Particle Characteristics and Metal Release From Natural Rutile (TiO2) and Zircon Particles in Synthetic Body Fluids ()
1. Introduction
Titanium oxide (rutile, TiO2) and zircon (ZrSiO4), known insoluble ceramic materials [1-5], play a large role for implant materials and corrosion protection (coating) of implant materials [2,5,6]. For example, the spontaneous growth of rutile on titanium-based implant surfaces is favorable from a biocompatibility perspective and reduces furthermore the extent of metal release. Even though stated insoluble, very low amounts of metals, depending on the surrounding solution (e.g. the solution pH), have been reported to be released in human body fluids [3-5]. Potential adverse effects induced by particles (e.g. released from implant materials) of rutile and zirconia (ZrO2) have been studied in the literature and mostly ascribed to their particle characteristics (size and shape), rather than to their solubility [1,7,8]. Adverse effects have also been reported for nanometer-sized [8-12] and micron-sized particles [1,8,12] of anatase (TiO2) of slightly lower solubility compared with rutile [3]. When the particles are small enough to be phagocytized (<5 µm), they show a significant increase in toxic response [1,8]. Below this size limit, cytotoxicity for anatase and rutile TiO2 and for ZrO2 particles (pigments) has been reported to be size-independent when normalized to volume, but to increase with increasing particle size when normalized to surface area or particle number [8]. However, no surface area or size dependence of pulmonary toxicity in rats have been observed for TiO2 pigments of different structure and size (10 to 300 nm) [13]. The lack of surface area dependence on toxicity for nanometer sized particles has also been observed for TiO2 nanoparticles [14] showing a higher (100 times) cytotoxicity for anatase compared to rutile TiO2 pigments. The structure of TiO2 was reported important for the induced toxicity with slightly higher levels of oxidative DNA damage induced by a mixture of rutile and anatase ultrafine particles compared with anatase or rutile alone [15]. Examples of reported adverse effects caused by nanoand micron-sized particles of TiO2 and ZrO2 observed in vitro and in vivo are cytotoxicity [1,7-8,14], DNA damage [12,15], release of inflammatory cytokines from macrophages [6], liver function damage in mice [9], oxidative stress in the brain of mice [11], decreased recognition memory in mice [10], and toxicological effects on major organs and knee joints of rats [16]. However, compared with other nanoor micron-sized particles, observed acute toxicity is generally low [12,17].
Literature on release mechanisms of rutile, zirconia, or zircon particles in human or artificial body fluids is however scarce, mainly due to their very low solubility. It is evident that the growth of titanium dioxide (rutile structure in comparison to anatase structure) on titanium implant materials reduces the release of soluble titanium species [2,5] and improves its biocompatibility properties [6]. Rutile and anatase particles (TiO2) have been shown soluble up to 0.2% - 1.2% in very acidic aqueous solutions when boiled for 30 minutes [3]. After 30 days in serum, implant materials have shown titanium to be released to an extent of 3.5% of the material (titanium) and 4.6% (TiAl6V4) [18]. Other investigations have focused on the dissolution kinetics of micron-sized zircon particles [4], for example at 25˚C in distilled water at pH 5.0 up to 714 hours. Non-measurable levels of dissolved zirconium (while silicon could be measured) were explained by the precipitation of ZrO2 and later ZrSiO4.
Toxicity studies alone can however neither explain observed differences in the induced toxic response of “insoluble” particles of different structure nor their induced toxicity when taken up by the cell or when remaining in the tissue over a long time period. Such investigations require additional studies on material properties in vivo, studies of the change of surface properties such as structure, composition and charge, and studies on the mechanisms of metal release.
Several factors may be important for the metal release mechanism of rutile and zircon particles in the human body: 1) surface structure and composition; 2) solution pH; 3) solution composition, including organic species, proteins, oxidants and reductants. Ligand-induced metal release mechanisms, expected to play an important role in vivo and in complexing artificial solutions [19], have been shown to be dependent on protein adsorption and zeta potential of the particles, parameters both influenced by pH and surrounding chemical species (e.g. Ca ions) [20] and by the surface structure [21].
In this study, we investigate the release of zirconium, titanium, aluminum, iron, and silicon from different natural micron-sized (median diameter 104 µm - 130 µm) rutile (TiO2, 2 types) and zircon (ZrSiO4, 4 types, of which one was micronized to less than 6 µm) powders. Main focus of the investigation is placed on the importance of bulk and surface composition for the release mechanisms into five different artificial body fluids covering a pH range from 1.5 to 7.4 (different possible endpoints), and with different solution complexities (artificial gastric fluid, artificial lysosomal fluid, artificial sweat, phosphate buffered saline, and Gamble’s solution). Generated metal release data were performed within the Registration, Evaluation and Authorisation of Chemicals (REACH) regulation even if rutile and zircon were exempt from REACH as they are naturally occurring substances. They were therefore mainly investigated to reach a deeper understanding of chemical-physical properties of these materials.
2. Materials and Methods
2.1. Materials and Particle Characterization
Six different materials, 2 natural rutile (TiO2) and 4 zircon powders were investigated, denoted “a”, “b”, “c”, “d”, “e”, and “f” (micronized zircon < 6 µm).
The specific surface area (m2/g) per mass unit was determined by means of BET-analysis (adsorption of nitrogen at cryogenic condition) using a Micromeritics Gemini V instrument. Nitrogen adsorption measurements were performed at five different partial pressures (p/p0 0.10 - 0.25) with a standard deviation between replicas of less than 1%. The specific surface area of all particles is given in Table 1.
Triplicate measurements of particle size distribution in solution (PBS, see next section for composition) were conducted by means of laser diffraction using a Malvern Mastersizer 2000 equipment with a Hydro SM dispersion unit. Refractive indices of zirconium dioxide or titanium dioxide, and water (since it was the solvent of the test medium), were used as input parameters applying standard operational conditions. In Table 2, the measured median particle diameter, and the 10% and 90% size distribution cut off points (by volume%) are presented for the different particles.
Surface compositional analyses were performed by means of X-ray Photoelectron Spectroscopy, XPS. Spectra were recorded using a Kratos AXIS UltraDLD x-ray photoelectron spectrometer (Kratos Analytical, Manchester, UK) using a monochromatic Al x-ray source (150 W) on areas approximately sized 700 × 300 μm. Wide spectra were run to detect elements present in the outermost surface of the test items. Wide and detailed high resolution spectra (20 eV pass energy) were acquired for carbon (C 1s), oxygen (O 1s), zirconium (Zr 3d), silicon (Si 2p), titanium (Ti 2p), iron (Fe 2p) and aluminium (Al 2p).
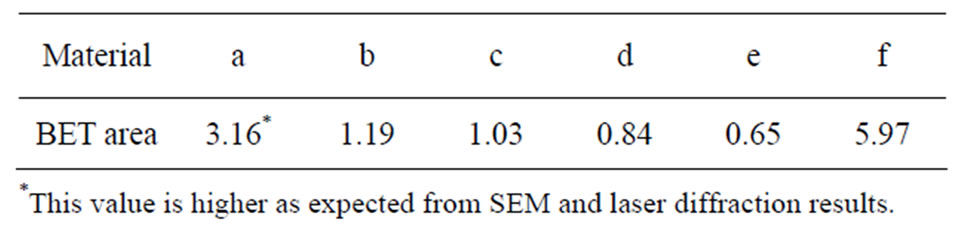
Table 1. Specific surface area [m2/g], measured by means of BET.
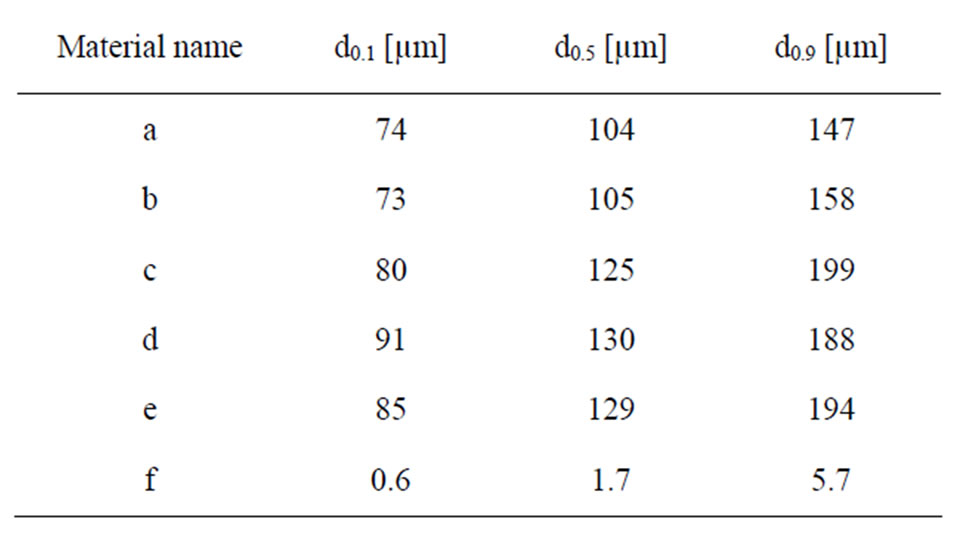
Table 2. Measured median particle diameter (d0.5) and the 10% (d0.1) and 90% (d0.9) size distribution percentiles, presented as a percentage of volume (mass) of the different particles in PBS using LD.
The test items were fixed on copper tape to avoid any dispersion of the powder particles in the vacuum inside the instrument chamber. All binding energies were calibrated by assigning the carbon C1s contamination peak to 285 eV. All peak areas were determined by assigning a linear baseline. Average results were calculated from several local areas, for each powder, respectively.
Differences in surface morphology of the investigated particles were studied using a tabletop scanning electron microscope (SEM) with backscattered electrons (Hitachi TM-1000). The powder samples were fixed on carbon tape to avoid any dispersion of particles inside the instrument chamber and to assure appropriate conduction. The morphology is shown in Figure 1 for all materials.
The chemical composition and structure (bulk information) of the materials were investigated by means of Raman spectroscopy in reflection mode. This configuration is in essence similar to the Total Internal Reflection Raman spectrometer described elsewhere [22]. Briefly, a highly stable 532 nm laser (Quantum lasers) is delivered to the sample in an external reflection configuration at an angle of incidence of 78 degrees. The polarization of the beam was S (perpendicular to the plane of incidence). The Raman scattered light is collected using an ultra-long high numerical aperture objective with 50×
magnification attached to a modified upright Axio microscope from Zeiss. The scattered light is then passed through a sharp long pass filter that blocks the 532 nm light and finally focused to a spectrograph (ShamrockAndor) and detected with a CCD camera (Newton 940Andor).
2.2. Test Fluids
The powders were exposed to five different test fluids at a pH range from 1.6 to 7.4. The composition of the fluids and the pH prior to and after exposure is given in Table 3. The solutions were selected to cover a wide and relevant
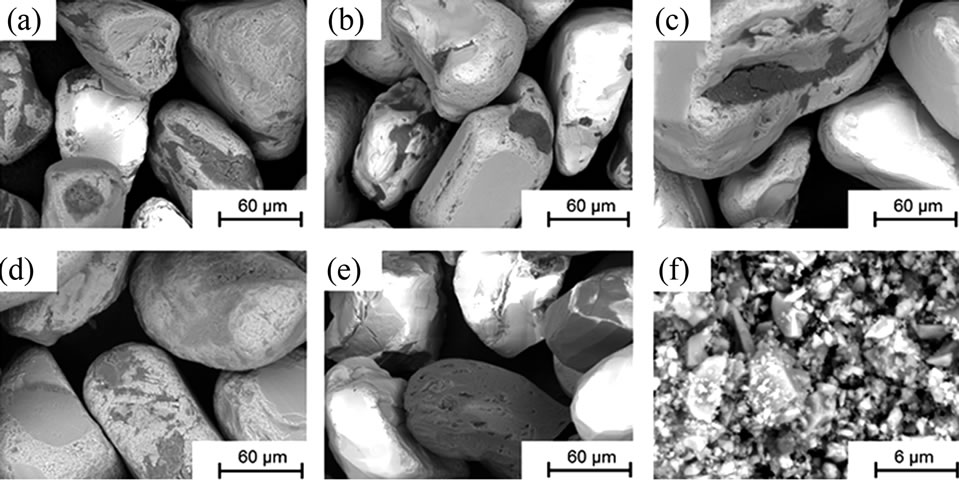
Figure 1. SEM images (backscattered electrons) at a magnification of 1000× (powders (a) to (e)) and 10,000× (powder (f)).
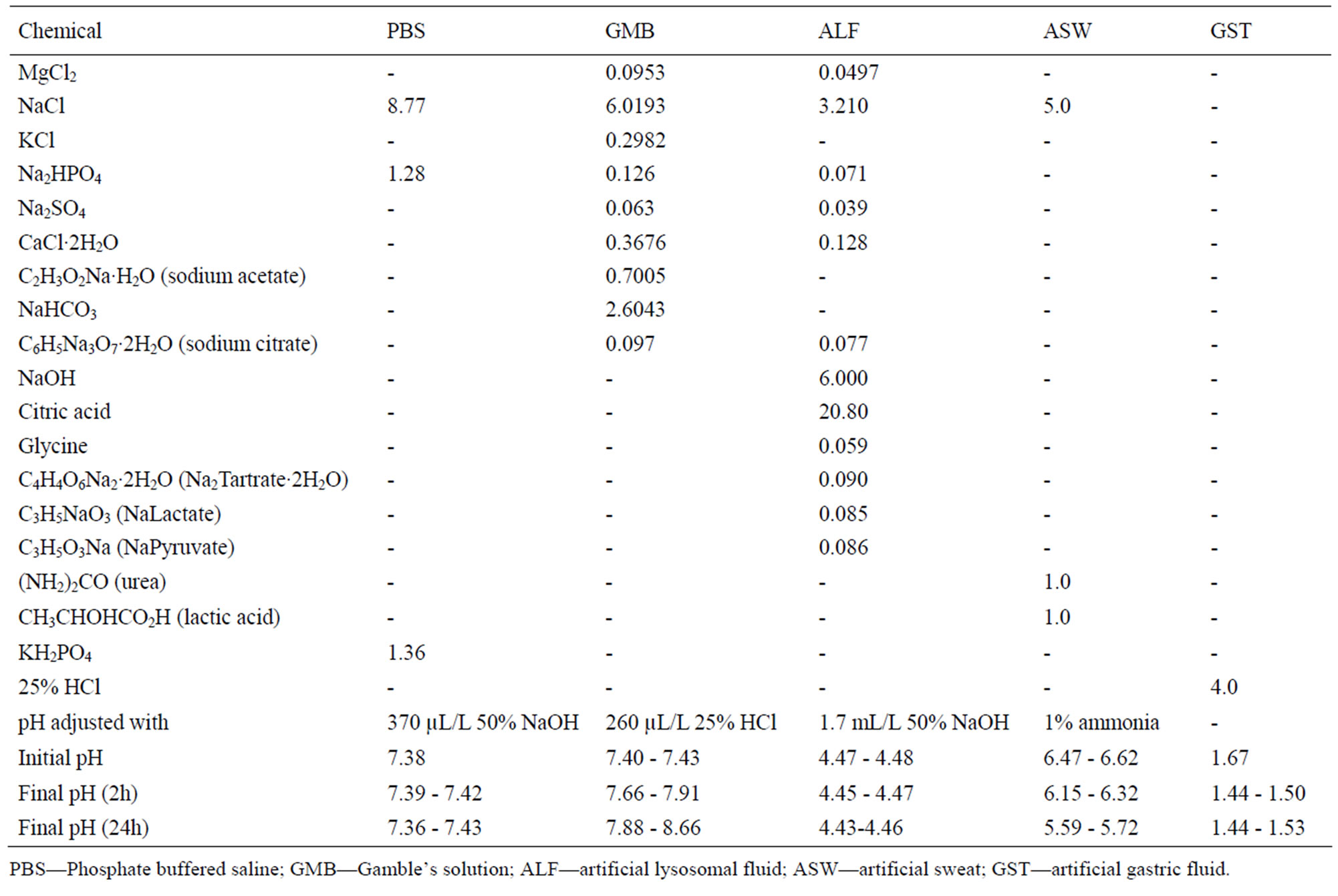
Table 3. Composition of the different synthetic body fluids (in g/L), pH adjustment, and pH prior and after exposure.
range of pH and relevant artificial body fluids for human exposure via inhalation, ingestion, and skin contact. Phosphate-buffered saline (PBS, pH 7.4) is a standard physiological solution that mimics the ionic strength of human blood serum. Gamble’s solution (GMB, pH 7.4) mimics the interstitial fluid within the deep lung under normal health conditions [23]. Artificial sweat (ASW, pH 6.5) simulates the hypoosmolar fluid, linked to hyponatraemia (loss of Na+ from blood), which is excreted from the body upon sweating [24]. Artificial lysosomal fluid (ALF, pH 4.5) mimics intracellular conditions in lung cells occurring in conjunction with phagocytosis and represents relatively harsh conditions [25]. Artificial gastric fluid (GST, pH 1.6) simulates the very harsh digestion environment of high acidity in the stomach [26].
2.3. Experimental Procedure and Metal Analysis
Triplicate samples were prepared for exposure in different test fluids, each for two different time periods. In addition, one blank sample (without addition of any particles) containing only the test solution was incubated together with the triplicate samples for each time period. 5 ± 0.5 mg of each material was weighed using a Mettler AT20 balance with readability of 2 μg, and placed in a PMP Nalge® jar. 50 mL of the test solution was then added to the Nalge® jar containing the material of interest, before incubated in a Platform-Rocker incubator SI 80 regulated at 37˚C ± 0.5˚C. The solution was gently shaken (bi-linearly) with an intensity of 25 cycles per minute for 2 and 24 hours, respectively. After exposure, the samples were allowed to cool to ambient room temperature before the final pH of the test solution was measured. The test fluid was then separated from the powder particles by centrifugation at 3000 rpm for 10 minutes (704 relative centrifugal force, r.c.f.), resulting in a visually clear supernatant with remaining particles in the bottom of the centrifuging tube. The supernatant solution was decanted into a polypropylene storage flask and acidified to a pH less than 2 (not required in the case of artificial gastric fluid) with 65% pure HNO3 prior to solution analysis. All vessels for exposure, centrifugation and storage of samples were acid-cleaned in 10% HNO3 for at least 24 hours, then rinsed four times with ultra-pure water and dried in ambient air in the fume hood within a few hours to avoid any risk of contamination.
Dissolved/released concentrations of metals were analysed by means of inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES), using a Varian Vista Ax with an axial plasma with CCD detector using standard operational procedures with multiple standards for calibration and triplicate measurements of each sample (relative standard deviation 1% – 8%). The sample matrixes and standard matrixes were adjusted to the same sodium chloride content by eventually adding sodium chloride on-line. The released elements considered in this paper are zirconium, titanium, aluminium, iron, and silicon. Calcium, magnesium, and phosphorus, measured to significant extent, were not considered, since these elements are present in the test fluid, are non-toxic, and easily dissolved. Limits of detection (calculated from the three-fold background concentration) for the metal elements taken into account were: Al-0.68 µg/L, Fe-0.80 µg/L, Si-5.1 µg/L, Ti-0.12 µg/L, Zr-0.34 µg/L.
3. Results and Discussion
3.1. Particle Characterization
The chemical composition and structure of the natural rutile TiO2 and zircon (ZrSiO4) powders were investigated using Raman spectroscopy. Peak positions and relative intensities are given in Table 4 and Raman spectra shown in Figure 2. For the rutile powders (“a” and “d”), main peaks were as expected assigned to rutile TiO2 (145, 246, 440, 607 cm–1) [21,27,28]. Differences in relative peak intensities, and also the occurrence of peaks in the 700 cm–1 - 800 cm–1 region for the rutile powder “a” could be due to differences in grain size implying bulk TiO2 for the rutile particle “d”, and smaller grains (possibly nanosized) for the rutile powder “a” [29].
The spectra of the zircon powders “b”, “c”, and “e” were similar (Figure 2), with main peak positions at approximately 215, 355, 439, 822, 995, 2250, and 2540 cm–1, all except the band at 822 cm–1 assigned to zircon [30]. A significantly different Raman spectrum was generated for the zircon powder “f” with broader and shifted peaks, but also with differences in relative peak intensities when compared with the other three zircon powders “b”, “c”, and “e” (Figure 2). The latter three powders revealed few weak bands at 461 cm–1 (only “b”), at 503 cm–1 - 510 cm–1, and at 572 cm–1 - 577 cm–1 (only “c” and “e”), possibly assigned to monoclinic ZrO2 [31]. The same bands, of significantly stronger relative intensity (462, 510, and 575 cm–1), were observed for the micronized zircon powder “f”. The strong peaks observed for the zircon powders “b”, “c”, and “e” were in contrast very weak or not present for the zircon powder “f”. The only exception was that the band at approximately 995 cm–1 (“b”, “c”, “e”) was slightly shifted to 978 cm–1 in a broader peak. It is unclear why the zircon powder “f” disclosed a different spectrum. However, similar to the other zircon powders, most peaks were assigned to zircon and monoclinic ZrO2. A plausible explanation could be a larger fraction of monoclinic ZrO2 and smaller grain size (broader peaks), compared with the zircon powders “b”, “c”, and “e” [31].
Differences in bulk (Table 5) and surface composition (determined by means of X-ray Photoelectron spectroscopy, XPS), are shown for the investigated powders in
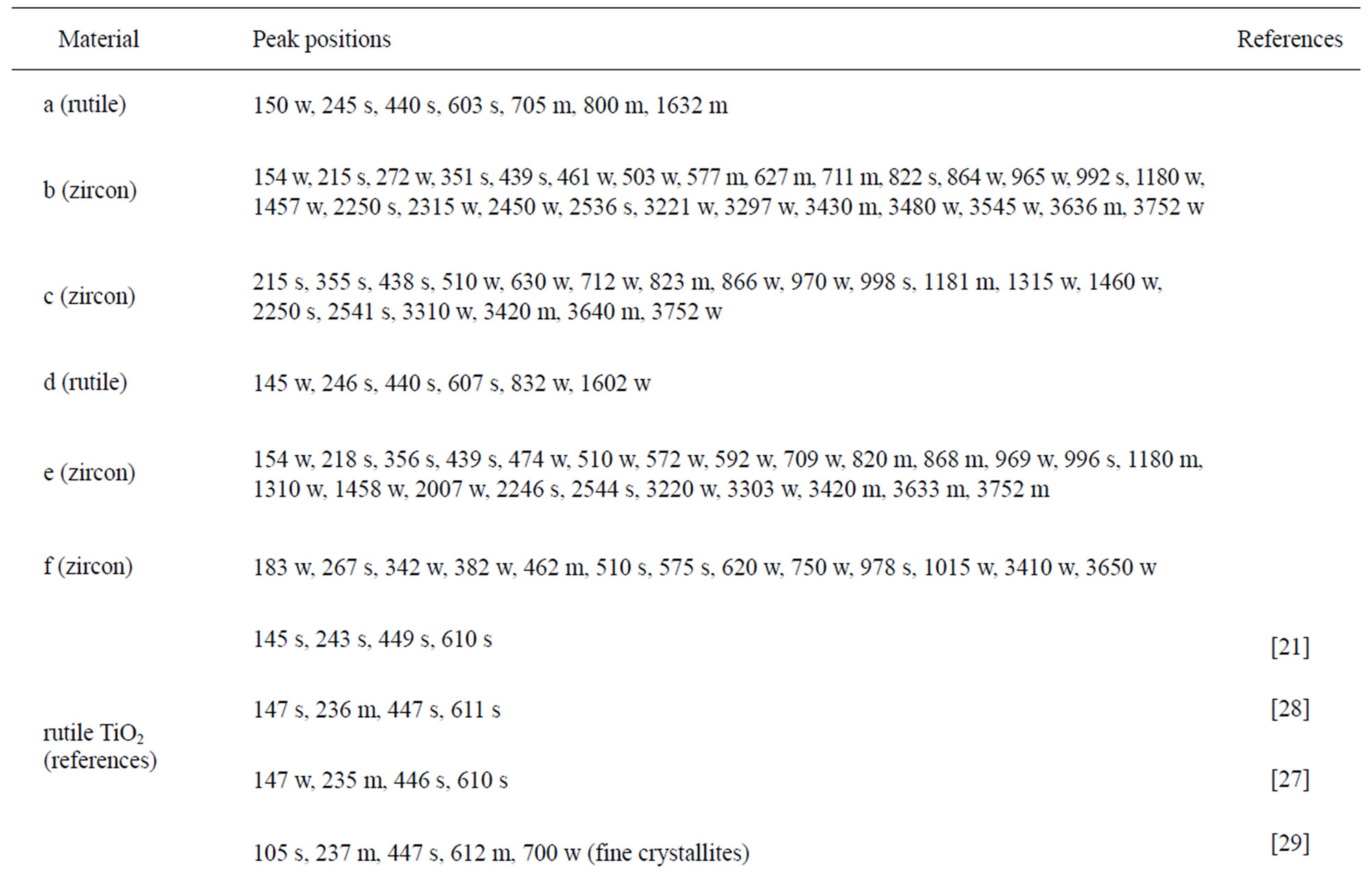
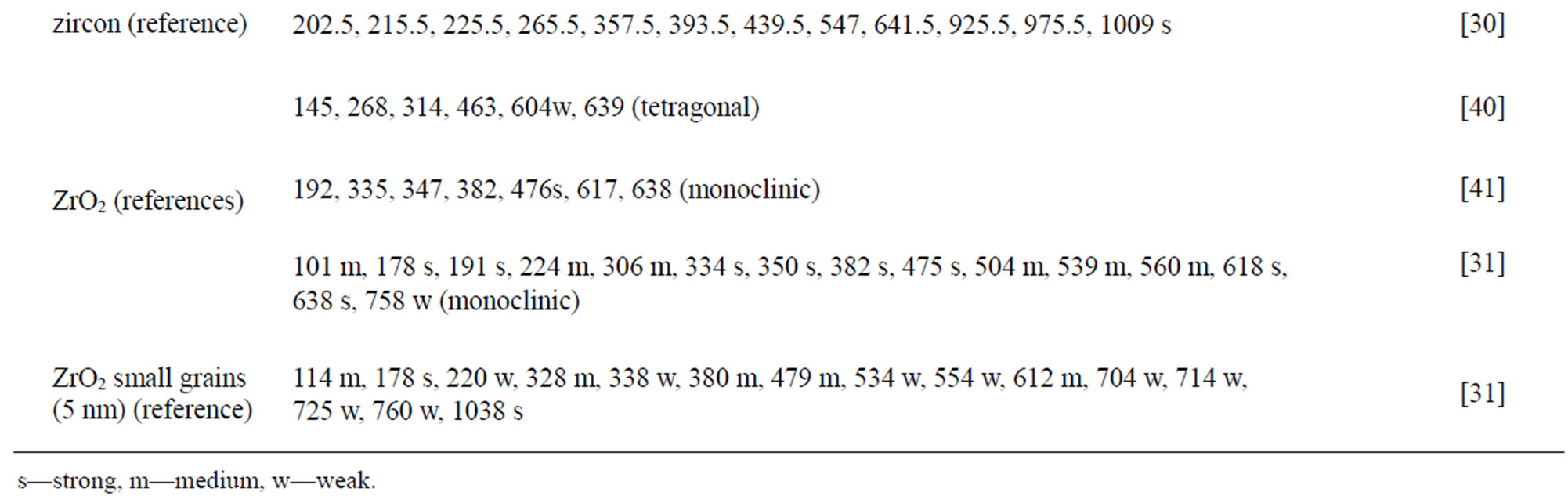
Table 4. Raman peak positions and relative intensities (strong, medium, weak) of the two rutile powders (“a” and “d”) and the four zircon powders (“b”, “c”, “e”, and “f”). Reference peak positions are included for comparison.
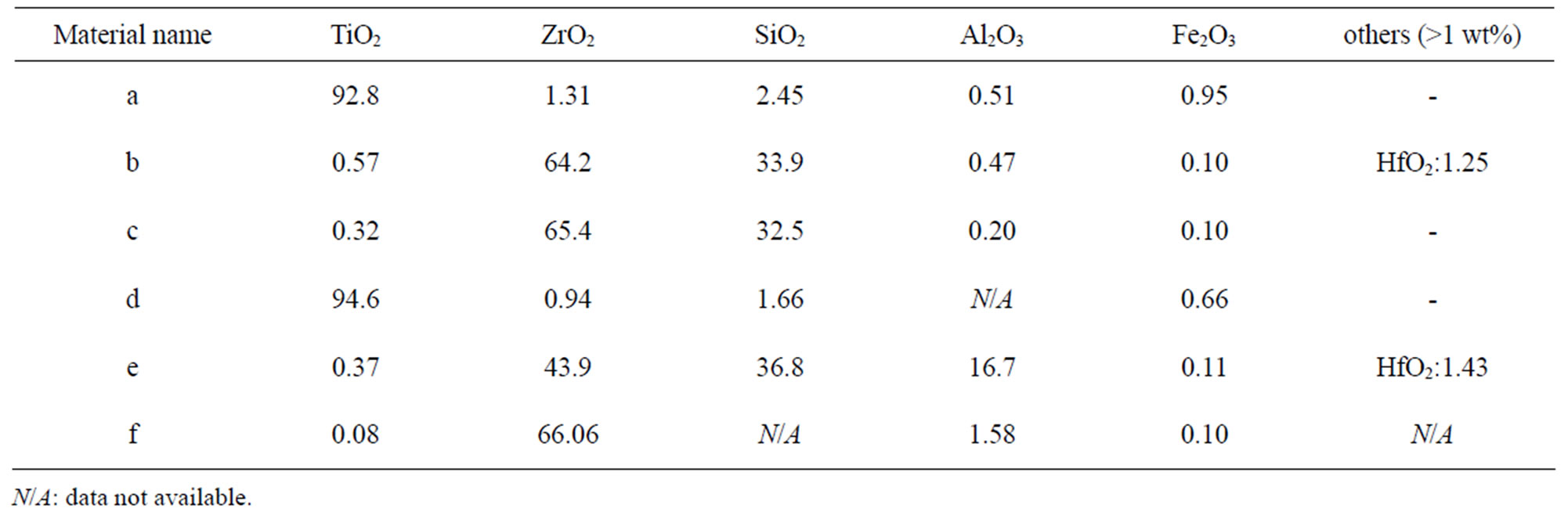
Table 5. Nominal bulk composition [wt%] of natural rutile and zircon powders investigated based on supplier information.
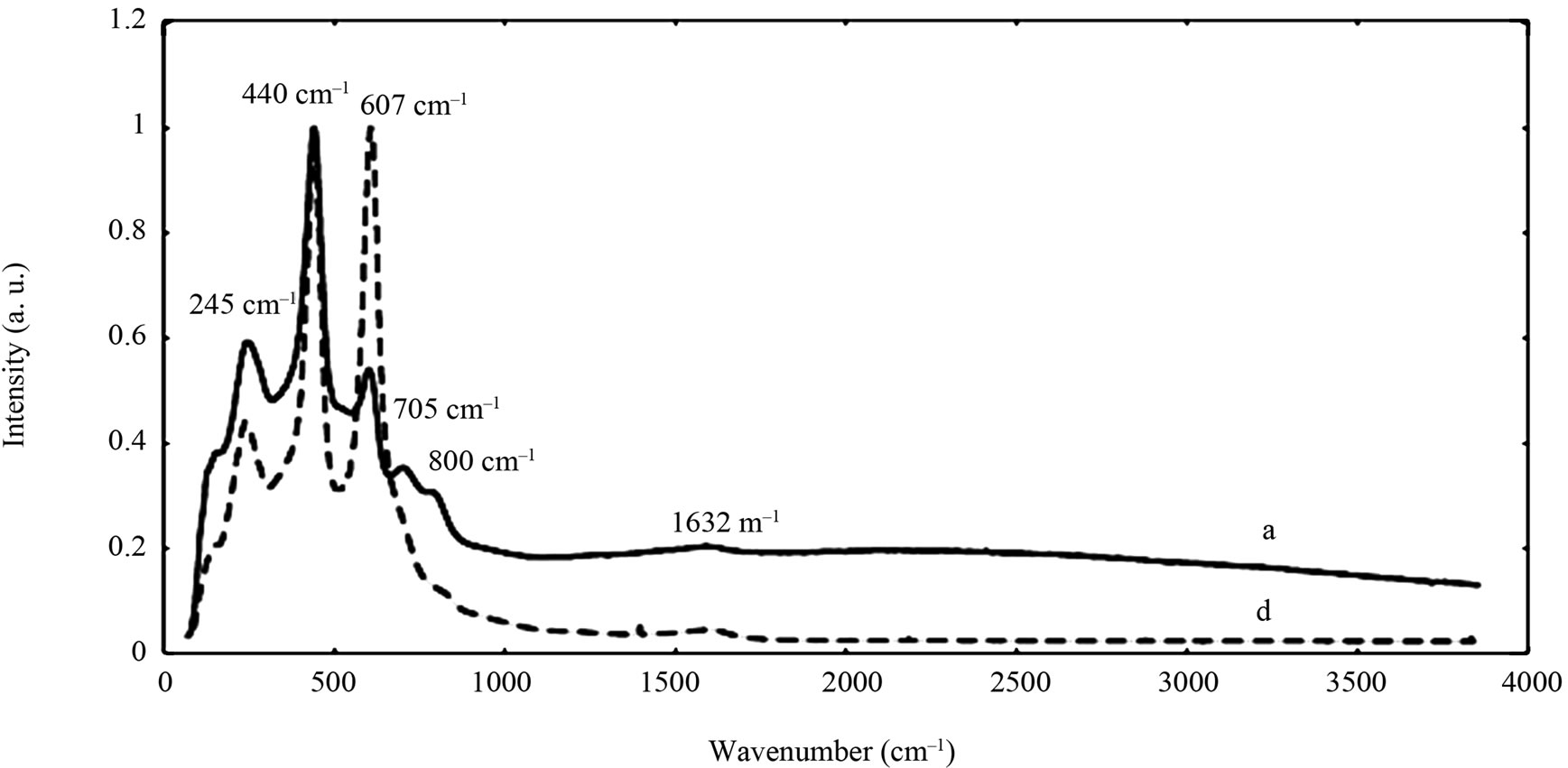

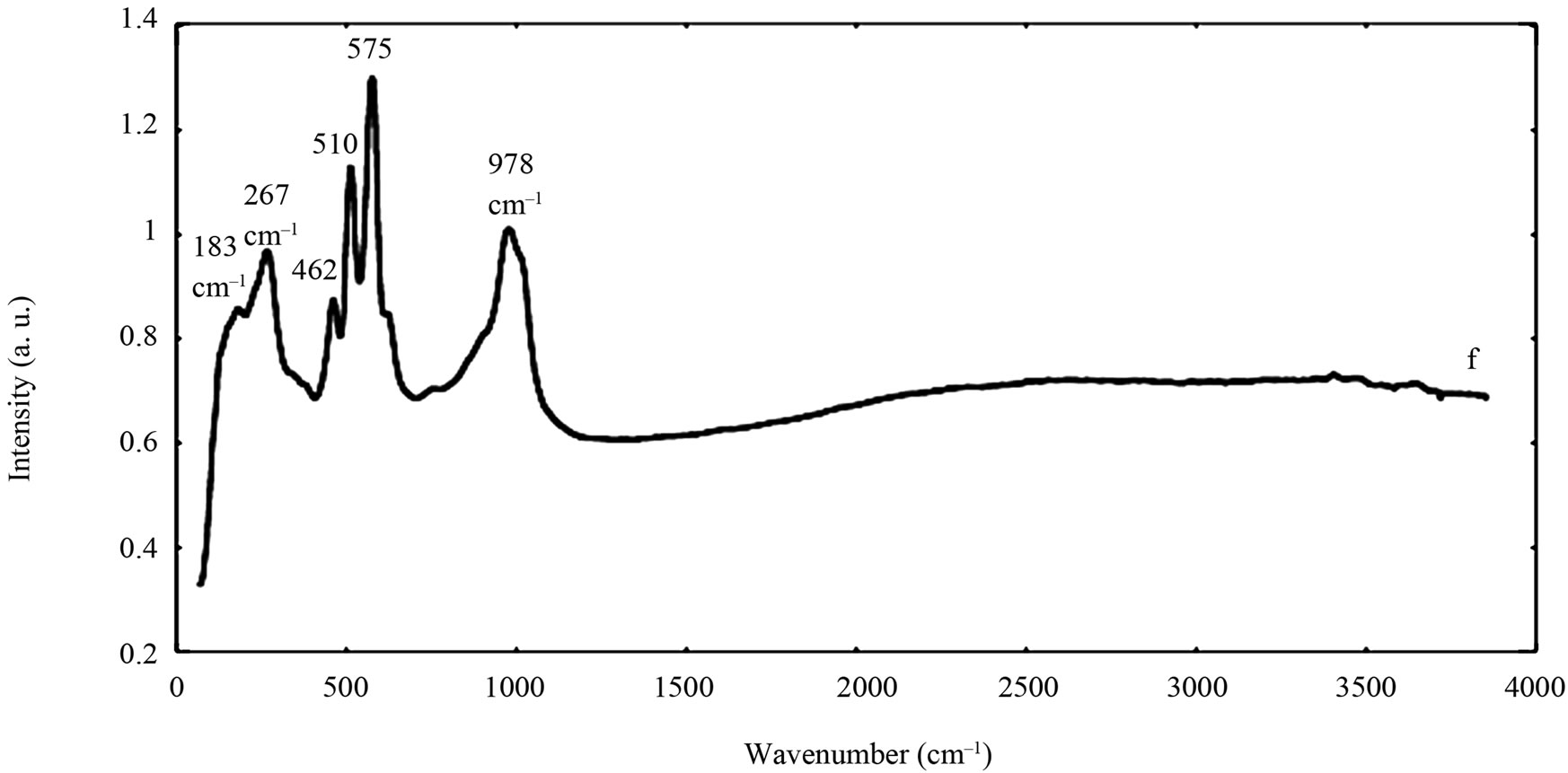
Figure 2. Raman spectra of the natural rutile (“a” and “d”—top) and zircon powders (“b”, “c”, and “e”—middle, and “f”— bottom). The spectra were offset for clarity.
Figure 3. The main oxygen 1s peaks correspond both to oxidized metal compounds and to a small extent also to oxidized carbon components in the layer of atmospheric surface contamination. The oxygen peaks for Fe2O3/Fe3O4/ FeO (≈530.2 eV) and TiO2 (530.0 eV) occurred at similar binding energies and were therefore difficult to distinguish. Therefore, data presented in Figure 3 is based on the metal content only.