Lead Ions Sorption from Waste Solution Using Aluminum Hydroxide Modified Diatomite ()
1. Introduction
The release of heavy metals to the surrounding environment, caused by the development of industries, such as ceramics, metallurgy, mining and battery manufacturing, is becoming a major concern and threatened a lot to the human health nearby [1] . Among them, lead ion is one of the biggest issues due to its high toxicity and mobility. In 1988, Nriagu and Pacyna estimated that 635 × 106 kg·yr−1 lead had been released into the environment (atmosphere, aquatic systems and soil) due to the mining and metallurgy industry [2] -[4] . Lead spreads into the environment through soils and water streams and accumulates along the food chain, resulting in a high risk to human health, as lead can affect red blood cells, the nervous system, and the kidneys. The lower IQ values and other neuropsychological deficits among the children exposed to higher lead levels have been well documented [5] . Therefore, there was an urgent need to find a solution to reduce lead from water for contamination control and environmental protection. It was reported that there are many approaches for lead removal from water, such as coagulation precipitation, adsorption, ion exchange method, electrolytic process. Among them, adsorption is commonly used and comparatively low cost. The previous reported absorbents that are suitable for the removal of lead ions included natural and modified bauxite tailings [6] , diatomite [7] , bentonite [8] , attapulgite [9] , palygorskite [10] , and sepiolite [11] . Diatomite is a type of adsorbent with large specific area, high adsorption capacity, and good mechanical properties, broadly utilized in wastewater treatment [12] . Diatomite is regarded as a mineral of organic origin, where the silica of fossilized diatom skeleton resembles opal or hydrous silica in composition (SiO2·H2O). The silica surface contains silanol groups that spread over the matrix of silica [13] . The silanol group is an active one which tends to react with many polar organic compounds and various functional groups. Al-Degs et al. [14] studied the sorption of lead ions on diatomite and manganese oxides modified diatomite. The result demonstrated that manganese oxides modified adsorbent showed a higher tendency for adsorbing lead ions from solution at pH 4. Khraisheh et al. [15] examined the effectiveness of raw and modified diatomite for the uptake of Pb2+, Cu2+ and Cd2+ from wastewater. Irani et al. [2] compared lead sorption onto natural perlite, dolomite and diatomite, in which, BET analysis of clays showed that the pore size, surface area and pore volume of diatomite were greater than those of perlite and dolomite. Even so, to our knowledge, little study has been reported for adsorption of lead on aluminum modified diatomite. In this work, the preparation and characterization of aluminum modified diatomite were reported.
2. Experimental
2.1. Adsorbent Preparation
The diatomite sample was obtained from Tianjin Fengchuan chemical technology co., LTD (China) and it was composed of SiO2 (92.20%), Fe2O3 (1.40%), Al2O3 (2.83%), CaO (0.60%), MgO (0.05%), K2O (0.52%), Na2O (1.91%) and LOI (0.09%). Firstly, 3 g of diatomite (80 mesh) were immersed in 10 ml of 1mol/L AlCl3∙6H2O, a certain amount of 3mol/L NaOH was immediately added into the mixture and then shook at room temperature for 2 h in the oscillators. Then, the mixture was exposed to air for 12 h and discard the supernatant and afterwards dried in an oven at 105˚C for 12 h. Finally, the samples were grinded to 80 mesh and stored in the valve bag for further study. The prepared sample was named as Al-diatomite. The surface of diatomite and Al-diatomite was observed by using a scanning electron microscope (SEM) (FEI Quanta 200, Netherlands). The mineral phase of diatomite was characterized by X-ray diffraction (XRD) (Rigaku TTR Ⅲ, Japan). X-ray fluorescence spectrometer (XRF) (Rigaku ZSX100e, Japan) provided us the element contents.
2.2. Adsorption Experiments
A stock solution (1000 mg/L) was prepared by dissolving 1.598 g Pb(NO3)2 (analytical grade) in 1 L of deionized water. All the solutions for lead removal experiments and analysis were prepared by an appropriate dilution of the stock solution. The pH of the lead solution was adjusted using 5 M NaOH and HCl solutions. 0.3 g of diatomite sample and 100 ml of Pb(NO3)2 solution were added in 150 ml conical flask and then were placed in a shaker with a speed of 100 rpm. Pb(II) remaining in the supernatant was analyzed by flame atomic absorption spectrophotometry (FAAS) (Varian AA240FS, American). Effects of adsorbent dose, pH of suspension, initial concentration and the reaction temperature were investigated
3. Result and Discussion
3.1. Characterizations of Adsorbents
The scanning electron microscope (Figure 1) shows that the main pattern of the diatomite (Figure 1(a)) was
 (a)
(a) (b)
(b)
Figure 1. SEM images of diatomite (a); Al-diatomite (b).
discoid and the surface was porous structure. However, Al-diatomite (Figure 1(b)) was relatively uneven and a number of pores were filled, it illustrates that the expected aluminium compound formed in the interspaces. As can be seen from Figure 2, the main composition of diatomite and Al-diatomite were opal (SiO2∙nH2O) [16] , nevertheless, after the modification, Bayerite knowing as Al(OH)3 was found in the XRD peak of the Al-diatomite, and the result of XRF in Table 1 also indicated the percentage of Al increased from 1.28% to 9.30%. This is because of the coating process by the product of AlCl3∙6H2O and NaOH solutions, followed that aluminum hydroxide compound has been successfully loaded at the diatomite. In Figure 3, the general range 3600 - 3100 cm−1 may be assigned to asymmetrical and symmetrical O-H stretching vibration modes for water of hydration and the 1670 - 1600 cm−1 region to O-H bending vibration modes [17] . The presence of SiO2 is verified by ad-sorption bands at 1068 cm–1 of asymmetric Si-O-Si stretching vibrations, and Si-O-Si bending vibrations at 469 cm–1 [18] . In addition, the adsorption band at 791 cm–1 and 618 cm–1 are ascribed to Al-O absorption bands [19] . As Al-diatomite shown, the peaks at 3421 cm−1 and 1641 cm−1 were attributed to the stretching vibration of absorbed water and zeolitic water [7] . It indicated that the group composition of diatomite changes as modification and this explained why the adsorption capacity of Al-diatomite became stronger.
3.2. Effect of Adsorbent Dosage
The adsorbent dose is an important parameter for the determining of the adsorb-ability of adsorbent at a given initial condition. In this study, the effect of adsorbent dose on the lead removal was studied at pH 5.0 ± 0.1, room temperature and 10 mg/L of lead for 2 h. As the result shown in Figure 4(a), it was observed that the adsorption efficiency of Pb (II) increased from 20.27% to 99.02% with the increasing dose of Al-diatomite from 1 g/L to 20 g/L. However, the per unit of adsorption capacity declined with the dose increasing, as we can see, the adsorption capacity decreased slowly after a dose of 3g/L. This is because at low adsorbent content, all types of surface sites are exposed for sorption and the surface reaches saturation faster, leading to a higher sorption capacity. But at higher hematite concentrations the availability of higher energy sites decreases and with a larger fraction of lower energy sites occupied, a lower sorption capacity is found [20] . In addition, a higher amount of adsorbent increases the probability of collision between solid particles and thus particle aggregation, causing a decrease in the total surface area and an increase in diffusion path length, both of which contribute to the decrease in sorption capacity [21] .
3.3. Effect of the pH
The pH of a solution could obviously influence the extent of lead adsorption, especially the existential state of lead in the solution and charge the surface of adsorption. As lead ion precipitates when the pH of solution is alkaline, the effect of pH was studied by varying the pH of lead solution from 4.0 to 6.5, 3 g/Lad sorbent dose,
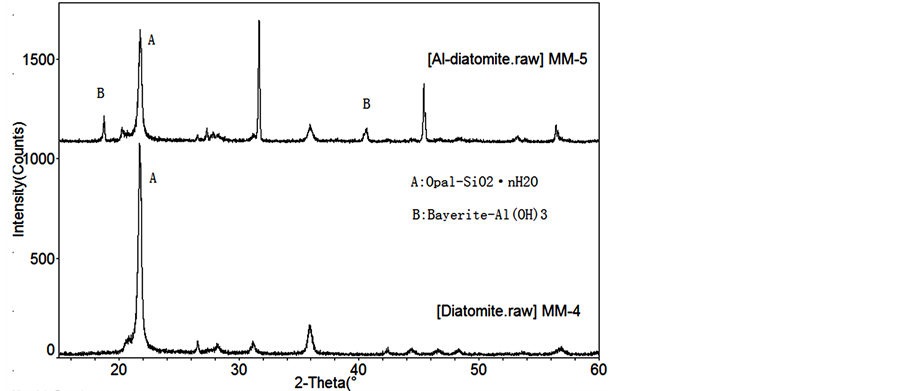
Figure 2. XRD of diatomite and Al-diatomite.

Figure 3. Infrared spectroscopy images of diatomite (a), Al-diatomite (b).

Table 1. Elementary composition of diatomite and Al-diatomite from XRF analysis.
room temperature and 10 mg/L of lead for 2h. The other experimental conditions were extremely same as described in the effect of dose. It can be seen from Figure 4(b) that the lead sorption by Al-diatomite was increased from 41.7% to 74.5% when the pH of the solution increased from 4 to 5.5 and from pH 5.5 to 6.6, the sorption maintains the maximum value of 74.5%. This may be due to the fact that a mass of H+ surrounded the drill way of adsorbent and occupied the adsorption site when the pH was low. Then lead was prevented into the drill way and it was adverse to the ion exchange adsorption between adsorption and lead.
3.4. Effect of the Initial Concentration
The effect of initial concentration on the lead removal was studied at 3 g/Lad sorbent dose, pH 5.0 ± 0.1 and room temperature for 2 h. It can be seen from Figure 4(c) that the adsorption capacity of lead ions by Al-diatomite increased with the raising of initial concentration, but the adsorption efficiency decreased. It was
 (a)(b)
(a)(b)  (c) (d)
(c) (d)
Figure 4. Effect of adsorbent dose (a); pH of suspension (b); initial concentration (c) and the reaction temperature (d) on lead adsorption percentage and adsorption capacity.
due to an increase of the amount of lead ions available to binding sites of adsorbents. Though at 100 mg/L lead concentration, the active sites were not saturated and adsorption capacity doesn’t approach a constant value.
3.5. Effect of Temperature
The effect of temperature on lead removal was studied at the solution temperature ranged from 298.15 to 328.15 K, 3 g/Lad sorbent dose, pH 5.0 ± 0.1, and 10 mg/L of lead for 2 h. It was found that the adsorption of Pb (II) on diatomite was promoted at higher temperature, adsorption capacity increased from 2.03 mg/g to 2.96 mg/g with the increase of temperature for Al-diatomite (Figure 4(d)), It demonstrated that the rise in temperature increased the binding tendency of the lead ions onto the interface and thereby strengthened the extent of adsorption [22] . One possible interpretation of this phenomenon was that the Pb (II) ions were well hydrated, in order to be adsorbed on the diatomite, they had to lose the hydration shell which needed energy, so the removal of water molecules from Pb (II) ions was an endothermic process in essence [23] .
Thermodynamics parameters for adsorption process, such as, the enthalpy change (∆H) and entropy change (∆S), were calculated from the slope and intercept of the straight line of ln versus 1/T according to the following form [24] :
 (1)
(1)
 (2)
(2)
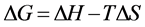 (3)
(3)
where CAd and Ce (mg/L) are the concentration of adsorbed and equilibrium lead, respectively, Kc is the equilibrium constant, R is the gas constant (8.314 J mol−1∙K−1), and T is the absolute temperature (K).
Values of ∆H, ∆G and ∆S were shown in Table 2. It was clear that the free energy (∆G) of Pb (II) adsorption on Al-diatomite was all negative and decreased with the rise in temperature, which demonstrated that the adsorption process was spontaneous. The endothermic process was confirmed by the positive value of enthalpy change (∆H). The positive value of entropy change (∆S) indicated the increase in randomness of the ongoing process and the adsorption reactions were spontaneous with a good affinity. As enthalpy change absolute value of physical adsorption is 0 - 20 kJ/mol. In another word, enthalpy change absolute value of chemical adsorption is greater than 20 kJ/mol. It was found that Pb (II) adsorption on Al-diatomite was chemical adsorption in consideration of enthalpy change absolute value in Table 2.
3.6. Adsorption Isotherm
Langmuir and Freundlich isotherm models described the relationship between the concentrations of absorbed lead and lead in solution when the adsorption is in dynamic balance with that on the liquid-solid interface at a given temperature. Langmuir isotherm was based on the theory that the monolayer adsorption and assumes that the surface of sorbent was composed of amounts of homogeneous adsorption sites. Langmuir isotherm equation [25] was given as:
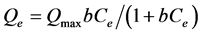 (4)
(4)
where Qmax(mg/g) and Ce(mg/L) are maximum adsorption capacity and equilibrium concentration, b represents a constant related to affinity and energy of blinding sites.
Freundlich isotherm equation is derived to describe the multilayer adsorption and the heterogeneous surfaces of absorbent illustrate that the adsorption equilibrium constants are related to the coverage of surfaces. Freundlich isotherm equation [26] was given as:
 (5)
(5)
where KF and n are Freundlich coefficients relating to adsorption capacity and intensity respectively.
The Langmuir and Freundlich isotherm of Pb (II) on Al-diatomite are shown in Figure 5 (The experiment conditions can refer to 3.4). As the parameters shown in Table 3, Compared with Langmuir isotherm (R2 = 0.933), Freundlich isotherm (R2 = 0.960) model was better fitted to describe the ad-sorption characteristics of Pb(II) on Al-diatomite. In Freundlich parameters, n value (1.650) indicated the high bond strength between absorbate and adsorbent, and it also illustrated the adsorbent surface was heterogeneous [27] .
3.7. Lead removal by Natural Diatomite
In order to compare the effect of lead removal between diatomite and Al-diatomite, The conditions on lead removal was studied at 3 g/Lad sorbent dose, pH 5.0 ± 0.1, room temperature and 10 mg/L of lead for 2 h. It canbe seen from Table 4. It was found that the percent adsorption of Pb (II) on diatomite was only 12.06% and ad

Table 2. Thermodynamic parameters for the adsorption of lead on Al-diatomite.

Table 3. Isotherm parameters for the for lead adsorption onto Al-diatomite.

Table 4. Lead removal by natural diatomite.
 (a) (b)
(a) (b)
Figure 5. Isotherm modeling of lead adsorption on the Al-diastomite (a) Langmuir isotherm; (b) Freundlich isotherm.
sorption capacity was 0.402 mg/g. However, in the same experiment conditions, the percent adsorption of lead removal by Al-diatomite could reach to 61.36%.
4. Conclusion
In this paper, aluminum was utilized to coat on the surface of diatomite for the efficient removal of lead. FTIR, XRD, and SEM results show that aluminium com-pound was successfully coated on the surface of diatomite. Comparing with the natural diatomite, the adsorption efficiency for the lead ions increased from 12.06% to 61.36%, indicating that Al-diatomite was an effective absorbent for removing lead ions from wastewater. The free energy (∆G) of Pb (II) adsorption on Al-diatomite was all negative and decreased with the rise in temperature. It demonstrated that the adsorption process was spontaneous. The enthalpy change absolute value illustrated that Pb (II) adsorption on Al-diatomite was chemical adsorption. The adsorbent surface was heterogeneous as the equilibrium data of Al-diatomite samples well fitted to Freundlich isotherm model. Therefore, Al-diatomite presents a good potential application in the treatment of lead wastewater.
NOTES
*Corresponding authors.