Fish-Zooplankton—A Predator-Prey Relations as a Key Factor for the Design of Zooplankton Distribution Sampling Program in Lake Kinneret, Israel ()
1. Introduction
The long-term Record of Zooplankton, as part of the scientific routine comprehensive limnological research of the
Lake
Kinneret
ecosystem [1] , was started by the team of Kinneret Limnological Laboratory, Israel Oceanographic and Limnological Research Company Ltd., in 1969. The responsibility of the author of this paper was the Zooplankton study. Location of sampling stations was fixed within the routine sampling program (Figure 1): the principal station at the deepest point and 5 - 7 other stations were fixed according to bathymetrical sutures at 15 - 25 m and one at 10 m depth. Consideration was given to the Epilimnion thickness during stable Thermal stratification and the Thermocline is allocated at the shallowest depth. When Thermocline is allocated at the shallowest depth, hypolimnetic samples could be collected only at the central (A) and 1 or 2 other deep stations if WL is high enough. The reason for allocation of sampling stations was defined by bathymetry (depth contour lines) (Figure 1). Additional requirements were considered for allocation of sampling station, such as the
Jordan River
inlet and outlet or sub-lacustrine salty spring inflows or the Intake of the National Water Carrier. Nevertheless, fish distribution was not concluded in the consideration of the sampling station spatial distribution. More- over, the study of fish distribution in Lake Kinneret and stock assessment by Acoustic Technology was initiated almost 20 years later [2] [3] . In this paper, the impact of the relationships between the zooplankton biomass density (LKDB 1969-2016) and fish assemblage distribution on sampling program design is discussed. Two periods were analyzed: 1) 1969-1985; 2) 1986-1995. The reason for the split between these two periods is technical: the formal data set of the 1st period include partial Hypolimnetic sampling and it was necessary to remove hypolimnetic data (below Thermocline); after 1985 due to financial limitations
![]()
Figure 1. Sampling stations (A)-(G) in lake Kinneret.
reduction of sampling stations was essential. Daily Spatial and Bathymetrical distributions of zooplankton in Lake Kinneret were previously studied as well as zooplankton ecological trait and diurnal migrations were previously documented [1] [4] - [11] . The present paper represents a long term (1969-2015) analysis of the related distributions of fish and zooplankton.
2. Material and Methods
The zooplankton sampling procedure is given in Gophen [11] and Gophen and Azoulay [12] . The data in tables 1 & 2 illustrate the sampling capacity and statistical evaluation (mean) of zooplankton (Table 1-annually; Table 2-monthly) in
Lake
Kinneret
during 1969-1985. The spatial definitions of the station locations are Periphery: C, G, F, K, D, L; Center: A; North: F, G, C; South: K, D, L (Figure 1). All data discussed in this paper were due to a similar water layer thickness (Epilimnion) and are, therefore, feasibly comparative. The documented reports of the original zooplankton raw date were recorded and computerized on tapes
![]()
Table 1. Annual means (all stations, all dates) of Zooplankton Biomass and annual means of C.V. (see text) and total number of sampled stations, in Lake Kinneret during 1969- 1985.
@Each sampled Station is a subsample removed from mixed 7 samples collected; total samples collected at fixed depth during 1969-1985 was app. 1765 × 7 = 12,355. *C.V. = Coefficient of Variation.
![]()
Table 2. Monthly means (all stations, all dates) of zooplankton biomass (g(ww)/m2) and C.V. values, during 1969-1985.
but only Epilimnetic data were filtered for the present study. Original zooplankton data presented here were converted into Biomass units (g(ww) per m2) [12] . The usage of biomass parameters of fish and its food consumption in an ecosystem with respect to long-term impacts is essential. Nevertheless, due to technical difficulties (calibration), fish data is not given by biomass but numerically. The biomass approach to the ecosystem long-term analysis of zooplankton-fish interrelation is essential because fish food (zooplankton) consumption rate is more biomass than numerically dependent. The study was restricted just to the Epilimnion because neither zooplankton nor fish densities in the Hypolimnion are negligible.
Such a retrospective analysis of data, including numerous items, require suitability of statistical methods. Raw date was taped and mean values per station, per periodical intervals (monthly, annual), were computed using indicative parameters of Coefficient of Variation (C.V). CV parameter expresses the Relative Standard Variation (RSD), i.e. the Ratio of the Standard Deviation (SD) to the Mean (X). C.V. shows the extent of variability in relation to the Mean of the population:
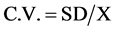
and:
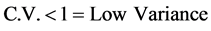
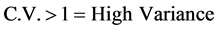
Comparative analysis between stations and periods was carried out by Test of significance known as “null hypothesis” which is assessing the strength of the evidence against it. The “null hypothesis” is a definition of “no effect” or “no difference”. The test of significance calculates the probability (p) of having an outcome at least as far from expected if “no difference” (null hypothesis; H0) was true, the computed value of p (probability) assuming H0 is true. Therefore, the smaller the p-value is, the stronger is the evidence against null hypothesis (H0) provided by the data. Practically, if p < 0.05, H0 is rejected, indicating that differences truly existed, and if p > 0.05, “no difference” is accepted.
3. Results
A summary of acoustic surveys carried out in
Lake
Kinneret
during 1987-2005 [2] are presented in Figure 2 and in Figure 3 [3] . The documented data are due to the most (>95%) common fish size frequencies monitored as <40 - 60 dB Transducer Recordings. These size frequencies are mostly due to Sardine (Lavnun; Mirogrex terraesanctae terraesanctae; Acanthobrama lissneri) and other
![]()
Figure 2. Acoustic surveys in Lake Kinneret during 1987-2005: Polynomial regression (p and r2 are given) between All Annual recordings of Fish number and years.
![]()
Figure 3. Acoustic surveys in lake Kinneret during 1987-2015: Polynomial regression (Confidence interval is indicated) between annual maximun recorded fish number and year.
species fingerlings and sub-commercial body length. Larger size frequencies are due to sub-commercial and commercial fishes of about larger than 15 cm (Total Length) (TL). Zooplankton predation pressure is mostly operated by the small sizes including larvae, fingerlings of all species and adult Sardines [13] - [24] . Presently, some of the Tilapias partly consume zooplankton. The data about stock assessment of fish in
Lake
Kinneret
is given in fish number qualified by Transducer, the acceptance of which is related to numerical density but not biomass. Calibration of dB to weight (Biomass) was partly carried out during 1987-2005 but not later. Therefore, the analysis of interrelationships between fish and zooplankton is parameterized as Biomass (Zoop.) Vs. Numerical (Fish) densities. Results of Polynomial regression shown in Figure 2 indicate a significant increase of fish densities (95% are small size frequencies) from the late 1980’s (App. 150 × 106/lake) to the early 2000’s (App. 900 × 106/lake). Detailed Data of Numerical Fish stock assessment based on Acoustic Survey [2] [3] documented in the Annual Reports of The Kinneret Limnological Laboratory [2] [3] is given in Figure 1 and Figure 2. Fish stock assessment as recorded and calculated was monitored by two different Acoustic Systems with partial temporal overlap; therefore, results are presented in separate Figure 1 and Figure 2: prominent increase of the stock community of fish is indicated since the 2000’s. Information given in the Annual Reports of LKDB [1] [2] [3] clearly indicates that the highest densities of small size fishes (>40 - <60 dB) were recorded in the peripheral regions of the lake. Fish assemblages in
Lake
Kinneret
inhabit mostly the peripheral (shallower) zones of the lake. The maps of fish density distribution in
Lake
Kinneret
confirm their very diluted density and they are mostly absent in the pelagic zone of the central and deepest part of the lake [2] [3] . The long-term (1969-2015) record of zooplankton biomass (Figure 4, and [1] ) prominently confirms a higher level during 1969-1980 and a decline later. The
![]()
Figure 4. Annual Means of all stations (A)-(G) of zooplankton biomass (g(ww)/m2). In the epilimnion of lake Kinneret during 1970-1995.
lake (all stations) averages of zooplankton biomass during the two periods 1970-1982 and 1983-1995 were 35 (SD 6) and 21 (SD 2) g(ww)/m2, respectively. Consequently, there is an overlap between the time of the decline of zooplankton biomass and the time the fish stock increase is confirmed. As a result of these observations, a periodical and spatial (station locations) analysis of zooplankton biomass was carried out. Results shown in Figure 5 indicate higher biomass of zooplankton in the central zone than in the peripheral stations of the lake and higher biomass in the Northern region compared to the Southern zone during 1969-1985. The probability value (p) that resulted from a comparative t-test (p < 0.05) between North and South stations indicated clear dissimilarity between those two lake regions. Correlation coefficients (r2) between monthly means of zooplankton biomass in all stations (each station Vs. all others) and grand total average were varied within the range of 0.4 - 0.9. Those values of correlation and others shown in Figure 6 indicate mutual ecological parameters other than fish predation which has an impact on zooplankton development. Fractional Polynomial Regressions of station A vs. all other stations, as well as northern Vs southern stations, are significantly similar (left and lower panels Figure 6). Nevertheless, results shown in the upper-left panel (Figure 6) indicate the highest biomass in the central region and higher biomass in the north than in the southern parts of the lake. For the indication of temporal effect, a periodical comparative study of individual stations was carried out (Figures 7-9): Stations D and K (south) (Figure 7), Station A (center) and station G (north) (Figure 8), as well as Stations D, G, and K, (Figure 9 lower left panel) confirm higher biomass in 1969-1985 than in 1986-1995. Moreover, the zooplankton biomass as averaged for the whole lake (Figure 9 upper left panel) and separately (1970-1982 and 19873-1995) clearly indicates the decline of zooplankton biomass from mid-1980’s and onwards. Periodical (1:1972-1994; 2: 1995-2005) Linear regression was tested between monthly lake means of zooplankton biomass (g(ww)/ m2: LKDB [1] ) record and fish stock (106 fishes /lake; [2] [3] ). The result indicates
![]()
Figure 5. Monthly mean of Epilimnetic zooplankton biomass (g(ww)/m2) during 1969-1985, in the Peripherial stations: Southern-L, K, D; Northern-C, G, F. And A (Central). Left: A-Blue (upper), Peripherial-Brown (lower); Wright: Southern-Blue (lower), Northern-Brown (upper).
![]()
Figure 6. Monthly mean of Epilimnetic zooplankton biomass (g(ww)/m2) during 1969-1985; Left: in the Peripherial stations: Southern-L, K, D; Northern-C, G, F. And in A (Central); and, Wright: Fractional Polynomial Regression of Station A Vs. all Peripherial stations. Lower: Northern (C, G, F) Vs. Southern (L, K, D).
![]()
Figure 7. Monthly mean of epilimnetic zooplankton biomass (g(ww)/m2) during 2 periods: 1969-1985 (Blue) and 1986-1995 (Brown) in two southern peripherial stations: left: station D and Wright station K.
non-significant (r2 = 0.0004) and significant (r2 = 0.5325) relations for the earlier and later periods, respectively. Diet composition for Bleak fishes of all ages in
Lake
Kinneret
comprised mostly of Zooplankton. Feeding competition does not exist if food availability is not limited as occurred in the first period and consequently prey-predator stocks are not statistically related. On the contrary, during
![]()
Figure 8. Monthly mean of epilimnetic zooplankton biomass (g(ww)/m2) during 2 periods: 1969-1985 (Blue) and 1986-1995 (Brown): Left: Station A (Central); Wright: Station G (Peripheral North).
![]()
Figure 9. Zooplankton biomass (g(ww)/m2). Wright: monthly means (all stations) of Epilimnetic zooplankton during two periods: Blue-1970-1982, Brown-1983-1995; Left: Monthly means in Peripherial Stations: Blue―1969-1985; Brown―1986-1995.
the 1995-2005 period, zooplankton biomass significantly declined and fish stock (mostly Bleaks) increased. It is likely that food source became limited and competition forcefully affected, making prey-predator relations significant in the second period.
4. Discussion
The inverse relation between Zooplankton Biomass and fish (mostly Bleaks) densities was widely documented in previous studies. An increase of fish densities in the lake started from 1998 (Figure 2) when zooplankton (mostly prey favoured Cladocerans) started a decline [30] , Figure 3). Moreover, during 1970- 1993 a significant high harvest (app. 1000 tons per annum) of bleaks was recorded. Probably reflecting a productive stock biomass producing intensive pressure on zooplankton which continuously declined. Nevertheless optimal conditions for zooplankton growth dynamics was indicated except fish predation. Three major factors affecting Zooplankton ecophysiology in
Lake
Kinneret
were considered: 1) Food availability; 2) Thermal impact and 3) predatory pressure. The study of herbivore food resources in
Lake
Kinneret
confirmed conditions of no food (algae, protozoa, detritus, and Bacteria) limitation. On the other hand thermal impact was found to have an influence but on a seasonal level and long term fluctuations were too small to produce significan conditional effect. Therefore fish predation was concluded the major factor which is significantly affect fluctuations of Herbivore zooplankton biomass in Lake Kinneret. The research team of the Kinneret Limnological Laboratory started, for the first time in
Israel
, a comprehensive integrated limnological research of the
Lake
Kinneret
ecosystem in 1968. The research of the zooplankton in the lake was given to the author of this paper. As a collaborative group of scientists, one of their first missions was to fix sampling stations to be followed routinely for the long-term achievement of a monitoring program implementation. This mission was accomplished and the monitoring program operated. The routine monitoring of limnological parameters was directed through those stations from 1969 and continues at present. A daily spatial, bathymetrical and diurnal distribution of zooplankton was previously documented ( [1] [4] [6] [7] [8] [9] [10] ). Those studies were carried out in a short time range: days and hours. But none of them gave an insight into a multiannual significance of long-term frame. This paper is the first approach to the issue of zooplankton spatial distribution in
Lake
Kinneret
as reflected in 30 years of documentation of routinely collected samples integrated with fish abundance. Ecological factors in
Lake
Kinneret
which have an impact on the natural zooplankton densities were widely explored but the analysis of zooplankton distribution as an outcome from multiannual sample data was never done before. The sampling program is first of all the outcome of available budget and manpower. These two parameters are the limiting factors for the decision about number of stations and frequency of sampling. The requirement for innovated design-based follow-up of the sampling efficiency and relevance to the research objective is obvious and the longer the term of implementation the higher the quality of the decision. The design of the present study advanced through double statistical evaluations: 1) each sampling station annually and monthly; 2) annually and monthly, including all stations. Each one of the analyses was done independently. In other words, the likeness of each station solely as a representative of the whole lake. It was documented in previous studies that zooplankton food availability is optimal and the limiting factors for zooplankton development are temperature and fish predation ( [25] - [32] ). The natural multiannual (not monthly) fluctuations of the Epilimnetic temperatures are too low to have any significant impact on zooplankton growth rate as confirmed experimentally. Therefore, the abundance of fish in
Lake
Kinneret
was taken as a major impact on the fluctuations of zooplankton density.
The first step in attempting the evaluation of the relation of zooplankton distribution to fish stock data was done by the multiannual fluctuations of both fish and zooplankton densities (Figures 2-4). The data support the suggestion that zooplankton decline was due to Fish stock enhancement. The relevance of the measured fish stock size comprises from >95% of the recorded targets to small and sub-commercial body sizes which are known as zooplanktivores. Figures 2-4 confirm the inverse relation between Zooplankton density and the documented fish stock.
The second step of the study was an attempt aimed at spatial allocating of fish shoals and zooplankton population. The acoustic surveys clearly confirmed assembling of fish flocks in the peripheral parts of the Kinneret Pelagial. It is, therefore, suggested that zooplanktivory pressure is more intensive in the peripheral stations and lower in the central part of the lake. Consequently, zooplankton biomass is higher in the central parts of the Kinneret pelagial and lower in the peripheral zones. In all stations zooplkankton biomass in winter is higher than in summer. It is the result of the lower energy investment in winter by zooplankton and higher reproduction efficiency [12] [33] [34] together with the lower feeding rate of the fish.
The next step forward in the investigation was due to the dissimilarity of zooplankton density found between the northern and southern parts. Water Current and water-mass moving directions in Lake Kinneret were widely studied and documented as well as the distribution of the Jordan River input waters in the lake [35] [36] . The direction of the flow of the
Jordan
waters is mostly from the river mouth to the west interlocked in the dominant direction pattern which is anticlockwise. It is suggested that such a current pattern enhances higher densities of
Jordan River
fluxed suspended particles in the northern stations. Reduction of water clarity by the enhanced concentration of suspended matter also reduces prey (zooplankters) visibility and consequently suppress fish zooplanktivory [37] . The suggested lower concentration of suspended matter in the southern stations (Figure 1) probably enhances zooplankter vulnerability. The outcome is lower zooplankton biomass in the southern part of the Kinneret pelagial. The long-term data record of zooplankton biomass and fish distribution in
Lake
Kinneret
clarified that the previous results are an underestimation. Multi-variable complicated modeling attempts that were carried out aimed at a quantitative chart of energy flow pattern in the Kinneret ecosystem [14] [38] revealed underestimated zooplankton biomass values [31] . The previous data of zooplankton biomass density are based on sampled regions where fish density is high and, therefore, zooplankton predation is intensive. Weighting averages of 7 stations (or even less) where only one of them (Station A, central lake zone) represent most of the Kinneret Epilimnion is fairly unbalanced. Fish density in the central part of the Kinneret Epilimnion is low and the zooplankton biomass is, therefore, high. For a fairly justified representation of the entire lake value, additional sampling stations in the central zone are required. Conclusively, it is therefore suggested to distribute same number of sampling stations for zooplankton study in the peripherial and in the central parts of the Kinneret Pellagial. An alternative option is to give each station a value of aerial coefficient (promoter) for the calculation of total lake biomass.
5. Summary and Conclusions
The grand total average of zooplankton biomass in all sampling stations (A, C, F, D, G, K, L) indicates its low level in the peripheral stations and its even lower levels in the southern region of the lake. Conclusively, the design of sampling program aimed at representing the entire Kinneret Pelagial Epilimnion should include at least one station in the southern part (stations D, K, L). If not, the lake mean value will be higher than reality. Furthermore, if sampling program eliminates the central zone (station A) the resulting value will be lower than real.
A future suggestion aimed at improvement of zooplankton sampling design is leaving 3 northern and 3 southern peripheral stations and an additional sampling station in the central region south of station A where the depth is about 30 meters.