The Effect of Intraocular Pressure Lowering Medications on the Pressure Spike Associated with Intravitreal Injection ()
1. Introduction
The most exciting and innovative advance in ophthalmology in recent years is the introduction of intravitreal injection of anti-VEGF drugs. These drugs have been shown to be sight-saving in a variety of retinal pathologies, including wet age-related macular degeneration and diabetic macular oedema [1] -[3] . One of the established side-effects of intravitreal injection is a temporary rise in the intraocular pressure (IOP) [4] - [6] . This has been attributed to volume expansion; however the exact mechanism remains unclear [7] [8] . Even a short-lived spike in the IOP can have potentially devastating consequences on an eye which is already compromised in terms of its vasculature. The Royal College of Ophthalmologists recommends routinely checking that the patient can see objects immediately after the injection, to ensure that the central retinal artery is patent (http://www.rcophth.ac.uk). Routine IOP measurement before and after injection is generally not necessary, however it should be considered in certain patients at risk of having a high IOP [9] .
Several authors have addressed the issue of prophylaxis in reducing the post-injection IOP spike. Frenkel et al. carried out a retrospective study of 71 patients, which did not show any significant benefit of pressure-lowering medications [10] . El Chehab prospectively evaluated different regimens in 210 patients, and showed a significant reduction in the pressure spike with several topical medications but not with oral acetazolamide [11] . Theoulakis had a series of 88 patients and found a reduction of the pressure spike after the use of brimonidine/ timolol [12] . To date, no prophylactic regimen has been established to be clearly effective and beneficial in patients undergoing intravitreal injection. Indeed, the question remains whether it is at all advantageous to use prophylactic pressure lowering medications prior to intravitreal injections, and if so, in which patients. The objective of our study is to determine whether the IOP spike is modifiable by the prophylactic use of the combination of dorzolamide and apraclonidine 1%. Both of these drugs are readily available in single dose units, which have useful infection control advantages.
2. Materials and Methods
A prospective, randomised controlled trial was performed between October 2011 and April 2012 in a single treatment centre. Ethical approval was obtained from the Clinical Research Ethics Committee of the Cork Teaching Hospitals.
80 consecutive patients due to undergo intravitreal injection of ranibizumab (0.5 mg/0.05ml) for a variety of retinal pathologies were included in the study. We selected patients based on specific inclusion and exclusion criteria. Inclusion criteria were patients aged 18 and over presenting for intravitreal anti-VEGF injections for the treatment of wet AMD, diabetic macular oedema, or macular oedema secondary to retinal vein occlusion. Exclusion criteria included a history of ocular hypertension or glaucoma, and intravitreal injection of agents other than ranibizumab. One eye only was included per patient. Written informed consent was obtained from all patients included in the study.
A random number generator assigned patients to either study or control group before the injection. The control group received no IOP lowering medications. The study group received guttae apraclonidine 1% (Iopidine, Alcon) and dorzolamide 2.0% (Trusopt, MSD) 30 to 40 minutes before the injection. IOP measurements were taken with the Perkins tonometer (Clement Clarke, Essex, United Kingdom) at baseline before the administration of drops (T-0). Subsequent measurements were 1 minute before injection (T-1), 2 minutes after injection (T-2), 5 minutes after injection (T-3), and 15 minutes after injection (T-4). To minimise inter-observer error, the same physician carried out all measurements for a given patient, (there were 4 such physicians over the 6 month period of data collection). Physicians were not blinded to the group of the patient. The IOP measurement technique and endpoint were clearly defined and standardised for all physicians involved prior to data collection. Identical injection technique of 0.05 ml of ranibizumab was used across all cases. A sterile cotton tip was applied to the injection site to prevent subconjuctival reflux. In between IOP measurements, the tonometer was made aseptic using alcohol swabs, and then dried using sterile gauze. Guttae chloramphenicol was administered after each IOP measurement.
The main outcome measure was the area under the curve (AUC) with respect to ground. This method is useful for detecting possible associations between repeated measures and other variables, over several time points [13] . We calculated this using a formula derived from the trapezoid formula [13] .
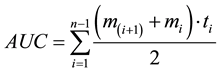
AUC is area under the curve with respect to ground. mi represents the mean IOP values for study and control groups from immediately before the injection (T-1) to 15 minutes after the injection (T-4). ti denotes the individual time intervals between measurements. n is the total amount of measures.
Statistical analysis was carried out using SPSS version 18. Data was tested for normality using the Shapiro- Wilk test and the appropriate statistical tests used to compare means (Independent samples t test for parametric data, and Mann-Whitney U test for non-parametric data). A p value less than 0.05 was considered statistically significant.
3. Results
The study and control groups did not differ significantly in terms of baseline IOP, with a mean (±standard deviation) of 14.17 ± 3.82 mmHg in the study group, and 13.88 ± 3.83 mmHg in the control group (p = 0.77). The mean age was 72 years in the study group, and 71 years in the control group.
Thirty to forty minutes post administration of IOP lowering prophylaxis to the study group, these patients showed a mean IOP drop of 4.09 mmHg (Figure 1, Table 1). Immediately pre-injection the study group had a
![]()
Figure 1. This figure illustrates the trend of IOP (intraocular pressure) over time in the study and control groups. The time intervals are baseline (T-0), immediately before the injection (T-1), immediately after the injection (T-2), 5 minutes after the injection (T-3), and 15 minutes after the injection (T-5). The IOP was significantly lower in the study group compared to the control group at T-1 (p < 0.01), at T-2 (p < 0.05) and at T-4 (p < 0.06); *p < 0.05; **p < 0.01.
![]()
Table 1. Mean IOP (mmHg) and difference between study and control groups.
Table 1. This table summarises the mean IOP (intraocular pressure) in study and control groups at various time intervals―at baseline (T-0), immediately before the injection (T-1), immediately after the injection (T-2), 5 minutes after the injection (T-3), and 15 minutes after the injection (T-4). The groups are compared and significance levels shown. CI = Confidence interval; SD = Standard deviation; *Study versus control group, independent t test.
significantly different mean IOP of 10.08 mmHg, compared to the control group’s mean IOP of 13.53 mmHg, with a p value of <0.001 (Independent samples t test).
The mean IOP immediately post injection in the study group was 26.71 mmHg, and in the control group it was 32.73. Using the mean values for study and control groups at T-1 to T-4 we constructed a curve and calculated the AUC. The values for AUC were not normally distributed (Shapiro-Wilke, p = 0.053), so we used non- parametric tests to compare the study and control groups (Mann-Whitney U test). The study group had a lower AUC than the control group (Mann-Whitney U test, p = 0.046).
A significant positive correlation was found between T-1 (IOP immediately pre-injection) and the AUC―Kendel’s tau: r = 0.268; p < 0.001. Data on axial length was available for 15 patients. No significant correlation emerged between the IOP spike and the axial length (Pearson’s correlation: r = −0.001; p = 0.997).
In both study and control groups, the IOP showed rapid normalisation post-injection. 79 of 80 eyes had an IOP of less than 30 mmHg within 15 minutes post injection. The patient who didn’t was in the control group and achieved an IOP of 28 mmHg twenty minutes following injection.
4. Discussion
Acutely raised IOP in an eye which already has compromised vasculature is one of the most hazardous complications of intravitreal injection. Prophylactic IOP lowering medications are effective in preventing IOP spikes following procedures such as ALT-trabeculoplasty and nd:YAG laser capsulotomy [14] - [16] . To our knowledge, this is the first prospective, randomised controlled trial investigating the effect of dorzolamide and apraclonidine on the post intravitreal injection IOP spike.
In our study we used a single regimen―the combination of dorzolamide and apraclonidine. These agents are available in single-dose formulation and hence are a cost-effective method of reducing intraocular pressure when only a single administration is required in each patient. It is interesting to note the extent of IOP reduction recorded in our study with this combination, just 30 minutes after administration. The IOP was reduced from 14.17 mmHg to 10.08 mmHg―approximately a 28% reduction. Such a pronounced reduction in IOP at just 30 minutes is greater than previously expected. Perhaps this phenomenon was due to the concurrent administration of local anaesthetic drops and their effect on corneal permeability, however no firm evidence for this was found in the literature.
Our study suggests that a lower starting IOP pre-injection is associated with lower pressure following the injection. We excluded glaucoma and ocular hypertension patients from our study, as this would confound our results. Erratic diurnal IOP fluctuation is more common in eyes with glaucoma and ocular hypertension than in a healthy eye, and hence the IOP may be more likely to reach a higher peak post intravitreal injection [17] . To the patients included in our study, prophylaxis has little clinical advantage―data showed that in both study and control groups the IOP spike was transient, with the vast majority of patients returning to a pressure of less than 25 mmHg within 15 minutes post-injection. This finding is similar to that of other authors―El Chehab, Falkenstein, and several other authors all found the IOP spike to be short lived [4] [11] [18] [19] . Consistent with this, is our finding that the difference in IOP between study and control groups seemed less at 5 minutes and 15 minutes post injection, as oppose to immediately before and after injection. El Chehab and colleagues reported similar findings [11] . This may be due to the fact that IOP normalises rapidly post the immediate pressure spike. Within a few minutes of the injection, the difference between normal and control group IOP is less, as the IOP in both groups is rapidly returning to normal.
It is interesting that the post injection IOP spike was higher in Frenkel et al’s study than in our present study. This discrepancy may be partially explained by the high number of glaucoma patients included in Frenkel’s study (36.6% in the pegaptanib group, 33.3% in the ranibizumab group, and 21.2% in the bevacizumab group). Furthermore, the volume of injection of pegaptanib is 0.09 ml, almost double the standard injection volume of ranibizumab and bevacizumab (0.05 ml) used both by Frenkel et al. and ourselves, so it is not surprising that the pegaptanib group had generally higher IOP spikes than the other two groups.
Why the post-injection IOP spike is much greater in some patients than others, is uncertain. Possible factors which influence the magnitude of the post-injection IOP spike include pre-existing glaucoma (excluded from this study), axial length, age, and reflux of synergetic vitreous/drug.
We postulated that the magnitude of the IOP spike post-injection may be related to the axial length, since the injection of 0.05 ml into a smaller, hyperopic eye would represent a greater proportion of the total ocular volume than in a larger emmetropic or myopic eye. Data on axial length was only available on 15 of our 80 participants, and did not show a significant correlation between magnitude of IOP spike and axial length. The phakic status of patients is also an interesting consideration―since during intravitreal injection in phakic patients the lens-iris diaphragm may shift forward and reduce aqueous outflow. El Chehab recorded the axial length and phakic status of patients, and did not find a significant correlation between the IOP spike and either of these variables [11] .
We also considered the role of age as a confounding factor. Previous literature reports a positive correlation between ocular rigidity and age [20] . It is reasonable to suppose that older eyes may have less ocular compliance, and hence respond with a greater IOP spike to ocular volume increase. Interestingly, our data showed no such correlation.
Our trial’s strengths and advantages lie largely in its prospective nature. Consistent technique is used for all injections and IOP measurements, lending reliability to the findings. Limitations include that greater patient numbers are needed to more accurately determine the possible factors leading to adverse IOP spikes. This was not a double-blind study, which allows for potential bias in the results. The study is subject to selection bias, and to measurement error and bias, as physicians were not blind to the status of the patient (study or control group). Our trial demonstrates the effect of relatively mild IOP prophylaxis, and we would be interested to see whether a greater effect on the IOP spike would be possible with other regimens.
Our study adds evidence that prophylaxis is unnecessary in those without glaucoma or ocular hypertension who are undergoing intravitreal injection of 0.05 ml. Given current evidence that the patients with glaucoma may have greater IOP fluctuations than those without glaucoma, and that the optic nerves of glaucomatous eyes are sensitive to these IOP fluctuation [17] [21] - [23] , future studies should focus specifically on the magnitude, duration, modifiability and potential deleterious effects of the post-injection spike in glaucomatous eyes. A future trial could also examine the rate of visual field progression in patients with glaucoma who are also receiving serial intravitreal injections, versus those who are not.
5. Conclusion
Our study demonstrates that topical dorzolamide and apraclonidine can indeed modify the IOP spike associated with intravitreal injection. Clinicians may use this evidence when considering IOP lowering prophylaxis in patients with high baseline IOP or compromised vasculature.
Acknowledgements
Dr. Jean Saunders, consultant biostatistician, University of Limerick, Ireland.
NOTES
*Corresponding author.