1. Introduction
Among abnormal skin scarring, keloids, and hypertrophic scars, represent the most extreme examples of pathological response to wound healing. Their clinical characteristics included inflammation, cell proliferation, hyperpigmentation, and matrix remodeling [1]. Therapeutic options for the treatment of these scars consist of intralesional steroid injection, surgery, radiotherapy, 5-fluorouracil, and silicone sheeting. However, modern medicine does not offer very effective treatment ways [2]. Recently it was demonstrated that the inhibition tyrosinase activity was a promoting way for dermatological toxicities and tumor treatment; and the natural products were indexed like some tyrosinase inhibitors [3] [4] [5]. African traditional medicines offer several remedies including plant oleoresins for the efficient treatment of cicatrizations [6]. In West Africa, oleoresins are found in some traditional remedies used to heal or to prevent keloids and hypertrophic scars [7]. Oleoresins are mainly constituted by mono-, sesqui- and diterpenes with interesting pharmacology properties [8]. In Burkina Faso, there are various ethnopharmacological indications, including the treatment of gastrointestinal complaints, diversities of genitourinary tract diseases, wound healing, and skin ailments [9]. According to the previous literature, most of the pharmacological activities of Daniellia oliveri oleoresins might be assigned to the diterpene acid [8] [10]. Labdane-type oleoresins compounds have already been linked to several reported biological activities including antioxidant, anti-inflammatory, antibacterial, antinociceptive, wound healing and cytotoxicity [11] [12] [13].
This study aims to evaluate the benefit of traditional use of Daniellia oliveri oleoresins in the healing of keloids. It attempts to complete the scientific literature with chemical characterization of daniellic acid, to investigate molecular properties, to evaluate antioxidant property, the cell proliferation, and the cell free mushroom tyrosinase inhibition activity.
2. Material and Methods
2.1. Chemicals and Tumor Cells Origins
All chemicals, substrates, standards, and analytical grade solvents used for the isolation of daniellic acid and bioactivities assays were supplied by Sigma (Belgium) and Chemlab (Belgium). Various tumor cells including melanomas (human A375, Mel-juso, Malme-3M, SK-MEL-28, UACC-257 and murine B16F10), carcinomas (lung A549 and brest MCF-7) and glioblastoma (U373) were from American Type Culture Collection (ATCC; Manassas, VA, USA).
2.2. Plant Material
The Daniellia oliveri plant oleoresin was obtained from authorized traditional healer, at Burkina Faso central region in October 2012. Specimen (NA_152) is available at the plant biology and ecology laboratory herbarium (University Joseph Ki-Zerbo, UFR/SVT, Ouagadougou, Burkina Faso).
2.3. Extraction and Isolation of Daniellic Acid from Daniellia oliveri Oleoresins
100 g of Daniellia oliveri oleoresins were placed at −80˚C before extraction. The oleoresins were grinded, and the powder was stirred twice with 1 L and 500 mL of CHCl3 respectively for 24 h. The organic phases were pooled and evaporated under reduced pressure to yield 92 g of dark yellow visquous liquid. This terpenoid-rich extract was used as a solid deposit for column chromatography (300 g silica gel). Oleoresins chloroformic extract was eluted with gradient of CH2Cl2/CH3OH (100/0 to 90/10 v/v). Fractions containing the major compound (Rf = 0.08 with CH2Cl2 as eluent for the TLC) were evaporated to yield 45 g of yellow visquous liquid. Furthermore, 6 g dissolved in CH2Cl2 was precipitated in methanol and 3 g of the white precipitation was chromatographed on silica gel with CH2Cl2. Pure fractions (as seen by TLC with CH2Cl2) were evaporated to dryness to yield 200 mg of daniellic acid. The purity of the compounds was checked on 13C-NMR and estimated for more than 95% w/w [14].
2.4. Daniellic Acid Structural Elucidation
2.4.1. Spectra Data Obtention
The chemical structure of daniellic acid isolated from Daniellia oliveri oleoresins was determined by using UV, 1H-NMR, 13C-NMR, HSQC, HMBC, and IR analysis. NMR spectra (CDCl3) were recorded using a Bruker 300 MHz spectrometer. IR spectrum was recorded with a PERKIN-ELMER 1420 Ratio Recording Infrared spectrometer as KBr disc (γ, cm−1).
2.4.2. Molecular Properties
The online chemo-informatics software Molinspiration (version 2018.03) was used to establish the molecular properties and drug-likeness/bioactivity score of daniellic acid compared with standards, kojic acid, and quercetin [14]. This analysis provides a molecule activity score (a number, typically between −3 and 3; and molecules with the highest and positive activity score have the highest probability to be active.
2.5. Antioxidant Assays
2.5.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
The DPPH radical scavenging activity of compounds was determined according to Nacoulma et al. (2012) methods with modifications by using 96 wells multiplate reader [15]. The assay mixture contained 200 µL of DPPH radical in methanolic solution (20 mg/L) and different concentrations of compound daniellic acid or quercetin (100 µL) were rapidly mixed and incubated for 30 min at room temperature. The absorbance was measured at 517 nm and DPPH-scavenging activity was calculated by using the standard Equation (1). In this assay quercetin is used as positive control and the experiments were performed in duplicate (n = 2 × 3).
(1)
B was the absorbance in presence of compounds and A the blank absorbances.
2.5.2. Ferric Reducing Antioxidant/Power (FRAP) Assay
The ability of the compound to reduce iron (III) was measured as described by Nacoulma et al. [15]. In brief, 500 µL of compounds daniellic acid, quercetin or ascorbic acid (0 - 100 µg/mL) was mixed together with 1.25 mL of phosphate buffer (0.2 M, pH 6.6) and 1.25 mL of 1% potassium hexacyanoferrate [K3Fe(CN)6] aqueous solution. After 30 min of incubation at 50˚C, 1.25 mL of 10% trichloroacetic acid were added and the mixture was centrifuged for 10 min (3000 rpm). The upper layer of the solution (0.625 mL) was then mixed with 125 µL of FeCl3 solution (0.1% in H2O) and 625 µL of H2O. The absorbencies were recorded at 700 nm and ascorbic acid was used to produce the calibration curve (y = 0.008x − 0.064; r2 = 0.99; p < 0.05). The iron (III) reducing activity was expressed in mg ascorbic acid equivalents per gram (mgAAE/g). Quercetin was used as positive control and the experiments were performed in duplicate (n = 2 × 3).
2.6. Inhibitory Effect on Cell-Free Mushroom Tyrosinase Activity
To investigate the effect of daniellic acid and some standards (kojic acid and quercetin) on the tyrosinase activity, different concentrations of each compound were pre-incubated with enzymes at room temperature. Tyrosinase activity was measured by replacing the phosphate buffer with daniellic acid or standards (kojic acid and quercetin). Briefly, 100 µL of aqueous solution of mushroom tyrosinase (50 units) was added to a 96-well microplate in a total volume of 300 µL containing L-DOPA (5 mM) solution in 50 mM phosphate buffer (pH 6.5) without or with compounds in different concentrations. The assay mixture was incubated at 25˚C for 30 min. Following incubation, the amount of dopachrome produced in the reaction mixture was determined spectrophotometrically according to Chiang et al. previous methods with slight modifications [16]. IC50 refers to the concentration of a substance that inhibits a standard response by 50% of the activity. IC50 values were derived from concentrations where the Y-axis revealed a 50% inhibition. IC50 was determined from dose-response-inhibition curves, by fitting experimental data to a non-linear regression using a polynomial curve (GraphPad Prism 5.0). A nonlinear regression of the Michaelis–Menten curve was used to obtain the most accurate values of Michaelis–Menten constant (Km) and maximum velocity (Vmax). Statistical significance level and linear regression were carried out with GraphPad Prism 5.0. The experiments were performed in duplicate (n = 2 × 2).
2.7. Anti-Proliferative Studies
2.7.1. Cell Culture Conditions
Six melanoma cell lines (five human and one murine) and 3 other tumor cell lines (A549 lung carcinoma, MCF-7 breast adenocarcinoma and U373 brain glioblastoma) from ATCC (USA) were used to investigate antiproliferative activity of the isolate compound. Cells were propagated in red RPMI-1640 medium supplemented with 10% heated-inactivated FCS, penicillin, streptomycin, gentamicin, and L-glutamine (Gibco, USA), and incubated in a 5% CO2 incubator at 37˚C. The medium was renewed every 72 hours. The cells are treated with trypsin and suspended in medium to obtain a density allowing a specific cellular count for biological analysis.
2.7.2. Cell Viability Assay
Cell viability was determined by using the MTT (3-[4,5-dimethylthiazole-2-yl]- 2,5-diphenyltetrazolium bromide) colorimetric assay (MTT, Sigma, USA). Mitochondrial dehydrogenases metabolize MTT salt to an orange formazan dye, which was measured at 570 nm by using a scanning multi-well spectrophotometer. Briefly, 2.104 cells were seeded in 96-well plates; after 24 h stay in the CO2 incubator, different dilutions of daniellic acid were added. After 72 h incubation, the media were replaced by an MTT solution in white RPMI-1640 medium (0.5 mg/mL) and the plates were left for 3 h in the 5% CO2 incubator at 37˚C. The MTT solution after centrifugation was then replaced by DMSO to dissolve the crystals of reduced formazan (15 min under low agitation, 700 rpm) and the absorbance was measured at 570 nm versus 620 nm reference [16]. Concentration of sample which inhibited 50% (IC50) of cell proliferation was determined from dose-response-inhibition curves, by fitting experimental data to a non-linear regression using a polynomial curve (GraphPad Prism 5.0). The experiments were performed in duplicate (n = 2 × 6).
2.7.3. Compounds Interference in Melanin Production Evaluation
Determination of melanin content was performed by using a modified method described by Chiang et al. [16]. The blank did not contain IBMX (3-Isobutyl-1- methylxanthine). After washing twice with PBS, samples were dissolved in 100 µL of NaOH (2N), incubated at 37˚C (1 h) and then mixed to solubilize the melanin. The optical density of the mixed solution was measured at 405 nm. The melanin content of each treatment was corrected with the total protein content measured from a standard curve prepared with BSA concentrations (Sigma, USA). The controls were normalized to 100% and treatments were expressed according to the percentage of the control (untreated melanocytes). The results are obtained from three independent experiments and the melanin content; pondered by the total protein that is compared with result of the control group.
2.8. Data Analysis
All data are represented as the Mean ± Standard deviation (S.D) of replicates. Group means were compared using the Kruskal-Wallis or Mann-Whitney non-parametric test (Statistica 7, USA). Values were determined to be significant when *P < 0.05 and **P < 0.01.
3. Results and Discussion
3.1. Daniellic Acid Structural Elucidation
3.1.1. Spectra Data Analysis
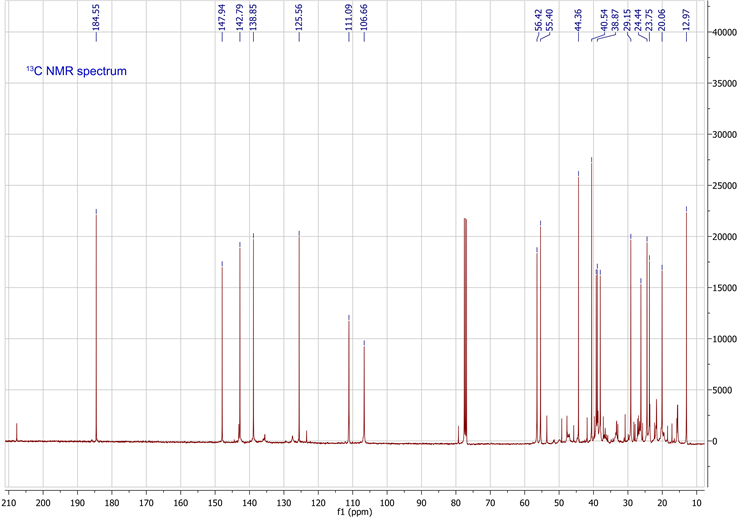
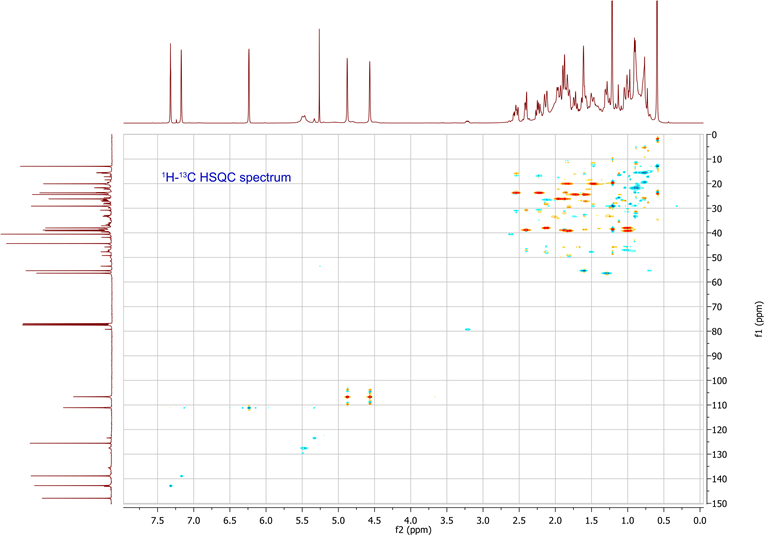
The molecular formula was determined in conjunction with its UV, IR, and NMR spectra (Table 1). 1H-NMR, 13C-NMR, HSQC and HMBC data are available in supplementary data. The UV spectrum showed a maximum absorption at 235nm, while the IR spectrum showed absorption bands, indicating the presence of a γ-substitute furan ring (ether C-O at 1070 cm−1 and aromatic C=C at 1470 cm−1), unsaturated vinyldiene (-C=CH2 at 1664 cm−1) and carboxylic acid (C-O at 1270 cm−1, C=O at 1720 cm−1 and O-H at 2960 cm−1) functional groups. The 1H-NMR and 13C-NMR spectrometric properties were globally superimposable onto those of the isomer polyalthic acid [12]. The 1H-NMR spectrum of daniellic acid in CDCl3 in Table 1 exhibited signals for two methyl groups [δH 1.22, 0.59 ppm, (3H each, all s, H-19 and 20)], five methine groups with a characteristic oxymethine group in furan moiety presenting 2 protons in alpha position and one in beta position [δH 7.32, 7.17 and 6.23 ppm (1H each, m, H-15; s, H-16;
![]()
Table 1. NMR data for daniellic acid in CDCl3 (300 MHz, δ in ppm; J in Hz).
and d, 1.5 H-14)], one methine group in cyclohexane ring [δH 1.28 ppm, (1H, m, H-5)], and another cyclic methine group [δH 1.61 ppm, (1H, m, H-9)]. It was found seven methylene groups with three in cyclohexane ring [δH 1.81, 1.01; 1.84, 1.48; 2.13, 1.00 ppm, (2H each, all m, H-1, 2 and 3)], two cyclic methylene groups [δH 1.96, 1.87; 2.40, 1.87 ppm, (2H each, all m, H-6 and 7)], and two non-cyclic methylene groups [δH 1.73, 1.60; 2.54, 2.23 ppm, (2H each, all m, H-11 and 12)], which were characteristic of labdane-diterpene skeleton. In addition, 1H-NMR spectrum also indicated presence of carboxylic acid group [δH 9.73 ppm, (1H, s, H-18)] and an ethylene group [δH 4.88, 4.57 ppm, (2H, d, d, J = 1.5, 1.5 Hz, H-17)]. By using 13C-NMR spectrum and HSQC spectrum data, the planar structure was clarified unambiguously (Table 1), and was elucidated the presence of 20 carbon signals assigned to two methyls with tertiary carbons [δC 29.1, 13.0 ppm (C-19, 20)], seven methylenes carbons in fives from saturated ring [δC 39.2, 20.1, 38.0, 26.2, 38.9 ppm (C-1, 2, 3, 6, 7)]; and two from aliphatic bridge [δC 24.4, 237.7 ppm (C-11, 12)], four vinylic carbons with one from decalone [δC 106.7 ppm (C-17)] and three from furan ring [δC 111.1, 142.8, 138.8 ppm (C-14, 15, 16)], two methine carbons from decalone [δC 56.4, 55.4 ppm (C-5, 9)], four non protonated carbons in two olefinic [δC 147.9, 125.6 ppm (C-8, 13)] and two quaternary carbons [δC 44.4, 40.2 ppm (C-4, 10)] and a carboxylic carbon [δC 184.5 ppm (C-18)]. Figure 1 showed the chemical structure of daniellic acid and 3JC-H correlations observed in the HMBC spectrum. Besides, 13C-NMR spectrum showed an impurity of the same family (<5% approximately). The molecular weight of daniellic acid was 316.2038 and estimated for C20H28O3. According to the best of our knowledge, this is the first report of the isolation and elucidation of a labdane-type diterpene from resins in Burkina Faso. Daniellic acid, the major diterpene acid found in Daniellia oliveri oleoresins
![]()
Figure 1. Daniellic acid and the 3JC-H HMBC correlations used to confirm its structure.
from India, belongs to the labdane-type diterpene [17]. The same compounds and isomers were isolated from Copaifera duckei Dwyer and Copaifera reticulata Ducke oleoresins from Brazil [18] [19].
3.1.2. Molecular Properties
Drug-likeness analysis in-silico screening was useful for drug development for improving the activity and the selectivity, as well as drug-like properties [20] [21]. These properties influenced the absorption, bioavailability, reactivity and toxicity, metabolic stability of the molecule and are related to its potential to be used as a pharmaceutical drug [20] [21]. Daniellic acid, kojic acid, and quercetin properties were shown in Table 2. Similar properties of such compounds were previously demonstrated [10]. The in-silico screening indicated that daniellic acid showed the highest hydrophobicity (LogP) compared to quercetin and kojic acid. Besides, this labdane-type diterpene exhibited the lowest electron distribution and hydrogen bounding characteristics that limits the oxydo-reduction potential. The Lipinski’s rules were not violated in any evaluated compounds (Table 2). The bioactivity of these compounds evaluated by calculating the activity score for G-protein coupled receptor (GPCR) ligand, ion channel modulator, kinase inhibitor, and nuclear receptor ligand were shown in Table 2. The positive bioactivity score of daniellic acid, especially its ability to act as nuclear receptor ligand and enzyme inhibitor (score > 0.50) is a clear indication that this compound is a potential lead for drug development through structure-activity relationship studies (Table 2). Daniellic acid was presented as having the best bioactivity score
![]()
Table 2. Molecular properties and bioactivity scores of daniellic acid, kojic acid and quercetin.
Log P is based on octanol-water partition coefficient, H bond acceptors include O and N atoms, H bond donors include OH and NH groups, GPCR is G-protein Coupled Receptor.
compared to kojic acid, and quercetin. Similar observations concerning daniellic acid as a stereoisomer polyalthic acid, were already proved by Atolani and Olatunji studies [10].
3.2. Biological Activities
3.2.1. Antioxidant Effect
Daniellic acid antioxidant capacity was investigated by using DPPH-radical scavenging ability and Ferric reducing antioxidant power. As indicated in Figure 2(A) and Figure 2(B), daniellic acid showed an antioxidant activity 4-fold and 2-fold less than quercetin in DPPH and FRAP assay respectively (p < 0.01). This result was consistent with the predicted molecular properties which indicated that daniellic acid possesses a limited oxydo-reduction capacity, related to H bounding characteristics compared to quercetin. The anti-DPPH activity of daniellic acid could be a justification of the oleoresin antioxidant capacity which was previously demonstrated [8].
3.2.2. Inhibitory Effects on Cell-Free Mushroom Tyrosinase Activity
Tyrosinase is a copper-containing enzyme widely distributed in microorganisms, animals, and plants, and it is a key enzyme in melanin biosynthesis, and it determines the color of skin and hair. Tyrosinase inhibitors are clinically used for the treatment of several dermatologic disorders associated with melanin hyperpigmentation [22]. Kojic acid and quercetin, well-documented tyrosinase inhibitors were used as model compounds for comparison with daniellic acid [23] [24]. So, the potential of daniellic acid as enzymatic inhibitor was then evaluated on cell-free mushroom tyrosinase. The effect on the oxidation of L-DOPA to melanin production was shown in Figure 3. Daniellic acid inhibited mushroom tyrosinase activity in a linear concentration-dependent (Figure 3(A)). These results indicated that the enzymatic interaction type of these two compounds was different (Figure 3(B)). The concentration of daniellic acid required for 50% inhibition of mushroom tyrosinase activity rate (IC50) was 1.2 mM which was 6-fold less active than kojic acid (0.2 mM) (Figure 3(A)). This was the first time daniellic acid
![]()
Figure 2. Antioxidant effect of daniellic acid. (A) DPPH; (B) FRAP.
![]()
Figure 3. Inhibitory effects on cell-free mushroom tyrosinase activity and enzyme kinetics. (A) Michaelis Menten curves; (B) Line Weaver-Burk curves.
tyrosinase inhibition effect was studied with the similar activity found in other labdane type-diterpene compounds [25] [26].
3.2.3. Enzyme Kinetic Characteristics
The kinetic behavior of mushroom tyrosinase during their diphenolase activity was studied for characterizing daniellic acid inhibition type. The kinetic parameters of daniellic mushroom tyrosinase inhibition obtained from a Lineweaver-Burk plot showed that enzyme Michaelis–Menten constant (Km) was 0.28 mM and maximum enzymatic rate or Velocity (Vmax) was 7.34 mM/min (Figure 3(B) and Table 3). The double reciprocal plot in Figure 3(B), indicated that daniellic acid exhibited the kinetic characteristics to a non-competitive mixed type inhibitor (Figure 3(B)). Kinetic studies showed that both Michaelis-Menten constant (Km) and maximum Velocity (Vmax) were affected by the daniellic acid. These kinetic behaviors were typical of a mixed-type inhibitor that means that daniellic acid interacted with the enzyme-substrate complex with high affinity compared to free enzyme (Ki <
; p < 0.05). The affinity to the enzyme-substrate complex was related to the inhibitor concentration compared to the affinity for
![]()
Table 3. Mushroom tyrosinase inhibition characteristics.
the free enzyme which remained constant when the inhibitor concentration increased (Table 3). Daniellic acid inhibition of tyrosinase was like quercetin’s but differed to kojic acid’s which exhibited a non-competitive pure-type inhibition. The difference between kinetic parameters (
~ Ki; Km and IC50) in kojic acid tyrosinase inhibition was not significant (p > 0.05). It is meaning that kojic acid interacted similarly with the enzyme-substrate complex compared to free enzyme (Table 3). These data proved that the daniellic acid and quercetin enzymes’ inhibitory properties were found in-silico screening.
3.2.4. Effects on Cell Proliferation and Melanogenesis Inhibition in Cell
Excessive cell proliferation was involved in the abnormal wound healing causing keloids and hypertrophic scars [1]. As shown in Table 4, daniellic acid had cytotoxic activity on cell lines and showed a selectivity index when there is comparison between melanomas cell lines with other cell lines (p < 0.05). IC50 values were in the range from 10 to 100 µM except for Malme-3M cell line (IC50 > 100 µM). These data characterized some moderate cytotoxic activities (IC50 from 10 to 50 µM) or weaker activities (IC50 from 50 to 100 µM) and could explain D. oliveri oleoresin previous cytotoxicity in PC3 cell line [8]. Polyalthic acid, stereoisomer of daniellic acid, labdane and abietane diterpenoids had weak to moderate cytotoxic activities against several tumor cell lines [18] [19].
Melanin plays a major role in skin protection from ultra-violet (UV) damage and an excess production was reported in abnormal skin hyperpigmentation in keloids [1] [27]. The evaluation of melanin content was used as an index of in cellulo melanogenesis inhibition analysis. Melanin and total protein content were attempted to be measured in melanocytes (B16F10 and Malme-3M) treated with daniellic and kojic acids at concentration of 45 µM and 20 µM, respectively (Figure 4). According to this data, B16F10 melanin production was significantly stimulated by IBMX rather than Malme-3M (p < 0.05). Kojic and daniellic acids lead to a half-reduction of melanin’s production in B16F10 cell under IBMX stimulation (p < 0.05). The same observation was effective in Malme-3M melanin production with a significant daniellic acid action more than kojic acid (p < 0.05)
![]()
Table 4. Daniellic acid cytotoxicity.
![]()
Figure 4. Anti-melanogenesis effect of daniellic acid. (+: presence, −: absence)
without reducing cell viabilities. It is suggested that the enzyme inhibition by daniellic acid could be one of the mechanisms of inhibition in anti-melanogenesis. The antiproliferative and anti-melanogenesis could justify partially the traditional uses of Daniellia oliveri like as gastrointestinal complaints, diversities of genitor-urinary tract diseases, wound healing treatments.
4. Conclusion
Daniellic acid, is a labdane type-diterpenoid isolated for the first time from Daniellia oliveri oleoresins of Burkina Faso. It showed some antiradical and antiproliferative activities with tyrosinase inhibition activities. This compound possessed anti-DPPH and iron (III) reducing abilities. It was able to inhibit 9 tumor cells with IC50 going from 0.03 mM to 0.14 mM. Remarkably it was inhibited tyrosinase activity and presented noncompetitive mixed type mechanism. Daniellic acids induced a half-reduction of melanin production in B16F10 cell in IBMX stimulation. The current results could be significant information to validate the traditional uses and the pharmacological significances of oleoresins. Chemical and biological activities of daniellic acid will be useful for the upcoming research in this area.
Supplementary Data Daniellic Acid