Deterministic Parsing Model of the Compound Biological Effectiveness (CBE) Factor for Intracellular 10Boron Distribution in Boron Neutron Capture Therapy ()
1. Introduction
Many types of pilot innovative accelerator-based neutron source for neutron capture therapy with lithium target were designed [1] - [3] and many inventions for the progressive power run-up were reported [4] [5] . In Japan, implemented deployment of accelerator-driven neutron source for Boron Neutron Capture Therapy (BNCT) is scheduled in 2014 in National Cancer Center, of which system was designed with the production of neutrons via threshold 7Li(p, n)7Be reaction at 25 kW proton beam with energy of 2.5 MeV, which was designed to dovetail the narrow peak band resonance of lithium target and started its installation at middle of 2013. This BNCT device is expected to offer the potential for achieving the objects of which any treatment capable of sterilizing the primary tumor locally will result in a high probability of cure.
BNCT is a targeted radio-therapeutic modality used for the treatment of brain tumors and melanoma and a bimodal approach to cancer therapy. Before BNCT, Boron-10(10B)-enriched compounds are used to deliver 10B to tumors. Once tumor uptake of a given boron delivery agent relative to the surrounding normal tissues and blood has been maximized and then irradiation with low-energy neutron takes place. An alternative boron delivery agent, p-borononphenylalaine (BPA) instead of administration of the boron delivery agent borocaptate sodium (BSH), is being used together with mode deeply penetrating epithermal neutron beam [6] . BNCT was extensively reviewed in two recent articles [7] [8] and the targeting effectiveness of BNCT was dependent upon the preferential delivery of 10B to the primary tumor and its metastatic spread.
In defining the biological effects of the 10B(p, α)7Li neutron capture reaction relative to photons, the term compound biological effectiveness (CBE) factor was used as an alternative to RBE. Calculation of the CBE factor is similar to that of the RBE factor [9] . Equating the X-ray ED50 dose with a BNC dose (beam + BSH) that gives the same end point of a 50% incident of ulceration produces the following equation:
The CBE factor = [(X-ray ED50) − (thermal beam component of ED50 × RBE]/10B(p, α)7Li component of ED50.
Recently, the CBE factors concerning to tumor, skin lung, liver [10] [11] , heart [12] and oral mucosal tissues [13] were reported and prospect of actually using BNCT for the patients has been developing under the right cir- cumstances. However, there is no theoretical unified explanation of the CBE factors for normal tissues and tumor, despite the fact that significance of high precision of the CBE factor evaluation is requested for the patients.
The purpose of the present investigation was to demonstrate the deterministic parsing model of the CBE factor for intracellular 10B distribution and discover the unified methodology for the evaluation of the CBE factors for normal tissues and tumor in BNCT.
2. Materials and Methods
2.1. 10B Concentration and the CEB Factors of BPA
As for the CBE factor and boron concentration data set of BPA previously obtained in biological test for normal tumor, skin lung, liver [10] [11] , heart [12] and oral mucosal tissues [13] [14] , we can classify these data into two main groups, tumor and normal tissue and no relationship between NB-max and CBE factor was not found in normal tissue group (Table 1, Figure 1).
2.2. Mathematical Analysis Model for the CBE Factor
2.2.1. Definition of the Duplicate Volume of the α Range and Proximity of Boron Atoms
Thermal neutrons or epithermal neutrons, which become thermalized at depth in tissue, are captured by 10B atoms, with the resultant fission reaction producing α-particles and lithium-7 (7Li) ions in BNCT.
These particles have a limited range of <9 μm in tissue. Thus, it is possible to selectively irradiate a tumor with high Linear Energy Transfer (LET) radiation, while sparing the adjacent normal tissues, theoretically. 10BPA is designed to be selectively spatially-integrated into the tumor cells and boron atoms are distributed in a
![]()
Table 1. The data set of the CBE factor and Nmax for normal tissues and tumour obtained in the case of BPA administration.
1)Fukuda et al., 1994, 2)Kiger et al., 2008, 3)Suzuki et al., 2000, 4)-7)Morris et al., 1997 with BPA experiments of mice.
![]()
Figure 1. The relationship between the CBE factor and Nmax in the case of 10BPA administration.
cell and to damage these area due to 10B(n, α)7Li reaction (Figure 2).
Distance r0 and 2r were defined as α range and distance between boron atoms in the figure. There are two conditions of boron atom exists beyond the α range (r0 ≤ r) (Figure 2(b)) and boron atoms within α range (r0 > r) mainly investigated in present study (Figure 2(c)).
In the case study of (c), there is the duplicate volume Vd, where is exposed under duplicated irradiation by α particles from the both side of boron atoms.
Here, we postulate the correlation between the CBE factor and Vd in the case of (c) as;
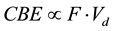 (1)
(1)
where proximity F is the number of boron atoms surrounding to in the centered born atom and the geometric duplicated volume of Vd is given by the formula of the volume of spherical cap with r0 and r;
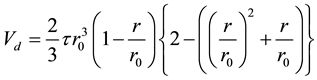 (2)
(2)
Here, Vd is normalized by the volume 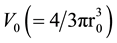 given as the α range volume, and the normalized duplicate volume Vdnor can be expressed as the ratio of Vd and V0 (Figure 3).
given as the α range volume, and the normalized duplicate volume Vdnor can be expressed as the ratio of Vd and V0 (Figure 3).
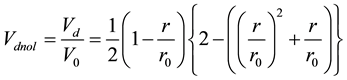 (3)
(3)
![]() (a) (b) (c)
(a) (b) (c)
Figure 2. Images of irradiation damage and its effective area caused by (a) γ ray, α particle irradiation due to 10B(n, α)7Li reaction in the (b) access distance (r0 < r) and (c) distance of closet approach (r0 > r) between boron atoms.
![]() (a) (b)
(a) (b)
Figure 3. Definition of (a) the duplicate volume of spherical cap of two α range volumes and (b) the change in the duplication volune as a function of the distance between boron atoms.
2.2.2. Space Factor n
After BPA administration, bio-distribution of 10B atoms is provided into a cell (Figure 4) and boron atoms become closer with each other, but exist beyond α range in the case of Figure 4(a).
In contrast to this case, boron atoms are spatially-integrated selectively in a cell as BPA administration pro- ceeds, very high dose of boron concentration is achieved as shown in the Figure 4(b) and Figure 4(c).
Therefore, it can be assumed that there is a relationship between boron concentration N and distance of boron atoms r within the limited volume and space of cells (20 - 200 μm);
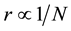 (4)
(4)
Thus, we expressed the relationship between normalized boron concentration N/Nth and normalized r/r0 here as;
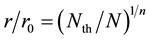 (5)
(5)
where n is the space factor (=1, 2, 3) and Nth is the threshold of N.
From Equation (3) and (5), Vdnol in Equation (3) is replaced as;
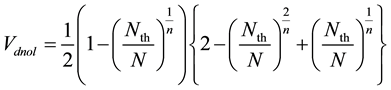 (6)
(6)
2.2.3. Definition of the Deterministic Parsing CBE Factor Model
From Equations (1) and (6), we defined the deterministic parsing CBE factor model as;
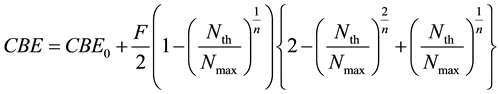 (7)
(7)
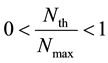 (8)
(8)
where Nmax is the saturated boron concentration mentioned-below and CBE0, F and n are constants.
2.3. Theoretical Calculation Method and Its Procedure
Iterative calculation technique was applied to obtain constants CBE0, F, n and the eigen values of Nth in Equation (7) for each cases (Figure 5).
We started iteration calculation with data set of (Nmax, CBE) in Table 1 and arbitrary values of CBE0, F and n in Equation (7) to obtain Nth value, where n was give as a number of 1 - 3 (process (a)). With the calculation results of Nth value obtained in each cases, first data set was rewritten as (Nth/Nmax, CBE) and calculated again to optimize constants CBE0 and F in the equation by least square method with these new data set (process (b)). Then, these calculation processes were terminated when the identical calculated CBE values to the original CBE values were obtained.
![]()
Figure 4. Concentration process of boron atoms into cell after BPA administration.
![]()
Figure 5. Iterative calculation process to obtain constants CBE0, F, n and eigen value Nth in Equation (7).
3. Results and Discussions
3.1. The CBE Factor Model for Normal Tissues and Tumor
The calculated CBE values obtained by the above-mentioned theoretical calculation procedure are listed with the original CBE values in the table (Table 2).
According to Table 2, it was found that the calculated CBE values obtained are almost identical to the original CBE factors, when CBE0, F and n are selected as 0.5, 8 and 3, respectively. Threshold values, Nth given by this calculation method are also listed for all cases in this table.
With these results, the original CBE factors are plotted as a function of the ratio, Nth/Nmax and the solid line in this figure was provided by Equation (7) (Figure 6).
It is clear that there is a good correlation between the original CBE factors and Nth/Nmax for all cases including tumor and it is concluded that the original CBE factors can be well expressed by Equation (7) with a parameter Nth/Nmax.
Here we emphasise that the a threshold Nth exists to surmount the potential barrier of BPA concentration, for which less than Nth, remarkable damages by α particles cannot occur in BNCT [15] .
3.2. Definition of Three Constants in the CBE Factor Model
3.2.1. Determination of the CBE Factor Depend on Boron Dose Level
The CBE0 was defined as a intercept constant in Equation (7) and the CBE factor is given by CBE0 value, which is determined as 0.5 for each intracellular distribution patterns (Figure 7).
![]()
Table 2. The results of iterative calculation of the CBE factor model presented in Equation (7).
![]()
![]()
Figure 6. Deterministic parsing Model for the CBE factor with Nth/Nmax.
![]() (a) (b) (c)
(a) (b) (c)
Figure 7. The CBE factor defined by CBE0 constant in the cases of intracellular distribution of boron. (a) r < r0; (b) r = r0; (c) r > r0.
In the figure, pattern a) is correspondent to lower boron concentration and boron atoms are distributed in a cell in heterogeneous condition. This condition can be defined in the case of r > r0 in Figure 2(b) and each boron atom induces irradiation damages individually and significant damage is not expected because of lack of boron concentration in the affected area. As BPA administration proceeding, born concentration increases to the level of conditions b) and c) in the figure. The condition b) is homogeneous and critical case of r = r0, in which boron dose N reaches to the threshold value Nth (N = Nth). In both cases of a) and b), the CBE factors are expected to be small value and given as constant level of CBE0.
In contrast with these conditions, the condition c) is homogeneous over-packed case and correspond to r < r0 (N > Nth) in Figure 2(c). In this case, the CBE factor can be expressed by boron concentration N in Equation (7).
Therefore, the CBE factor can be defined by CBE0 as following;
![]() (9)
(9)
3.2.2. Sterically-Oriented Intracellular Distribution of Boron Atom
There are many types of sterically congested mode of 10B atom distribution (Figure 8). Here, confront factor F in Equation (7) is defined as a sterically-oriented number of boron atoms surrounding center boron atom and is given as 8 in previous caption (2.1).
F = 8 (10)
This result indicates that the data case in present study is corresponding to octahedron type and 8 boron atoms confronted to the center boron atom (Figure 8(d)).
Higher mode pattern also suggests a higher level of boron concentration under surgically observation and it is interest that proximity F informs sterically-oriented intracellular distribution of boron atom concentrated in the affected area.
3.2.3. Intracellular Cubic Array of Boron Atom
To define the relationship between the distance between boron atoms in a limited cell space, space factor n was adopted in Equation (5). There are allowable three patterns of space factor under the Equation (5) (Figure 9).
The space factor n = 1, 2 and 3 addresses liner, planar and stereo type cubic array, respectively. The space factor n is calculated as 3 in caption (2.1), this fact indicates that high level of boron concentration are achieved by stereo type cubic array formed after BPA administration in normal tissues and tumor cases.
n = 3 (11)
The space factor n suggests cubic array structural extension of intracellular boron concentration, whereas F implied sterically-oriented intracellular proximity above-mentioned.
Two of these constant F and n are very important parameters to well-understanding boron concentration in normal tissues and tumor and to determine the effectiveness of BNCT.
3.3. Application of the CBE Factor Model for Human Brain Tumor
It is evident that the CBE factors for BPA, calculated using Equation (7) are the almost same values with those obtained in previous animal experiments [10] - [13] and well expressed without distinction of tissues and tumor. Considered overall, the CBE factor is given as a function of boron concentration ratio, Nth/Nmax.
In this section, we applied this calculation method to estimate the CBE factors for many types of human brain tumor.
Imahori reported dynamic PET analysis of 33 brain tumor patients contained of AII (8 patients), AIII (11) and GBM (14 Glio Blastoma Multiforme) [16] and presented typical change in 10B concentration of blood, brain tumor and normal brain measured by dynamical PET technique during L-BPA-18F administration (Figure 10).
To determine the CBE factor, the values of Nth and Nmax can be derived from the points on these typical dynamic PET curves. The Nth values were determined at the intersection points of two of fitting lines on 10B concentration curves in low dose level and Nmax values were defined at the peak value of the curves, respectively.
With these Nth and Nmax data, the CBE factors were calculated by Equation (7) for all grade of brain tumor (Figure 11).
![]() (a) (b) (c) (d)
(a) (b) (c) (d)
Figure 8. Typical intracellular distribution pattern of proximity F. (a) Dihedral; (b) Tetrahedron; (c) Hexahedron; (d) Octahedron.
![]()
Figure 9. Intracellular cubic array of boron atom as a function of r/r0.
![]()
Figure 10. Typical change in 10B concentration of brain tumour, blood and normal brain measured by dynamic PET technique.
From these results, it is found that all of these CBE factors on the lime of the CBE factor model in Equation (7), increases with tumor grade and categorized into three groups. These data were re-plotted to show more precise relationship between the CBE factor and tumor grade (Figure 12).
It is found that the CBE factors for AII and AIII vary widely whereas values for GBM display small variation and the same level of the CBE factor range of AIII.
After BPA administration, boron atoms are ingested into the cell model consisted of endoplasm and cell nucleus and Imahori reported [16] the kinetic analysis for these brain tumor patients by the Gjeddl-Patlak model [17] [18] using three-compartment rate constants (K1, k2 and k3) (Figure 13).
![]()
Figure 11. CBE factors calculated by CBE factor model in Equation (7) for three grade of brain tumour patients as a function of Nth/Nmax.
![]()
Figure 12. Distribution of the CBE factor range of brain tu- mour grade.
![]()
Figure 13. The rate constants of three compartment model for 33 brain tumor patients.
From the results, it is found that rate constant K1 into cell increases drastically as tumor grade proceeding, whereas k3 into cell nucleus decreases. This result means that the damage on toughness of these membranes deteriorates blocking capability and retention of BPA in cells due to attack of tumor to membrane of cell and nucleus, especially in GBM [16] .
This fact indicated that the effectiveness of BNCT treatment achieves improvement in the brain tumor patients of grade AII and AIII, however its effectiveness is saturated for the grade of GBM.
3.4. Application of the Calculation Method and Its Clinical Significance
Normally, cancer patients are given low doses of intravenous radioactively-labelled 18F-BPA before BNCT and diagnosed cancer by Positron-Emission-Tomography (PET). Physicians developed a treatment plan by BNCT based on PET diagnosis and then after administrates high dose of BPA to the patients.
In present paper, we emphasized existence of Nth and showed a calculation method of the CBE factor with Nth/Nmax in caption (2.1). Here, the most important thing for this calculation run is how to determined Nth and Nmax values, and we presented Nth and Nmax determination method in the case of brain tumor measured by dynamic PET technique in caption (2.3).
In practical use of this calculation method, 18F-BPA and BPA two-in-one medical mixture should be administrated into body and a small increment change of BPA concentration in tumor and normal tissue should be measured by dynamic PET technique simultaneously, as shown in the Figure 10. Then, Nth and Nmax for tumor and normal tissue can be determined respectively, and the CBE factors for tumor and normal tissue can be defined by the calculation formula in Equation (7).
4. Conclusions
In present study, deterministic parsing model of the CBE factor for intracellular 10B distribution was proposed in the following equation and at pretty much the same values as original CBE factor were obtained by the calculation to the original CBE factors for normal tissues and tumor by iteration calculation technique.
![]()
In this equation, constants values of CBE0, F and n are key factors for well-understanding of 10B structural distribution in the cells analytically derived as 0.5,8 and 3 respectively in present study.
This CBE factor model was applied to human tumor brain cases and derived good results dovetailed with empirical facts.