcohol, and Benzyl benzoate as per the test procedure, injected into a chromatographic system, and analyzed. Calculate the percent recovery for each preparation, with a mean percent recovery of about 50%, 100%, and 150% for each level.
2.5.7. Ruggedness
Six samples of Fulvestrant Injection 250 mg/5mL were made to specification of test solution and evaluated according to the analytical test technique for different HPLC, different column, different scientist, and different day. On different days, with the system suitability characteristics for both the systems and columns were evaluated. The percent relative standard deviations of fulvestrant, benzyl alcohol, and benzyl benzoate were calculated.
2.5.8. Stability of Solutions
The stability of standard and test preparation solutions was investigated. Standard and test preparation were prepared and stored at 25 degrees and in the refrigerator. These solutions were injected into the chromatographic apparatus and examined at the beginning, and three consecutive days. Calculated the percent assay for test preparation against newly prepared standard solution and the similarity factor for the standard solution. From the initial results, the difference was calculated.
2.5.9. Application of the Method
The assay of Fulvestrant, Benzyl alcohol, and Benzyl benzoate was tested using the in-process bulk solution 100 mg/mL and the optimised and validated method. The commercially available Fulvestrant injectable 250 mg/5mL was chosen to demonstrate the method’s application in injection formulation estimation.
This method is suited for the quantification of fulvestrant and co-solvents can be used to analyse drug product formulations, in-process samples, and active pharmaceutical ingredients.
Open the two Fulvestrant injections and thoroughly combine the contents. Weight around 150 mg of Fulvestrant injection into a 50 mL volumetric flask accurately. Shake for one to two minutes with 30 mL methanol, then dilute to volume with methanol and thoroughly combine. Standard and sample solutions were introduced into the HPLC as final solutions. The average peak areas of six injections of test sample were calculated (refer precision results).
3. Results
3.1. Optimization of Chromatographic Conditions
Each injection of FASLODEX 250 mg (Fulvestrant injection) comprises Benzyl alcohol (co-solvent), Benzyl benzoate (co-solvent), and Fulvestrant (active), according to the label. The goal of this study is to use the RP-HPLC technology to extract Fulvestrant from co-solvents and measure it in less time.
The resolution of the drug and co-solvents, tailing factor, and theoretical plates produced were used to optimise the mobile phase. At a flow rate of 1.5 ml/min, the gradient mixture of Deionised water and acetonitrile as described in chromatographic conditions (2.3) was found to be excellent, achieving good separation and showing symmetric peak for Fulvestrant and co-solvents.
3.1.1. Optimization of Wavelength
The maximum wavelength of Benzyl alcohol, Benzyl benzoate, and Fulvestrant were used to optimize the detector wavelength. The spectra revealed that the 254-nm wavelength was best for detecting Benzyl alcohol and Benzyl benzoate, while the 280-nm wavelength was best for detecting Fulvestrant.
3.1.2. Optimization of Column
The following columns (Table 1) tried for the separation of Benzyl alcohol, Benzyl benzoate and Fulvestrant.
The separation and peak shape in the Luna column were deemed to be satisfactory among all of the above columns. As a result, the Phenomenex Luna C8, 150 × 4.6 mm, 3µ column was chosen for testing.
3.1.3. Optimisation of Sample Concentration and Injection Volume
A sample concentration of 150 µg/mL of Fulvestrant, 300 µg/mL of Benzyl Alcohol, and 450 µg/mL of Benzyl Benzoate with an injection volume of 10 µL is sufficient for detection using the above specified chromatographic conditions. The linearity of Fulvestrant, Benzyl alcohol, and Benzyl benzoate in this sample concentration was established, and the data was judged to be adequate.
![]()
Table 1. Columns used for the method development.
3.1.4. Selection of Diluent for Sample Preparation
Fulvestrant is water-insoluble but easily soluble in ethanol and methanol, according to the literature. The solvent Methanol was tried based on this information. The medication is stable in methanol. Hence For diluent preparation, methanol is chosen. The chromatographic peak shape and pattern were judged to be good in this diluent.
3.1.5. Optimization of Column Oven Temperature
Based on the separation of Fulvestrant, Benzyl alcohol, and Benzyl Benzoate, the column’s temperature was set to 35˚C.
3.2. Method Validation
The process was validated in accordance with ICH recommendations. Specificity, linearity, precision, accuracy, were all examined and found to be sufficient.
3.2.1. System Suitability
The results of the system suitability parameters, which were found to be in the appropriate ranges, are shown in Table 2.
3.2.2. Specificity
There was no interference were observed at retention time of all main analytes in the analysis when the standard solution, and placebo solution were all injected, refer Table 3.
3.2.3. Linearity
The detector response was confirmed to be within the appropriate range, as indicated in Table 4.
3.2.4. Precision
The relative standard deviations of six sample preparations were within the acceptable range, as shown in Table 5.
![]()
Table 2. System suitability results.
![]()
Table 4. Results of linearity study.
![]()
Table 5. Results of precision study.
3.2.5. Accuracy
Table 6 shows that the average percent recoveries from 50% to 150% of specification were determined to be within the limitations.
3.2.6. Ruggedness
The percent relative standard deviations of fulvestrant, benzyl alcohol, and benzyl benzoate were found to be within the limitations as shown in Table 7.
3.2.7. Stability of Solutions
Study of standard and sample solution stability on three consecutive days at 5˚C and 25˚C, a solution was used to calculate percent Assay of drug and co-solvent peak areas. The percent Assay was determined to be within the limits at 5 and 25 degrees, demonstrating that the drug and co-solvents were relatively stable at room and refrigerator temperatures. For quality control (QC) of samples, the stability of the drug product was tested under various conditions. When the test was compared to freshly analysed QC samples, no differences in assay were identified. As a result, no product stability issues were discovered during the analysis.
4. Discussion
The RP-HPLC procedure was used to create an assay method for determining the concentration of Fulvestrant and its co-solvents in Fulvestrant injection. The current study’s major challenges included obtaining optimum resolution between
![]()
Table 6. Results of accuracy study.
![]()
Table 7. Comparison results of Fulvestrant, Benzyl alcohol and Benzyl benzoate between two analysts.
% RSD-Percentage relative standard deviation.
the Benzyl benzoate and fulvestrant peaks with shorter run time, selecting suitable mobile phase composition using a gradient method to obtain optimum resolution between the co-solvent peaks, and optimizing the test concentration to obtain the desired response levels for the fulvestrant and its co-solvents. Fulvestrant injection was established employing a precise, accurate, and reliable approach. Fulvestrant injection formulation contains Fulvestrant (Active), Benzyl alcohol (Co-solvent), and Benzyl benzoate (Co-solvent), although only the Assay technique of Fulvestrant injection has been documented for measurement of Fulvestrant. As a result, a single analytical method for quantifying the three components Fulvestrant, Benzyl alcohol, and Benzyl benzoate has been developed and validated.
5. Conclusion
The proposed approach was successful in separating and determining Fulvestrant, Benzyl alcohol, and Benzyl benzoate. This method can be used to analyse Fulvestrant, Benzyl alcohol, and Benzyl benzoate in the Fulvestrant injection. The development of an assay method for quantifying fulvestrant and its co-solvents in fulvestrant injection has not yet been published. This is the first RP-HPLC method that can resolve all of the fulvestrant and its co-solvents in a fulvestrant injection sample.