Studies on Chemical Resistance of PET-Mortar Composites: Microstructure and Phase Composition Changes ()
1. Introduction
Degradation of concrete, as well as the related protection and ensuring of concrete structures against aggressive impacts by chemical agents, regardless of whether this concerns liquid, gas, or even solid phase under certain conditions, represents a complex problem of utmost importance for the economy in general, and especially for building construction and the construction industry [1]. Therefore, polymer-modified mortars have been popular construction materials because of their excellent properties in comparison with ordinary mortars. Polymers have been used for improving mechanical properties, adhesion with substrates, or waterproofing properties of mortars and concretes. The literature agrees that the properties of polymer modified mortar and concrete depend significantly on the polymer content or polymer-cement ratio, that is, the mass ratio of the amount of polymer solids in a polymer-based admixture to the amount of cement in a polymer-modified mortar or concrete [2-4].
A substantial growth in the consumption of plastic is observed all over the world in recent years, which has led to huge quantities of plastic-related waste. Recycling of plastic waste to produce new materials like concrete, mortar or composite appears as one of the best solution for disposing of plastic waste, due to its economic and ecological advantages. Several works have been performed or are under way to evaluate the properties of cement-composites containing various types of plastic waste as aggregate, filler or fibre [5,6].
Depending on the appropriate final target, varied types of waste can be used in concrete: Portella et al. [7] and Guerra et al. [8] have studied the properties of concrete with addition of ceramic waste; Ismail and Al-Hashmi [9] have studied concrete with recycled plastic addition; Angulo et al. [10] have characterized recycled materials from construction and demolition as an aggregate for concrete; and, amongst others, Hoppen et al. [11] have found in concrete the possibility to deposit the residual sludge from water treatment stations.
Different works, as of Rebeiz [12], Choi et al. [13,14] and JO et al. [15], have analyzed the effect of addition of recycled PET to the properties of concrete. The fibers of recycled PET easily mix in the concrete, giving new properties to the material [16]. Khaloo et al. [17] have observed that the addition of tire rubber particles provided the concrete with higher ductility in compressive strength testing, if compared with concrete without addition. J. C. A. Galvão et al. [18] have demonstrated a better performance to use of waste polymers (PET, LDPE and rubber from useless tires) in concrete for repair of dam hydraulic surfaces. Wang et al. [19] have analyzed a Performance of cement mortar made with recycled high impact polystyrene, which is a common component of consumer electronics. Corinaldesi et al. [20] have also showed a better mechanical behaviour and thermal conductivity of mortars containing waste rubber particles coming from wasted rubber-shoe outsoles (SR, acronym of “sole rubber”). In the previous work [21,22], the author studied the effects of PET polymer on the mortar properties, specifically to decrease the chloride ion penetration depth and apparent chloride ion diffusion coefficient of polymer-mortar composites. This may be explained due to the reduced volume of large-sized pores and the improved resistance to the absorption of the test solutions with an increase in polymer-cement ratio [22]. In addition, Gouasmi et al. [23,24] showed the improvement of the adherence strength and the resistance to aggressive solutions of composites using waste PET lightweight aggregates (WPLA). One of the advantages of the use of recycled plastic in concrete is the reduction of solid waste in landfills [5,6].
Polyethylene terephthalate (PET) is one of the most common consumer plastics used and is widely employed as a raw material to realize products such as blown bottles for soft-drink use and containers for the packaging of food and other consumer goods. PET bottles have taken the place of glass bottles as storing vessel of beverage due to its lightweight and easiness of handling and storage.
In 2007, it is reported a world’s annual consumption of PET drink covers of approximately 10 million tons, which presents perhaps 250 milliards bottles. This number grows about up to 15% every year [25]. On the other hand, the number of recycled or returned bottles is very low. Generally, the empty PET packaging is discarded by the consumer after use and becomes PET waste (WPET). The major problems that this level of waste production generates initially entail storage and elimination [26]. The recycling of PET bottles and the preservation of natural resources are priority items but to date, the recycling of PET bottles as a lightweight aggregate for concrete has not been studied because of the high melting cost [16].
Chemical degradation of concrete is the consequence of reactions between the constituents of cement stone, i.e., calcium silicates, calcium aluminates, and above all calcium hydroxide, as well as other constituents, with certain substances from water, solutions of soil, gases, vapors, acids etc. The most important aggressive agents are:  and
and  [1,27,28].
[1,27,28].
When speak about sulfate degradation, we primarily think of the impact by sulfate ions on cement stone. The sulfate ion is the cause of one of the most dangerous corrosions—the corrosion of expansion and swelling—because it causes the occurrence of expansive compounds, the most important of which is ettringite, C3A∙3CaSO4∙32H2O, in the shape of prismatic crystals [29,30].
For the process of concrete degradation under the impact of sulfates, it is essential which cation is linked with the sulfate ion. Namely, cations linked with sulfate ions can be divided into three characteristic groups [1]. The first group includes alkali metals Na+ and K+, which give extremely soluble hydroxides, while the second group comprises metals such as Mg2+ and Fe2+, which give poorly soluble hydroxides, and the third group consists of cations  and H+, which give volatiles or hydroxide. The third group of sulfates, that is (NH4)2SO4 and H2SO4, covers the most aggressive compounds. In case of impact by these compounds on concrete, there occurs not only expansion, but also intensive dissolution of cement stone [1].
and H+, which give volatiles or hydroxide. The third group of sulfates, that is (NH4)2SO4 and H2SO4, covers the most aggressive compounds. In case of impact by these compounds on concrete, there occurs not only expansion, but also intensive dissolution of cement stone [1].
The degree of aggressivity of an acid is dependent on the chemical character of anions present. The strength of acid, its dissociation degree in solutions and, mainly, the solubility of the salt formed are dependent on the chemical character of anion. With respect to concrete acidic attack, the solubility of calcium salts formed is of a great significance. Because the acidic attack is based on the processes of decomposition and leaching of the constituents of cement matrix, the conditions of transport phenomena, such as the supply of aggressive acidic solutions and draining of the products of the attack is of specific significance for the intensity of attack [31-33].
In addition, a parameter which is tightly connected with the properties of acids is that their pH reaches a value of approximately 5 and lower. The severity of the acidic attack is significantly dependent on the solubility of the calcium salt formed. In the case of the formation of highly soluble salts, the severity of the attack is very high. This is caused by the dissolving and leaching of the formed salt from the attacked material. A very porous layer of corrosive products remains on the surface, contributing to further development of the deterioration process. In the case of the formation of insoluble calcium salts such as calcium oxalate and sulfate the effect of the acidic solution is entirely different. A dense insoluble layer is formed enhancing the development of the acidic attack showing a protective effect. This is used in practice for the protective purposes [34].
The objective of the research reported here was to evaluate the chemical resistance of polymer-mortar composites containing a waste polyethylene terephtalate PET, as a substitute of the cement used in the mix, under hydrochloric acid, ammonium-chloride, ammonium-sulfate and sulfuric acid solutions exposure. The identification of the deterioration products’ which appear on the surface of the samples were analyzed by X-ray diffraction, FTIR, SEM, ATD, TG/dTG and DSC analyses. However, no pertinent data were previously found concerning the effect of PET against ammonium-chloride or ammoniumsulfate attack. So, in this paper the durability of PETmortar composites exposed to aggressive solutions has been investigated. If successful, such an application of waste PET, as substitute of cement, will be a major step towards reducing the solid waste disposal problem and reliance on natural resources, thereby reducing environmental pollution and energy consumption. Additionally, the immediate consequence is the anticipated necessity of maintenance and repairs of the concrete structures, which must have specific characteristics, mainly mechanical and chemical, based on the material of the base or its substratum.
2. Materials, Experimental Design and Methods
2.1. Raw Materials
The cement used was a blended Portland cement type CPJ-CEM II/A (pouzzolanic cement) delivered from Zahana factory located in the western Algeria, chemical and physical properties of cement are shown in Tables 1 and 2, respectively, according to the manufactories. The chemical composition was obtained by using an X-ray fluorescence spectrometer analysis type OXFORD MD X1000.
Two different aggregate types were selected for this study, sand (S) and PET particles. Those come from drinking water bottles that was first separated, washed and shredded. The particles thus derived were then shredded once again, using a propeller crusher in order to control granular limit with crushing. Moreover, they present irregular shape and rough surface texture in order to facilitate matrix-particles adhesion (Figure 1). Figure 2 showed the X-ray diffraction analysis of PET particles and indicated the presence of a backbone form of the polymer which generates sharp peaks at a high angle range ~10˚ - 35˚. The main mechanical and thermal properties of the plastic used in this study are presented in Table 3. Particle size distributions analysis (Figure 3) reveals the sand to be particularly coarse (Fineness modules = 3.66) and that the upper granular limit value of PET aggregates is 5 mm. After preliminary tests, polymer particles of size lower than 1 mm were used in this study. Absolute density of PET particles  is approximately 2.5 times smaller than that of cement
is approximately 2.5 times smaller than that of cement

Table 1. Cement chemical composition.

Table 2. Physical properties of cement.

Table 3. Physical properties of the PET plastic used.

Figure 1. Scanning electron micrographs of PET particles, (magnification, ×1200).
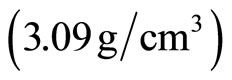 . This low density will allow the lightening of PET-mortar composite.
. This low density will allow the lightening of PET-mortar composite.
2.2. Composite Mixing Conditions
The mortar manufactured with PET particles was first optimized on the basis of mechanical criteria and then constitutes the reference composite. The composites containing PET particles were produced in accordance with the results of the previous work [35]. A massic ratio of 3 between sand (S) and the cement (C) has been respected. Various massic percentages of cement (2.5%, 5.0% and 7.5%) were substituted by the same weight of granulated plastic waste (Table 4). The water to binder ratio was kept constant at 0.5. The physical properties of the pastes of mortars were determined in accordance with EN 19-3 [36].
The PET particles and cement were first dry-mixed at 140 rpm during 1 min in a standard mixing machine (EN 196-1 [37]) to reach a homogeneous mixture. Then sand was added and the mixing continues during 1 min at low speed. Next, water was gradually added at 140 rpm during 1 min and after the whole is mixed during 1 min with 285 rpm. After pouring fresh material into the molds, samples were stored in both a hygrometrically-controlled and temperature-controlled room (98% relative humidity and 20˚C ± 2˚C) for 24 h. After removal from the moulds, at 24 h of age, mortar specimens were immersed in water saturated with lime at 20˚C ± 3˚C until the age of testing. Test results from the Table 4 of the hydraulic transport properties, sorptivity-value, revealed that the addition of PET particles tends to restrict water propagation in the cement matrix and reduces water absorption of the composite. This may be due both to the capability of PET to repel water (non-sorptive nature, water absorption % = 0, Table 3) and to the increase of air-entrainment, as manifested by closed empty pores, which are not accessible to water. This phenomenon serves to reduce the volume accessible to water and hence capillary porosity. The decrease in water absorption is also attributed to a reduction in the porosity near particle/matrix interfacial zone, due to the high bonding between PET additive and cement paste [35]. So, the decrease of the sorptivity-value is favorable to the durability of the specimen structures.

Figure 2. X-ray diffraction pattern of the PET particles.

Figure 3. Particle size distributions of polyethylene terephthalate (PET) and Sand.
2.3. Resistance to Chemical Attack Test
The relative acid attack was determined in accordance with ASTM C267-97 [38]. The mortar specimens were cured in water at 20˚C ± 3˚C for 28 days before being subjected to acid attack. Three specimens of each mortar and composite mixes  were immersed in three types of chemical solutions: 10% ammonium chloride NH4Cl; 10% ammonium-sulfate (NH4)2SO4; 5% sulfuric acid H2SO4. Before the test, the attacked specimens were cleaned with deionised water and then the acid attack was evaluated by measuring the mass loss
were immersed in three types of chemical solutions: 10% ammonium chloride NH4Cl; 10% ammonium-sulfate (NH4)2SO4; 5% sulfuric acid H2SO4. Before the test, the attacked specimens were cleaned with deionised water and then the acid attack was evaluated by measuring the mass loss  of the specimens, determined as follows:
of the specimens, determined as follows:
 (1)
(1)
where Wr is weight of the specimen before immersion and Ws is weight of the cleaned immersed specimen after test period. The solution was renewed every 7 days and the mass loss of the specimens measured.
After immersion in 0.5%, 1% and 1.5% HCl acids  , 5% H2SO4 acid (ASTM C267-97) and 10% (NH4)2SO4 solutions (ASTM C1012-04 [39]) for the required period of time, the specimens were capped and tested for residual compressive strength based on the original cross-sectional area. The compressive strength loss
, 5% H2SO4 acid (ASTM C267-97) and 10% (NH4)2SO4 solutions (ASTM C1012-04 [39]) for the required period of time, the specimens were capped and tested for residual compressive strength based on the original cross-sectional area. The compressive strength loss  is calculated as follows:
is calculated as follows:
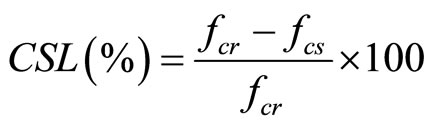 (2)
(2)
where fcr is the reference compressive strength of specimen before immersion in the acid or sulphate solutions in MPa and fcs is the average compressive strength of the specimens after immersion in acid or sulphate solutions for the required period of time.
After compression testing, scanning electron microscopy (SEM), X-ray diffraction (XRD), FT-IR analyses, DSC and DTA-TGA/dTG were conducted on selected surface fractures to investigate damage mechanisms.
An accelerated leaching test using an ammonium chloride solution (10% NH4Cl) was carried out on three samples  of each composite, after cured in water at 20˚C ± 3˚C for 28 days. Ammonium nitrate allows an equivalent leaching test with demineralised water to be simulated but increases the kinetics by a factor 100 [40,41]. The mortar and composites specimens were immersed in the solution at 20˚C for 480 days. For the required time of test, the leached depth (observed with phenolphthalein) and the mass loss were measured each time of test.
of each composite, after cured in water at 20˚C ± 3˚C for 28 days. Ammonium nitrate allows an equivalent leaching test with demineralised water to be simulated but increases the kinetics by a factor 100 [40,41]. The mortar and composites specimens were immersed in the solution at 20˚C for 480 days. For the required time of test, the leached depth (observed with phenolphthalein) and the mass loss were measured each time of test.
3. Results and Discussion
3.1. Hydrochloric Acid (HCl) Attack
3.1.1. Compressive Strength Loss (CSL%)
Mass loss is a simple traditional test in the context of acid attack. However, mass change results may depend on sample size and cement type, and are also influenced by the way the decomposed cement paste and other reaction products on samples are treated during testing [42, 43]. Therefore, along with mass loss test, compressive strength is considered to be a more reliable measure to judge the performance of mortar/concrete subjected to acid attack. Siad et al. [44] reported that there is some divergence between the mass loss and the compressive strength loss.

Table 4. Mix proportions and physical properties of polymer-mortar composites.
Hence, Figure 4 presents the results of compressive strength loss CSL% of the mixes at 56 days in different hydrochloric acid concentration (0.5%, 1% and 1.5%) solutions. The results indicate that the resistance of acidic attack of the composites (PET7.5) was increased with an increase in PET content. At day-56, the CSL% of PET7.5 was reduced by 68%, 41%, 10.7% for the 0.5%, 1% and 1.5% of HCl acid solutions, respectively, when compared to that of PET0.
The H2SO4 solutions lead to the formation of less water-soluble gypsum CaSO4,  of H2O at 20˚C, and ettringite on the surface in contact with the cementing matrix. With CH3COOH acid, there is a formation of the hydrate calcium acetate Ca(CH3COO)2,
of H2O at 20˚C, and ettringite on the surface in contact with the cementing matrix. With CH3COOH acid, there is a formation of the hydrate calcium acetate Ca(CH3COO)2,  of H2O. While, the chemicals formed as the products of reaction between hydrochloric acid and hydrated cement phases are some soluble salts, mostly with calcium chloride CaCl2 (dihydrate) that is very watersoluble:
of H2O. While, the chemicals formed as the products of reaction between hydrochloric acid and hydrated cement phases are some soluble salts, mostly with calcium chloride CaCl2 (dihydrate) that is very watersoluble: 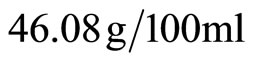 of H2O, which are subsequently leached out, and some insoluble salts along with amorphous hydrogels (iron hydroxide) which remain in the corroded layer. These chemical reactions are shown in Equations (3)-(5). These results are in agreement with those reported by elsewhere [45-47].
of H2O, which are subsequently leached out, and some insoluble salts along with amorphous hydrogels (iron hydroxide) which remain in the corroded layer. These chemical reactions are shown in Equations (3)-(5). These results are in agreement with those reported by elsewhere [45-47].
 (3)
(3)
 (4)
(4)
 (5)
(5)
Additionally, these results are confirmed by the change of surface samples before and after immersion in the HCl
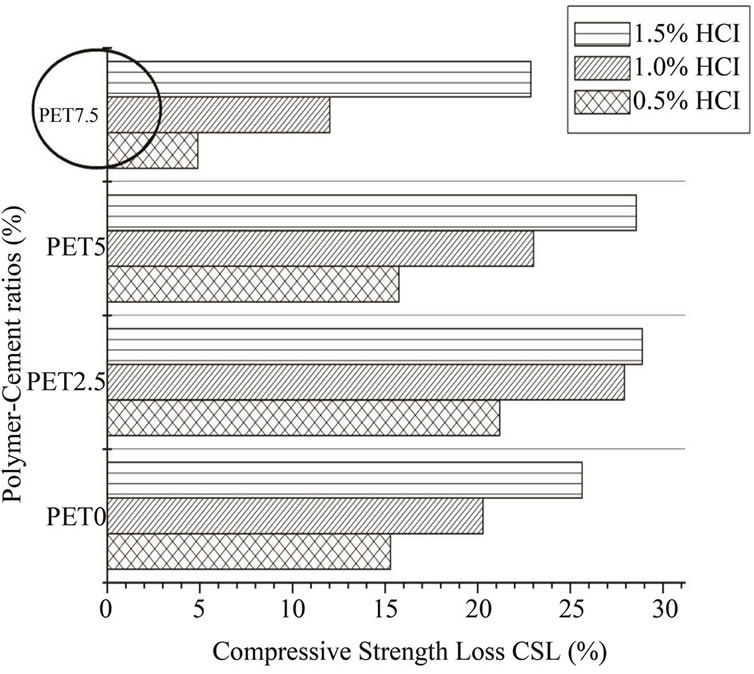
Figure 4. Compressive strength loss of specimens under HCl acid solutions exposure.
aggressive solutions as depicted in Figure 5. These mortars kept their rectangular forms more or less, but their dimensions decreased considerably from 0.5%, 1% to 1.5%. Hydrochloric acid attack is a typical acidic corrosion which can be characterized by the formation of layer structure [47]; its can be divided to three main zones: undamaged zone, hydroxide mixture zone or brown ring, and attacked zone. By hydroxide mixture zone, there is a layer formed by undissolved salts seen as a dark brown ring.
The chemical resistance of materials is more or less affected by the concentration and the nature of acids in the order with the most aggressive as given below:
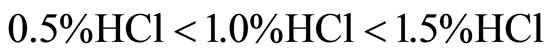 .
.
The increase in the resistance to hydrochloric attack of the composites is attributed to the impervious PET granules blocking the passage of the aggressive solutions and the reduction of the sorptivity of PET-mortar composites (Tables 3 and 4). Furthermore, the decrease in porosity due to the incorporation of PET in modified mortars [48] contributes to reduce the absorption of acidic solution accompanied by a reduction of loss in weight. These results are in agreement with those reported by Benosman et al. [46]. Additionally, different teams of researchers [49-51] reported that the incorporation of organic additions (polymers) increases chemical resistance in aggressive media.
3.1.2. X-Ray Diffraction (XRD) Analysis
Figure 6 presents the XRD analysis of composite PET0, as an example, before and after attack by different hydrochloric acid concentration (0.5%, 1% and 1.5%) solu

Figure 5. Deterioration of specimens after 56 days of immersion; (1) Tap water, (2) 0.5%, (3) 1%, (4) 1.5% HCl acids (From the left to right, respectively).

Figure 6. X-ray diffraction pattern of the specimens under HCl acids exposure. P: portlandite Ca(OH)2, C: calcite, G: gypsum,  (hydrated calcium chloride).
(hydrated calcium chloride).
tions. The stacking of the various spectra (initial state, after 0.5% attack, after 1% attack and after 1.5% attack) confirms the appearance of a trace of calcium chloride (CaCl2) on the specimens exposed to hydrochloric acid. The low quantity of calcium chloride found is due to its high solubility in water (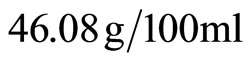 of H2O). Washing of each specimen after the immersion period leaves its surface nearly free from this salt. Additionally, the portlandite Ca(OH)2 was decomposed by different acids concentration following the chemical reaction (Equation (3)).
of H2O). Washing of each specimen after the immersion period leaves its surface nearly free from this salt. Additionally, the portlandite Ca(OH)2 was decomposed by different acids concentration following the chemical reaction (Equation (3)).
3.1.3. FT-IR Analyses
Table 5 illustrates the positions and intensities of infrared absorption bands and Figure 7 shows the FT-IR patterns of specimens exposed to: Tap water, 0.5%, 1% and 1.5% HCl acids solution. The FT-IR spectra of the composite hydrated up to 56 days and cured in water are presented in Figure 7 and Table 5. The major changes of the FT-IR spectra in the hydrated cement pastes are: Calcium hydroxide bands (~3635 cm−1) and also for the free OH groups, combined and adsorbed water of CSH, AFm and AFt phases (~3480 cm−1), molecular water (3440 - 3446 and 1614 - 1621 cm−1), carbonate phases (~1425, 870.03 and 708 cm−1). The broad band at ~1020 - 1016 cm−1 arises from C-S-H vibrations, in agreement with those reported by Martinez-Ramirez [52].
As the same for the X-ray diffraction, the FT-IR analysis of composite after attack by different acids (Table 5, Figure 7) confirms the appearance of a trace of calcium chloride (CaCl2) on the specimens exposed to various HCl acids. The low quantity of calcium chloride found is due to its high solubility in water.
Therefore, in Table 5 and Figure 7 no absorption bands corresponding to calcium hydroxide were detected in all of specimens exposed to acidic solutions, which is in agreement with XRD analysis. The Ca(OH)2 was consumed by different HCl acids following the chemical reaction (Equation (3)).
3.1.4. DTA-TGA/dTG Analyses
The simultaneously traced DTA-TGA curves of PET0 composite before and after HCl acids attack (0.5%, 1% and 1.5%) are presented in Figures 8-11.

Table 5. Fourier-transform infrared table of composite before and after attack by different HCl acids, in KBr pellet.

Figure 7. FT-IR spectra of the specimens under HCl acid exposure, (C5: Tap water, C6: 0.5%, C7: 1%, C8: 1.5%).

Figure 8. DTA/TG curves at 20 K/min of composite PET0 in tap water exposure.
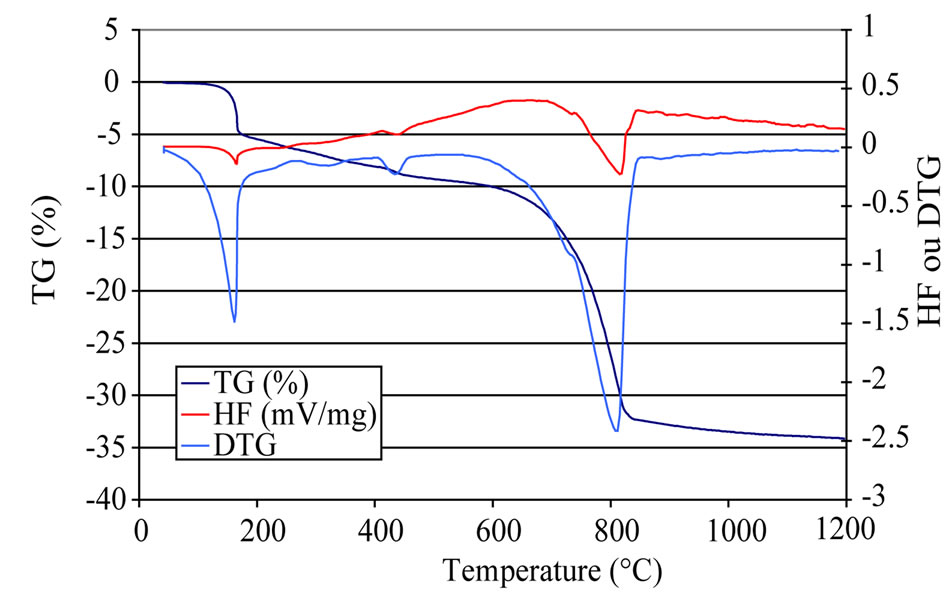
Figure 9. DTA/TG curves at 20 K/min of composite PET0 under 0.5% hydrochloric acid exposure.
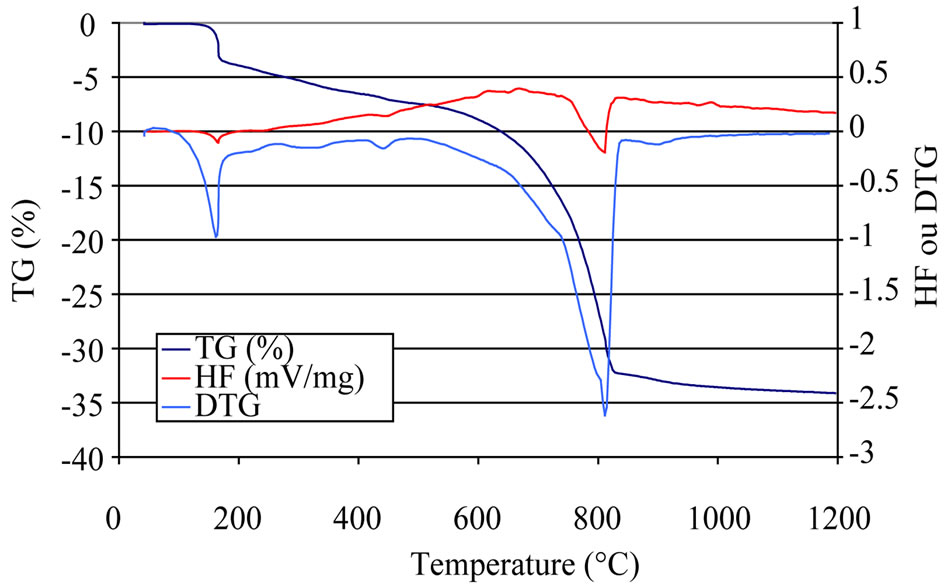
Figure 10. DTA/TG curves at 20 K/min of composite PET0 under 1.0% hydrochloric acid exposure.

Figure 11. DTA/TG curves at 20 K/min of composite PET0 under 1.5% hydrochloric acid exposure.
Figure 8 shows the DTA/TG curves of mortar without polymer PET0 in the tap water. It can be seen that DTA-TG/dTG curves for this mortar consist of four zones:
~100˚C - 150˚C: dehydration of pore water (CSH, ettringite)~225˚C - 230˚C: dehydration of calcium aluminates hydrates~450˚C - 550˚C: dehydroxylation of calcium hydroxide~700˚C - 850˚C: decarbonation of CaCO3.
Figures 9-11 show DTA-TG/dTG curves of PET0. As it is shown, the curves can be divided into four major parts, according to different reactions:
~100˚C - 145˚C: dehydration of pore water~225˚C - 230˚C: dehydration of calcium aluminates hydrates~440˚C - 540˚C: the reduction of the intensity of the peak corresponding to dehydroxylation of portlandite, which indicates that, with exposure all the Ca(OH)2 that was formed reacted with the HCl acid solutions (Equation (3)).
~700˚C - 850˚C: decarbonation of CaCO3.
All the weight loss data are expressed as a function of the ignited weight of the sample, as suggested by Taylor [53]. The calcium hydroxide content was determined from the following equation:
 (6)
(6)
where  is the content of Ca(OH)2 (in weight basis),
is the content of Ca(OH)2 (in weight basis),  is the weight loss occurred during the dehydration of calcium hydroxide (in weight basis), MWCH is the molar weight of calcium hydroxide and MWH is the molar weight of water.
is the weight loss occurred during the dehydration of calcium hydroxide (in weight basis), MWCH is the molar weight of calcium hydroxide and MWH is the molar weight of water.
When one attacks the PET0 composite by the hydrochloric acid at 0.5%, 1% and 1.5%, there is a diminution of the calcium hydroxide (CH) content, as can be seen in Figure 12. Because when the concentration of acid increases, there is less formation of portlandite Ca(OH)2, which is mainly consumed following the chemical reaction (Equation (3)). This is confirms our previous results.
3.1.4. Scanning Electron Microscope (SEM) Observations
In case of HCl attack, the damaged of PET0 composite was selected as an example to observe the microstructure of deterioration and the SEM images are shown in Figure 13. No portlandite crystal exists in the acid attacked composite. It is clear that exposure to solution of hydrochloric acid generates pores and micro-cracks in the mortar specimens. So, The SEM and ATD-TG/dTG analyses corroborate some of the results discussed above.

Figure 12. Effect of HCl acids concentration on the calcium hydroxide content (CH).

Figure 13. SEM images obtained for the external porous texture of PET0 sample after being attacked by the 1% hydrochloric acid solution (×600).
3.2. Ammonium Chloride NH4Cl Attack
Figures 14 and 15 show the evolution of the degraded thickness of composite immersed in a solution of ammonium chloride 10%, represented as a function of the polymer-cement ratios. Loss of mass followed the same trend. At 480 days, significant differences were obtained: compared to PET0, the depth of attack was 16.2%, 26.2% and 34.8% lower for PET2.5, PET5 and PET7.5, respectively.
Ammonium salts are generally more destructive than salts of other bases [54]. So, ammonium chloride is very aggressive and reacts following an exchange mechanism  given by the reaction:
given by the reaction:
 (7)
(7)

Figure 14. Depth of attack due to the leaching of composite immersed in 10% ammonium chloride.

Figure 15. Depth of reduced lixiviation of the composites PET0, PET2.5, PET5 and PET7.5 (from the left to right) indicated by the phenolphthalein solution.
This reaction leads to the formation of a highly soluble calcium chloride (  of H2O) and a release of ammonia. The resulting reduction of the pH impedes the reaction to reach a state of equilibrium. The conesquence is progressive leaching of portlandite and C-S-H, thus leading to a decrease of the mechanical properties of mortar. The reaction with aluminates leads to the formation of a hydrated calcium chloride aluminate
of H2O) and a release of ammonia. The resulting reduction of the pH impedes the reaction to reach a state of equilibrium. The conesquence is progressive leaching of portlandite and C-S-H, thus leading to a decrease of the mechanical properties of mortar. The reaction with aluminates leads to the formation of a hydrated calcium chloride aluminate  [55].
[55].
As for most of the other tests, the composites were the modified mortar most resistant to this acid attack (pH = 5.6). This might be due to impervious PET granules blocking the passage of the aggressive solutions and the reduction of the sorptivity of modified mortar (Tables 3 and 4). Furthermore, the decrease in porosity due to the incorporation of PET in composite [48] contributes to reduce the absorption of acidic solution accompanied by a reduction of loss in weight. However, no pertinent data were found concerning the effect of PET against ammonium chloride attack.
3.3. Ammonium Sulfate (NH4)2SO4 and H2SO4 Attacks
Figure 16 shows the percentage change with respect to time in the weight of cubes immersed in sulfuric acid and

Figure 16. Change (%) in weight with time under H2SO4 acid and (NH4)2SO4 solutions.
ammonium-sulfate. It can be seen in the same figure that there is an increase in weight of cubes immersed in ammonium sulfate for the period of experimentation. The augmentation in weight after 120 days of immersion was 4.5%, 4.8%, 4.86% and 6.9% for PET0, PET2.5, PET5 and PET7.5 respectively. However, in the cubes immersed in sulfuric acid it can be noted that the reduction in weight has occurred. So, the rate of reduction in weight was less in PET5 and PET7.5. The mass loss for PET7.5 is lower than the corresponding PET0 mortar by 5% (Figure 16).
The increase in the weight of composites immersed in sulfatic solutions can be explained by the formation of expansive products. In the presence of water, the sulfates ions react with calcium aluminates hydrate (C3A) and/or the components of the calcium hydroxide of hardened cement paste to form calcium sulfoaluminate hydrate commonly called “ettringite” [56] and calcium sulfate (gypsum). However, it is important to recognize that the end products of the various reactions, if they occur so as to damage the mortar or concrete, result in different types of damage [57]. Mehta [58] reported that the 5% (NH4)2SO4 solution proved to be more aggressive than 1% H2SO4; it appears that ammonium salts are able to decompose the calcium hydrate, which is the principal solid phase in hydrated portland cement pastes. In consequence, different preventive measures are required.
Compressive strength losses CSL% of PET-composites samples after curing in water for 28 days and exposed in 10% of ammonium-sulfate solution for 860 days and in 5% of sulfuric acid for 56 days are presented in Figure 17. It can be seen that the modified mortar samples containing polyethylene therephtalate have better behaviour in ammonium-sulfate solution. The results indicate that the resistance to (NH4)2SO4 solution of the composites was increased with an increase in PET content. At day-860, the CSL% of PET2.5, PET5 and

Figure 17. Compressive strength loss of specimens under H2SO4 acid and (NH4)2SO4 solutions exposure.
PET7.5 were reduced by 19.2%, 18.2% and 22.6%, respectively, when compared to that of PET0. In addition, at 56 days, there is a CSL% diminution around 7% for PET7.5 compared to an unmodified one in sulfuric acid solution (Figure 17).
Therefore, the increase in the resistance to ammonium-sulfate of the composites is attributed to the impervious PET granules blocking the passage of the aggressive solution and the reduction of the sorptivity of polymer-mortar (Tables 3 and 4). Consequently, the decrease in porosity due to the incorporation of PET in modified mortars [48] contributes to reduce the absorption of sulfates solution. These results are in agreement with those reported elsewhere [46]. The same explanation was also done for the PET7.5 in the sulfuric acid. Additionally, different teams of researchers [49-51] reported that the incorporation of organic additions (polymers) increases chemical resistance in aggressive media.
It can be concluded that adding PET to blended portland cement makes this cement become more resistant to the ammonium-sulfate aggressive environment. It is evident that the resistance of cement to sulfate aggression is also related to the content of C3A in them [27]. However, no pertinent data were found concerning the effect of PET against ammonium sulfate attack.
Ammonium sulfate is very aggressive and reacts following an exchange mechanism  given by the reaction:
given by the reaction:
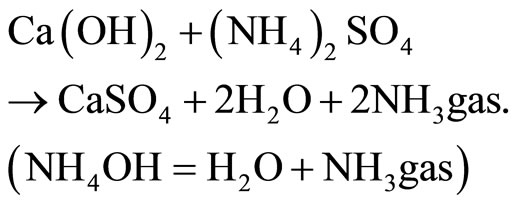 (8)
(8)
This reaction leads to the formation of less watersoluble gypsum CaSO4, 0.241 g/100 ml of H2O and a release of ammonia. Calcium sulfate formed as described above can subsequently react with C3A, usually via the formation of mono-sulfoaluminate, to form ettringite (Equation (5)). In addition, attack of hydrated Portland cement by H2SO4 acid is two-fold. The first one is by acid attack or hydrogen ions and the second is by the sulfuric ions. Two salts are formed: namely calcium sulfate and ettringite (Equations (4) and (5)). These are destructive salts and the pressure produced during their formation causes mortar to crack and disintegrate.
Comparing the exposure of mortars to sulfuric acid and ammonium sulfate, it is immediately apparent that the mechanisms involved are quite different. So, Rendell et al. [59] reported that sulfuric acid causes a heavy deposition of gypsum that acts as a protective layer; this surface protection is verified by the unchanged hardness beyond a depth of 2 mm, however, it is noted that gypsum is deposited at depths of up to 2 mm in fissures and voids. In this case the mechanism of attack is caused by the production of the expansive gypsum, this expansive effect being responsible for the progressive opening of the material structure by dislocation of surface material. It was noted from the SEM and microanalysis [59], that there is a filling of fissures and pores with gypsum, this deposition will have the effect of producing an internal blocking of the pore structure. It is, therefore, proposed that the surface deposit of gypsum, if not removed by scour forms a protective layer thus limiting the attack rate.
The samples exposed to ammonium sulfate experienced a significant deterioration in material hardness up to a depth of 5 mm [59]. However, from the microanalysis it was noted that there was little sulfur evident behind the surface; the major site of gypsum formation being at the surface. It is proposed that the mechanism of deterioration in this case is one of dissolution of Ca2+ from the mortar. Leaching of Ca2+ is principally the result of the dissolution of portlandite; this action causes an opening of the pore structure of the mortar and is, therefore, responsible for the reduction in mechanical strength and ultimate loss in durability [59].
3.3.1. Visual Inspection
Additionally, in case of H2SO4 and (NH4)2SO4 attacks, the damaged of PET0 composite was selected as an example to observe the macrostructure of deterioration. So, these results are confirmed by the change of surface samples before and after immersion in the aggressive solutions. A visual inspection of specimens as shown in Figure 18 revealed the deterioration of the samples, particularly for the mortars immersed in sulfuric acid. These mortars kept their cubic forms more or less, but their dimensions decreased considerably.
Photos of specimens stored in the ammonium sulfate solution for 120 and 860 days are presented in Figure 18. The samples stored for 120 days under ammonium sulfate exposure showed the first signs of deterioration, while the specimens stored in tap water did not show any
clear evidence of attack. The discussion below concerns the samples stored in (NH4)2SO4 solution. In all cases, the first sign of attack was the deterioration of the corners, followed by cracking along the edges. Progressively, expansion and spalling took place on the surface of the specimens.
After 860 days of immersion in a solution of 10% (NH4)2SO4, all 4 surfaces of the specimens developed a white cover that was friable and started to peel off, leaving the aggregates uncovered and reducing the connectivity of the paste. Thus, the extent of surface deterioration after 860 days of exposure had a tendency to decrease with the increased replacement level of the PET wastes.
Polymer-mortar composites modified with PET waste can be advantageous for special applications where the main request is not for mechanical properties, such as in the production of sound barriers and cement blocks for lightweight concrete walls. Also, these composites are often used as low-cost materials for preventing chemical attacks or repairing various reinforced concrete structures damaged by chloride-induced corrosion [21,22], as well as in structures exposed to aggressive environments where high resistance to ammonium-sulfate/-chloride, acid, basic and chloride solutions is required.
3.3.2. X-Ray Diffraction (XRD) Analysis
Figure 19 presents the XRD analysis of PET0 composite, as an example, before and after attack by different sulfuric acid and ammonium sulfate solutions. The common factor for all hydrated samples is the presence of portlandite Ca(OH)2 and ettringite 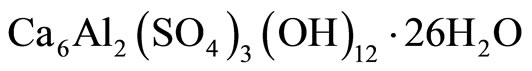 . Unlike thesam ples from the sulfuric acid and ammonium sulfate solution which are marked by considerable quantities of gypsum
. Unlike thesam ples from the sulfuric acid and ammonium sulfate solution which are marked by considerable quantities of gypsum , the samples which were in water contain minimal quantity of gypsum. As gypsum existed in the initial material in both cases, it is evident that during the process of hydration it served, together with other components, to form ettringite, for water cured samples.
, the samples which were in water contain minimal quantity of gypsum. As gypsum existed in the initial material in both cases, it is evident that during the process of hydration it served, together with other components, to form ettringite, for water cured samples.
Opposite to this, the quantity of formed ettringite is small and similar in all the samples exposed to ammonium sulfate, which points out to the assumption that mostly sulfate ions only from gypsum present in the initial samples participated in the forming of ettringite. These results are in agreement with those reported by Miletić et al. [27]. We also noted that portlandite Ca(OH)2 was completely decomposed by different acid and sulfate solutions following the chemical reactions 4, 5 and 8.
3.3.3. FT-IR Analyses
Table 6 illustrates the positions and intensities of infrared absorption bands and Figures 20 and 21 show the FT-IR patterns of specimens exposed to: Tap water, H2SO4, and (NH4)2SO4. The FT-IR spectra of the composite hydrated up to 28 days and cured in water are presented in Figure 20 and Table 6. The major changes of

Figure 19. X-ray diffraction pattern of the specimens under H2SO4 acid and (NH4)2SO4 solutions exposure. P: portlandite Ca(OH)2; E: ettringite; C: calcite; G: gypsum.
the FT-IR spectra in the hydrated cement pastes are: Calcium hydroxide bands  and also for the free OH groups, combined and adsorbed water of CSH, AFm and AFt phases
and also for the free OH groups, combined and adsorbed water of CSH, AFm and AFt phases , molecular water
, molecular water 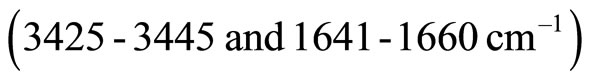 , carbonate phases
, carbonate phases  . The broad band at ~990 - 1020 cm−1 arises from C-S-H vibrations, in agreement with those reported by Martinez-Ramirez [52].
. The broad band at ~990 - 1020 cm−1 arises from C-S-H vibrations, in agreement with those reported by Martinez-Ramirez [52].
As the same for the X-ray diffraction, the FT-IR analysis of composites after attack by acid and sulfate solutions (Table 6, Figures 20 and 21) confirms the appearance of a large quantity of gypsum 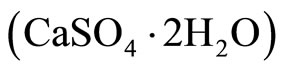 on the specimens exposed to H2SO4 and (NH4)2SO4 and a small quantity of ettringite on the specimens exposed to ammonium sulfate.
on the specimens exposed to H2SO4 and (NH4)2SO4 and a small quantity of ettringite on the specimens exposed to ammonium sulfate.
Therefore, in Table 6 and Figures 20 and 21 no absorption bands corresponding to calcium hydroxide were detected in all of specimens exposed to acidic and ammonium sulfate solutions, which is in agreement with XRD analysis. The Ca(OH)2 was consumed by aggressive solutions following the chemical reactions 4, 5 and 8.
To obtain further evidence to corroborate these observations from XRD and FT-IR, further investigation was made using SEM, DSC and TG/dTG analyses.
3.3.4. Scanning Electron Microscope (SEM) Observations
The composite of PET0 exposed to (NH4)2SO4 solutions was selected as an example to observe the microstructure of deterioration products, and the SEM images are shown in Figure 22. No portlandite Ca(OH)2 crystal existed in the sulfate-attacked unmodified mortar, and many club-shaped or needle-like crystals were embedded irregularly in the pulpy material with a very open microstructure. Figure 22(a) shows a large number of needlelike ettringite crystals, smaller than 2μm in diameter and 10 - 11 μm in length, and the existence of different morphologies of the C-S-H gel in the specimen covering the mortar surface before sulfation. In the pores or surface cracks, there were many club-shaped gypsum crystals, which had much larger sizes (smaller than 100 - 200 µm in diameter and 300 - 500 µm in length) than the ettringite, as shown in Figure 22(b). The formation of both ettringite and gypsum led to the cracking, spallingand decomposition of the unmodified mortar exposed to a sulfate environment (Figure 22(c)). The combination of XRD, FT-IR and SEM led to the positive identification of the deterioration products’ formation.
The surface that was exposed to sulfuric acid had a nature that was quite different. The scanning electron microscopy (SEM) observations were also carried out to identify the products formed by attack of PET0 in the
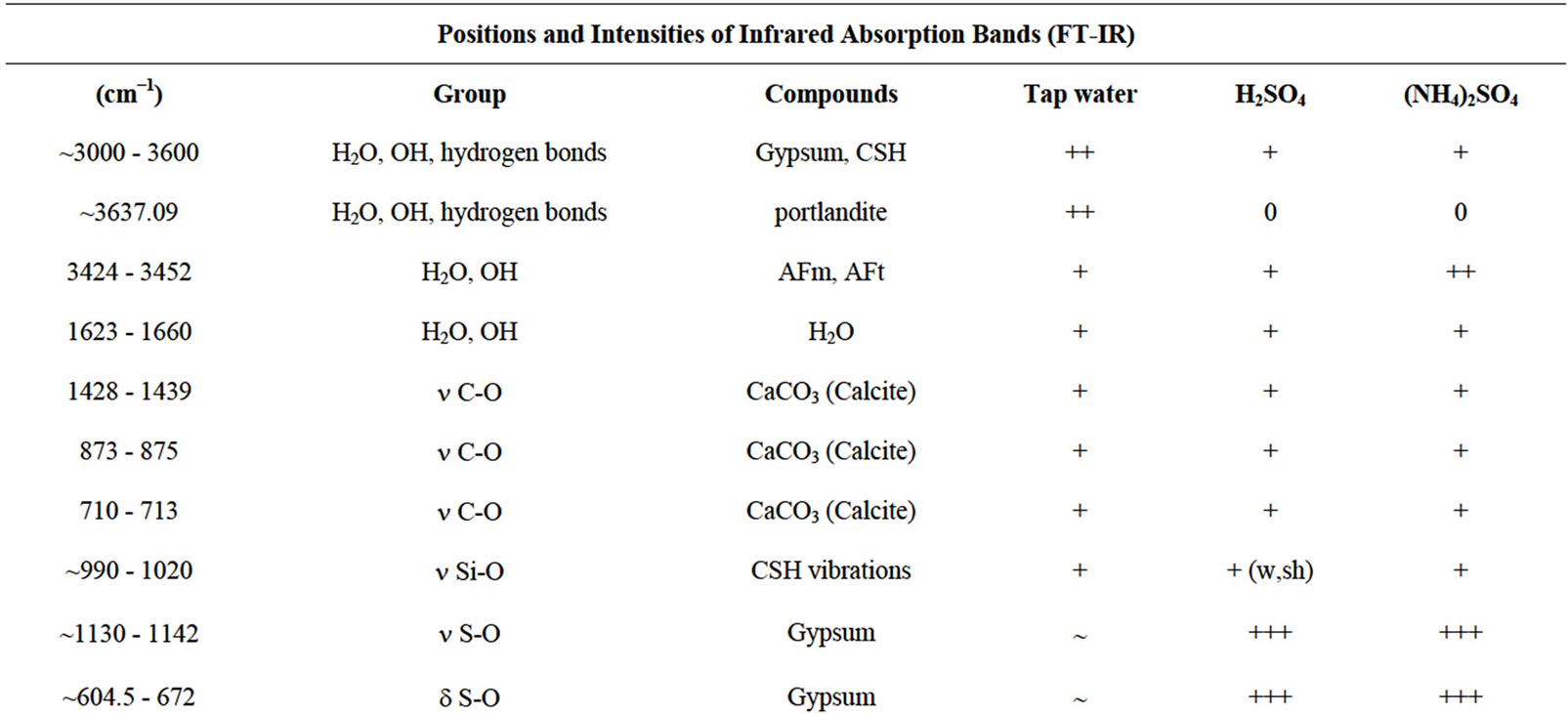
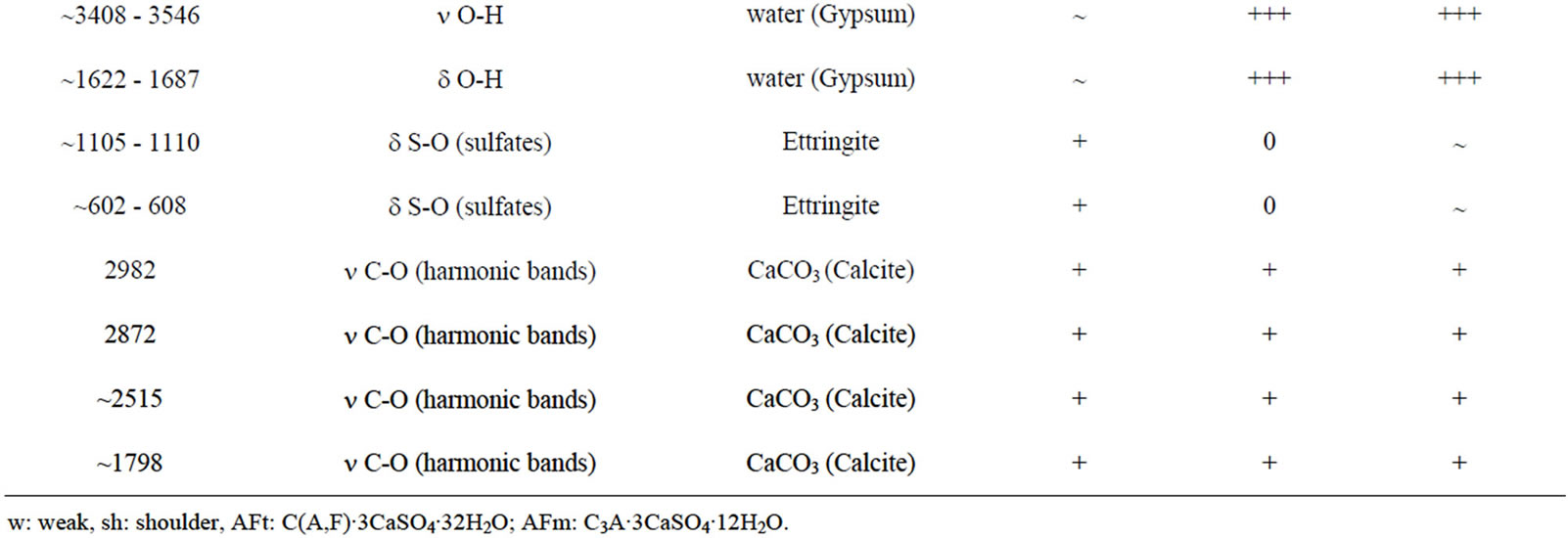
Table 6. Fourier-transform infrared table of composite before and after attack by different acid and sulfate solutions, in KBr pellet.

Figure 20. FT-IR spectra of the specimens under (a) Tap water and (b) (NH4)2SO4 solution exposure.

Figure 21. FT-IR spectra of the specimens under sulfuric acid exposure.
sulfuric acid solutions and the results are exhibited in Figure 23. In addition, Figure 23 taken around a crack on the surface of the core sample shows deposition of a large number of needle-like crystals (gypsum), smaller than 36 - 40 µm in diameter and 300 - 500 µm in length, covering the mortar surface. In the pores or surface cracks, there are many club-shaped gypsum crystals as shown in Figure 23. The formation of gypsum leads to the cracking, spalling, and decomposition of mortar exposed to sulfuric acid environment. The SEM images obtained by SEM corroborate some of the results discussed above.
Consequently, our results are in agreement with those reported by Rendell et al. [59] which showed that when cement mortar is exposed to sulfuric acid a dense layer of gypsum is formed; this is capable of retarding the deterioration process by acting as a surface sealing layer. Gypsum also exists in pores and fissures in the surface zone, indicating that the attack is due to expansive crystallisation. In ammonium sulfate the surface deposit of gypsum is sparse and the damage to the concrete occurs to a greater depth. The lack of sulfur found in the surface zone indicates that the mechanism of deterioration is due to the dissolution of Ca2+.
3.3.5. DSC and TG/dTG Analyses
The DSC and TG/dTG curves of PET0 composite before and after (NH4)2SO4 solution and H2SO4 acid attack are presented in Figures 24-26.
Figure 24 shows the DSC curves of unmodified mortar PET0 in the tap water. It can be seen that DSC curves for this mortar consist of three zones:
~90˚C - 115˚C: dehydration of pore water (CSH, ettringite)~145˚C - 157˚C: dehydration of calcium sulphate (Gypsum)~450˚C - 500˚C: dehydroxylation of calcium hydroxide, portlandite.
Figure 25 presents the DSC trace of PET0 after ammonium sulphate attack; it displays four endothermic peaks, according to different reactions:
~100˚C - 145˚C: dehydration of pore water~115˚C - 120˚C: ettringite  ~140˚C - 145˚C: the intensity of the peak due to calcium sulphate
~140˚C - 145˚C: the intensity of the peak due to calcium sulphate  is greater. So, gypsum was the dominant reaction product (Equation (8)) while the ettringite appeared as a trace element in the composite.
is greater. So, gypsum was the dominant reaction product (Equation (8)) while the ettringite appeared as a trace element in the composite.
~440˚C - 510˚C: In PET0 composite there was no endothermic peak at 462˚C, which indicates that, with exposure all the Ca(OH)2 that was formed reacted with the ammonium sulphate solution.
Figure 26 presents the TG/dTG trace for the surface part of the sample obtained from the PET0 composite stored in 5% sulphuric acid solution; it displays three endothermic peaks at 157 (High intensity), 210 and 850˚C - 900˚C, indicating gypsum, calcium monocarpboaluminate hydrated and decarbonation of the calcite CaCO3. Also, TG/dTG analysis showed no endothermic peak at 462˚C, which indicates that, with exposure all the Ca(OH)2 that was formed reacted with the sulphuric acid solution (Equation (4)).
Hence, the DSC, TG/dTG curves obtained by DSC and TG/dTG analyses corroborate some of the results discussed above.
It should be pointed out that, in practice, the durability of composites exposed to the chemical solutions investigated in this study should be much better than indicated by the test data given here. First, because the chemical solutions of high concentrations used in the test are not commonly encountered in food and most other industries. Second, the composite specimens in the test were fully submerged, whereas in the industrial practice the structural element such as floor is usually exposed to attack
 (a)
(a) (b)
(b) (c)
(c)
Figure 22. SEM photos of the PET0 composite after exposure to 10% (NH4)2SO4 solution for 860 days; (a) Needlelike ettringite crystals, (b) Club-shaped gypsum crystals and (c) deteriorate mortar.

Figure 23. SEM photos of the PET0 composite after exposure to 5% H2SO4 solution (×600) for 56 days; needle-like crystals (gypsum) in the pore.
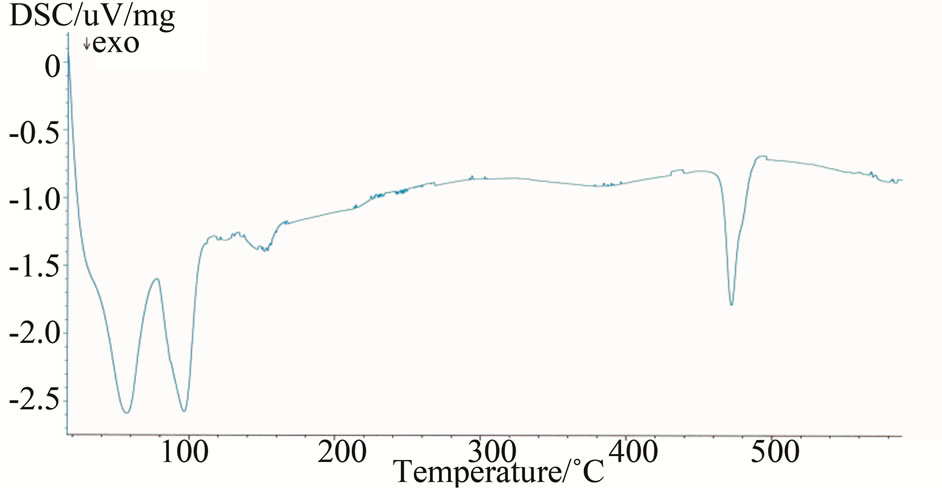
Figure 24. DSC curves at 10 K/min of composite PET0 in tap water exposure.

Figure 25. DSC curves at 10 K/min of composite PET0 in (NH4)2SO4 solution exposure.
from one side only. Third, the immersion solutions were kept in state of constant motion, and were frequently replaced which fresh brushing of the specimens once every week; this removed the reaction products and almost continuously forced new material to come into contact with aggressive solution.
4. Conclusions
The influence of the PET particles on PET-modified

Figure 26. TG/dTG curves at 10 K/min of composite PET0 in H2SO4 acid exposure.
mortar composites sorptivity, mechanical strength and the chemical properties under hydrochloric acid, ammonium-chloride, ammonium-sulfate and sulfuric acid attack solutions were determined. The results of testing the attack by aggressive solutions allow the following conclusions:
1) Test results of the hydraulic transport properties revealed that the addition of PET particles tends to restrict water propagation in the cement matrix and reduces water absorption of the composite. The decrease of the sorptivity-value is favorable to the durability of the specimen structures.
2) The results of specific indicators for ammonium chloride, ammonium-sulfate and acid attacks showed that an increase in the PET content led to better resistance of the composite. This may to the reduced volume of largesized pores and the improved resistance to the absorption of aggressive solutions with the addition of PET in the cement pastes, because of the impervious PET particles blocking the passage of the aggressive ions. In addition, to the reduction in the porosity near particle/matrix interfacial zone, due to the high bonding between PET additive and cement paste.
3) Among composite mixes in the HCl acid attacks, PET7.5 mixes perform better than other mixes. The chemical resistance characteristics of materials are affected by the concentration and by the nature of acids following the order: . Additionally, from the simultaneously traced DTA/TG curves there is a diminution of the calcium hydroxide (CH) content, because of the formation of calcium chloride which has a high solubility in water.
. Additionally, from the simultaneously traced DTA/TG curves there is a diminution of the calcium hydroxide (CH) content, because of the formation of calcium chloride which has a high solubility in water.
4) On the basis of the XRD, FT-IR and DSC analyses of the changes in the phase composition, it can be concluded that the main product of the ammonium-sulfate corrosion of cement is gypsum, accompanied by ettringite. The common factor for all hydrated samples is the presence of portlandite Ca(OH)2 and ettringite 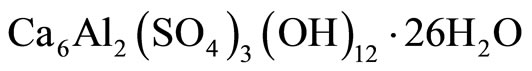 . The samples exposed to the ammonium-sulfate solution show considerable quantities of gypsum CaSO4∙2H2O. The quantity of ettringite formed is small and very similar in all the samples, which points out to the assumption that mostly sulfate ions only from gypsum present in the initial samples participated.
. The samples exposed to the ammonium-sulfate solution show considerable quantities of gypsum CaSO4∙2H2O. The quantity of ettringite formed is small and very similar in all the samples, which points out to the assumption that mostly sulfate ions only from gypsum present in the initial samples participated.
5) The SEM results show that the presence of sulfur ions is virtually existent in the matrix of composites exposed to ammonium sulfate. It is proposed that the reduction in mechanical hardness can be attributed to a migration of Ca2+ from the outer layers of the unmodified mortar.
6) The mechanism of attack in the case of composite exposed to sulfuric acid is principally a surface phenomenon, the expansive action of the gypsum acting to dislocate aggregate and to increase micro cracking at the surface. It is evident from the SEM that there is a filling of pores and micro fissures with gypsum (Tg/dTG). In the absence of erosion this layer has a surface blocking ability.
The utilization of the PET waste particles as a binder instead of cement in the manufacture of PET-mortar composites and as a sustainable building materials in the construction industry help to preserve natural resources and maintain the ecological balance and also to prevent or repair various reinforced concrete structures.
5. Acknowledgements
The authors acknowledge the financial support from the Ministry of Higher Education and Scientific Research of Algeria, under the grants CNEPRU J0405520120005. The authors greatly appreciated the technical support from the Laboratory of LUSAC EA 2607, University of Caen Basse-Normandie, Cherbourg Octeville (French) and also would like to thank Mr M. T. Gouasmi and the graduate students, Mrs. A. Herizi and A. Kerour for their help.
NOTES