1. Introduction
Humic substances (HS) are ubiquitous natural compounds comprising the major component of soil organic matter and sediments, as well as aquifers [1-3]. By the chemical nature HS can be considered to be irregular polymers of aromatic polyhydroxyl carbonic acids [3], and the peculiarity of the structure of HS consists of a coexistence of both polar and hydrophobic environments in the same molecule. As a result, HS are able to bind both polar and hydrophobic organic compounds and inorganic ions as well. Thus, HS are one of the main environmental factors controlling the fate and behavior of the compounds released into the environment. In particular, they are universally considered of great importance in determining soil extracellular enzyme activity and stability via association with essential soil enzymes such as ureases, proteases, phosphatases, hydrolases, laccases, and peroxidases, which have been detected in soil extracts as complexes with HS [4,5]. Contrary to the extracellular enzymes incorporated into a soil mineral matrix, where they often loose their activity, humus-immobilized enzymes can frequently retain their activity and display stability against environmental stresses.
Laccases (EC 1.10.3.2) are extracellular multicopper oxidases that catalyze the oxidation of a wide range of reducing substances with the concomitant reduction of O2. Because of their capability of oxidizing aromatic compounds, such as phenols and anilines, laccases have promising potential as industrial enzymes for various applications, such as wood fiber modification, biosensor construction, and water and soil remediation [6-10]. Recently laccase-based biocatalysis has been expanded, with the aid of small “mediators”, to pulp delignification [11- 13], textile dye bleaching [14,15], polycyclic hydrocarbon degradation [16-20], destruction of pesticide, insecticide, or chemical warfare [21-23], and organic synthesis [24,25]. Nowadays a special attention is paid to laccase in relation to so-called enzymatic treatment, which is currently considered as an alternative method for the removal of toxic xenobiotics from the environment [8,10]. Enzyme-mediated transformation reactions may result in formation of compounds where xenobiotics bind covalently to HS via oxidative coupling [26,27].
Despite the abundance of promising experimental data, a number of limitations still restrict the use of laccase to detoxify xenobiotics. One of them is a lack of knowledge in the field of laccase interaction with HS, which can influence both on the enzyme activity and stability in the environment. Only a few studies are available now in literature [28,29], and an investigation of association of HS with laccase is therefore of great interest. The objective of this research was to investigate laccase interaction with HS in a model system containing laccase and coal humic acids (HA) and to get insight into the nature of HA-laccase complex.
2. Experimental
2.1. Humic Acids
Humic acids (HA) used in this study was a commercial preparation of leonardite HA “Powhumus” (Humintech, Germany). Elemental analysis of HA was determined with a Carlo Erba Strumentazione analyzer and showed that HA contained (on ash-free and moisture-free bases) 45.9% C, 3.4% H, and 1.6% N. Ash content was 1.1%. Content of carboxylic and phenolic groups determined using potentiomentric titration technique was 2.9 and 3.0 mekv/g, respectively [30].
2.2. Laccase Isolation and Characterization
For this study laccase form the strain of Trametes hirsuta (Wulf.:Fr.) Pil. 072 was used. The fungal strain was kindly provided by Komarov Botanical Institute (St. Petersburg, Russia) and cultivated as described earlier [31]. Extracellular laccase was isolated from the culture medium and purified in accordance with [32]. The control of laccase homogeneity was carried out with polyacrylamide gel (PAAG) electrophoresis under non-denaturing conditions as described in [33]. The pH optima of the major isoenzyme determined using pyrocatechol as substrate was 4.5 [32].
2.3. HA-Laccase Complexes Preparation and Monitoring
The HA-laccase complexes were prepared by incubation (27˚C, 72 hours) of laccase and HA together at concentrations of 100 and 40 mg∙l−1, respectively. A 50 mM potassium phosphate buffer at pH 5.0 or 6.5 was used as a background electrolyte. In parallel, control solutions of laccase and HA solely were incubated under the same conditions. Because of the observed biodegradation of laccase (data not shown) toluene was added to the solutions to suppress microbial activity [34]. After 6, 24, 48, and 72 hours of incubation solutions were sampled for size-exclusion chromatography analysis. Additionally, during the incubation laccase activity and protein concentrations were monitored at regular intervals (after 1, 3, 6, 9, 12, 24, 48 and 72 hours). For isoelectric focusing laccase with HA were incubated at the same conditions, but for longer period of time (168 hours) and using higher concentrations of the compounds. Namely, concentration for laccase was 1000 mg∙l−1 and for HA 400 mg∙l−1.
2.4. Fractionation of the HA-Laccase Complexes by Size-Exclusion Chromatography (SEC)
SEC analysis was performed according to [35]. SEC system Abimed (Gilson, France) was equipped with online UV detector and included HPLC pump, auto sampler, and glass column. The column 25 mm × 20 cm packed with Toyopearl TSK HW-55S gel (Toso Haas, Japan) was used for separation. The 0.028 M phosphate buffer (pH 6.8) was used as a mobile phase. The flow rate was set at 1 ml∙min−1. The absorbance of eluate was detected at 254 nm. A void volume (V0 = 19.2 ml) and a total permeation volume (Vt = 74.5 ml) of the column were determined using Blue Dextran 2,000,000 and acetone, respectively. Sodium polystyrene sulfonates (PSS) of molecular weight of 2.29, 4.48, 14.00, 20.70, 45.10, and 80.84 kDa (Polymer Standard Service, Germany) were used as markers for molecular weight calculations. Data obtained was treated using approach published in [36]. To obtain laccase activity profiles of the HA-laccase complex 10-ml fractions were collected and analyzed for laccase activity.
2.5. Study HA-Laccase Complex Formation by Isoelecric Focusing (IEF)
To determine change of isoelectric point (pI) of laccase associated with HA compared to free laccase, IEF was carried out as described in [37]. Briefly, 20 ml of solution of fresh prepared HA-laccase complex, HA-laccase complex after 72 or 168 hours of incubation, HA or laccase without added HA was sampled and directly loaded in a standard PAAG (110 ´ 120 ´ 0.4 mm, T = 7.5%, C = 3%) containing 3% ampholytes pH 4.0 - 7.0 (Iso-Lyte 4 - 7, ICN Biomedicals). 1 M NaOH and 1 M H3PO4 were used as cathodic and anodic buffers, respectively. IEF was conducted at constant power of 6 Wt and temperature of 10˚C during 1.5 hours. Gels were stained with a solution of Coomassie Blue R-250.
2.6. Measurements of Laccase Activity
The laccase activity was determined spectrophotometrically or polarographically. In case of spectrophotometric approach [32] laccase activity was measured by following the rate of oxidation of pyrocatechol to quinone by plotting the absorbance at 410 nm (A410) against time. A stock solution contained 10 mM pyrocatechol in 0.1 M sodium acetate buffer at pH 4.5. One unit of activity was defined as a change in A410 per min per mg of protein.
Polarographic assay of laccase activity was carried out using a Clark oxygen electrode in a hermetically sealed sample chamber (final volume 0.7 ml) at 25˚C. The enzymatic reaction was initiated by the addition of laccase solution to 4 ´ 10−5 M syringaldazine (4-hydroxy-3,5- dimethoxybenzaldehyde azine) in 0.1 M sodium acetate buffer at pH 4.5. Laccase final concentration in the sample chamber was 25 nM. The initial oxygen concentration was 260 µM (according to the evaluation of the Henry coefficient). One unit of enzymatic activity was defined as the amount of laccase required for consumption 1 mmol∙min−1 of O2.
Protein concentration was measured with a use of bicinchoninic acid (BCA) protein assay kit (Pierce, USA).
2.7. Statistical Data Treatment
All results were presented as mean ± SD. Comparison between two means was performed using unpaired Student’s t-test. Differences were considered to be significant when p < 0.05.
3. Results and Discussion
3.1. HA-Laccase Association
Association of laccase with HA was monitored using SEC. SEC-profiles of HA and laccase solely did not change within 72 hours (Figure 1) demonstrating their stability during the time of the experiment. Chromatograms of both HA and laccase exhibited a sharp single symmetric peak. This finding confirms that the SEC-fractionation was conducted under conditions appropriate for the substances under study, as no artifacts such as ionic exclusion or specific adsorption were observed [35,38]. That was also indicative for complete suppression of the surface negative charge of HA (ibid.). Observed values of elution volume for HA [45.4 ml] and laccase [38.4 ml] corresponded to the peak molecular weight (MW) values of 9.6 and 21.9 kD respectively. The MW of laccase from T. hirsuta determined earlier using standard globular protein calibration kit was reported to be 55 kD [39], what was considerably higher than obtained in our experiments. The observed variance in MW values can be attributed to the difference in SEC system such as the charge density of the used calibrants. In particular, in [35] it was shown that the smaller the charge density of the chosen calibrants, the higher the values of MW will be.
SEC-profiles of HA-laccase complexes at different pH are presented in Figure 2. The chromatograms consisted

Figure 1. SEC chromatograms of laccase and HA solutions at pH 5.0 (a) and 6.5 (b) in fresh-prepared solutions and after 72 hours of incubation. Firm lines represent laccase, dotted lines represent HA.

Figure 2. SEC chromatograms of HA-laccase complexes after different time of incubation at pH 5.0 (a) and 6.5 (b).
of the first sharp peak followed by 2 - 3 diffused peaks. As it can be seen, profiles of HA-laccase complexes cannot be described as a superposition of SEC-profiles of these substances. Along with peaks corresponding to HA and laccase and additional peak at an elution volume equal to the V0 (19.2 ml) of the column appeared. Given that ionic exclusion arising from repulsive interaction between the charged analyte and the partially charged gel matrix can be excluded, a formation of HA-laccase complex with molecular weight larger that fractionation range of the SEC-system used should be proposed. Indeed, activity profile of laccase in the presence of HA obtained by SEC-fractionation of HA-laccase profile after 24 hours of incubation showed the main activity peak at the elution volume of about 20 ml (Figure 3). This behavior was interpreted to be a result of the formation of a stable and enzymatically active complex between HA and laccase.
Polymerization of HA in the presence of laccase could be also surmised to be responsible for the observed additional peak. Appearance of new HA peaks with corresponding peak MW of 18.8 and 40.9 kD at pH 5.0 (elution volumes 39.6 and 32.3 ml) or with 18.8 kD at pH 6.5 (elution volume 39.6 ml) was also evident for the formation of HA fractions with larger peak MW comparing with initial humic material. This is in agreement with recent results that have shown an increase in molecular size of humic matter following an oxidative polymerization reaction catalyzed by the peroxidase [40]. As laccase under study was demonstrated to have the maximum catalytic activity at pH 4.5 [39], the enzymatic activity was therefore supposed was greater at pH 5.0 than at 6.5. In comparison to pH 6.5, the intensity of the additional peak was higher at pH 5.0 (Figure 2). This finding could
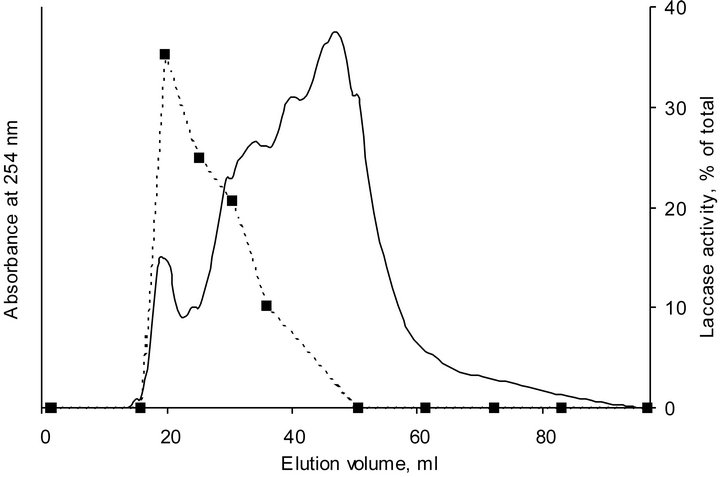
Figure 3. SEC chromatogram and activity profile of HA-laccase complex at pH 5.0 after 24 hours of incubation. Firm line represents absorbance profile, dotted line represents activity profile.
be attributed to the greater HA polymerization under conditions of the higher enzymatic activity of laccase. However, quantitative evaluations are prone to errors because of possible changes in the molar extinction coefficients of components in fractions belonging to different peaks.
During the experiment a slight increase in intensity of absorbance of the diffused peaks was observed. Assuming that chromophores possessing conjugated π-electron systems (e.g. C=C-C=C, C=C-C=O and aromatic compounds) mainly determined UV absorptivity at 254 nm, oxidation of HA in the presence of laccase following formation of quinonic structures from phenolic moieties in HA could be proposed. So, the observed polymerization of coal HA in the presence of laccase was possible due to the processes similar to the oxidative coupling of phenols.
Association of HA with laccase was supposed to alter pI of the enzyme due to formation of its complex with humics. Therefore, the IEF separation of the HA-laccase complex and laccase was conducted. The obtained results are presented in Figure 4. IEF showed no HA band under selected conditions, while for laccase a double band at pH ca 4.0 was observed reflecting the existence of two isoenzymes with close isoelectric points [39]. IEF of the blank solution containing laccase solely showed no changes in enzyme electrophoretic behavior during 7 days of incubation indicating laccase stability under selected conditions. It can be seen that over the experiment the pI of laccase in the HA-laccase complex did not change as compared to free laccase at both pH studied. In particular, neither appearance of an additional band nor its shift nor alteration in intensity of the bands was observed. This finding was an evidence of non-covalent character of laccase association with HA, as covalent bond formation would result in HA-laccase complex stable under IEF conditions. On the other hand, ionic interactions between HA and enzyme was supposed to be negligible under selected conditions as HA-laccase complex remained stable in SEC analysis where surface negative charge of HA was suppressed. The association between HA and laccase by weak dispersive forces such as van der Waals, hydrophobic, π-π, CH-π and others was therefore hypothesized.
3.2. Dynamic of Laccase Activity in the Presence and Absence of Coal HA
The study of laccase activity in the presence of HA has been carried out for 3 days and one could expect the interference of microorganisms in reaction mixture. To suppress microbial activity, toluene was added to the solutions, what resulted in inhibition of laccase activity in fresh prepared solutions both at pH 5.0 and pH 6.5 (Figure 5). The drop in enzyme activity comprised nearly 30% and 50% compared to laccase without added toluene at pH 5.0 and 6.5, respectively. This finding was in agreement with a generally accepted notion on an enzyme inhibition by organic solvents due to their interaction with the protein [41-43]. The investigations showed that at low and moderate concentrations (up to 3.5 M) of water-miscible solvents, the solvents caused a linear increase of the apparent Michaelis-Menten constant Km values for standard laccase substrates and rather low changes in the maximum reaction rate Vmax values. The authors supposed that organic solvents behaved like the simple competitive or mixed competitive inhibitors of

Figure 4. IEF analysis of HA, laccase and HA-laccase complex after different time of incubation at pH 5.0 (a) and pH 6.5 (b). 1 - HA, 2, 3 -laccase after 0 and 168 hours of incubation, 4, 5, 6 - HA-laccase complex after 0, 72, and 168 hours of incubation.
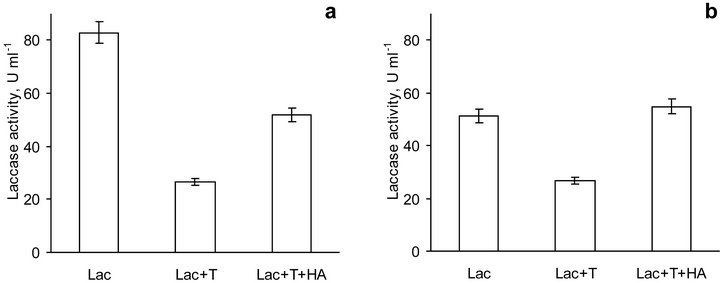
Figure 5. Activity of laccase solely (Lac), in the presence of toluene (Lac + T) and in the presence of toluene and HA (Lac + T + HA) at pH 5.0 (a) and 6.5 (b). Bars represent standard deviations.
laccase [42,43]. Another possible reason for laccase inactivation could be a loss of water and/or unfolding of an enzyme in the presence of polar solvents [41]. Laccase from T. hirsuta contains 284 molecules of water according to crystallographic study and the first assumption is rather reasonable. Besides, the difference in optical spectra of laccase solely and laccase with toluene was insignificant (data not shown). Therefore, only the nearest surrounding of the laccase active center was supposed to be affected by toluene.
In this study, coal HA did not have a negative effect on laccase activity (Figure 5), what was in agreement with recently published data [44]. The finding, nevertheless, contradicted the reported data on the inhibitory effect of coal HA on laccase [29]. The authors proposed that the inactivation of laccase was mainly due to depletion of the copper ion from the active site of the enzyme. However, it is widely accepted that laccase can loose its activity because of dissociation of Cu only in the presence of a strong chelating agent under anaerobic conditions. Even for elimination of copper type 2 (the most easily eliminating copper ion from laccase) it is necessary to use guanidine chloride under denaturing condition followed by bathcuproine treatment [45,46]. In this study interaction of native laccase with HA under non-denaturing conditions has been carried out.
Therefore, copper dissociation from laccase in the presence of HA can hardly occur in our experiments.
The dynamic of laccase activity at pH 5.0 and 6.5 (Figure 6) was characterized with two stages, namely, with an activation and an inhibition stages. This effect supposed to be connected with a rearrangement of laccase active center as well as with inter transition of laccase forms existing in the solution. The peak of maximum laccase activity was observed within 1 - 3 hours for laccase both at pH 5.0 and pH 6.5. In the presence of HA the peak of activation was shifted to 12 hours at both pH studied. This finding was probably attributed to the conformational changes in laccase structure due to its association with HA.
On the second stage gradual decrease in enzyme activity along with time of incubation was observed. Laccase activity after 72 hours of incubation decreased to 72% and 70% compared to initial activity at pH 5.0 and 6.5, respectively. In the presence of HA those values were 89% and 99%. Compared to the maximum-recorded values of laccase activity, laccase activity at the end of experiment reduced to 63% or 52% at pH 5.0 or 6.5, while in the presence of HA enzyme activity was 78% and 71%, respectively. So, laccase activity was concluded to be stabilized in the presence of coal HA.
So, the complex formation between laccase and HA
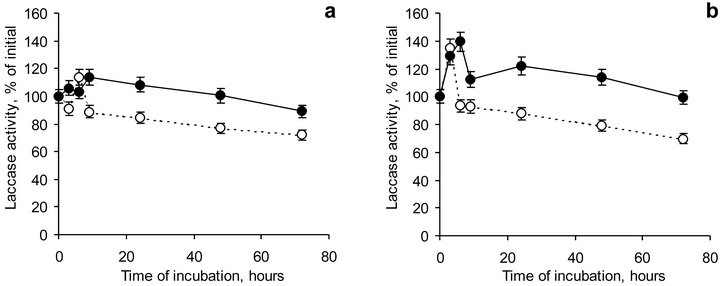
Figure 6. Dynamics of laccase activity solely (dotted lines) and in the presence of HA (firm lines) at pH 5.0 (a) and 6.5 (b). Bars represent standard deviations.
with preservation of laccase activity has been established. Assuming the stability of HA-laccase complex under conditions that provided compensation of partial negative charge both of laccase and HA, ionic interaction between those compounds can be excluded. On the other hand, IEF data allowed concluding on the non covalent binding. The association between HA and laccase by weak dispersive forces such as hydrophobic, van der Waals, π-π, and others was therefore hypothesized. The obtained data showed also that the interaction of laccase with HA included both complex formation and direct partial oxidation of HA structural fragments.
4. Acknowledgements
Authors thank Dr. Elena Stepanova for her kind assistance in fungi cultivation and laccase extraction and purification and Elena Belyaeva for the SEC measurements. This work was partly supported by the Ministry of education and science of Russia in the framework of the Federal Targeted Program “Scientific and research and educational cadres of innovative Russia in 2009-2013” (Agreement #8111).
NOTES