1. Introduction
The luminescent materials has been subject of large number of investigations due its wide range of applications, among which we can include fluorescent lamps, flat screens, lasers, and different opto-electronic devices [1-5]. These applications are often based on host materials with a wide band gap activated by rare-earth (RE) ions. Among the host materials the hafnium oxide (HfO2) has attracted much attention, because its wide band gap (Eg = 5.68 eV), high refractive index and low phonon frequencies [6-8], besides this hafnium oxide has a high density (10 g/cm3) and good transparency in the visible range. A considerable number of research on the photoluminescence properties of metal oxides doped with rare-earth ions have been reported [9,10] however, the majority of this investigations has been carry out on films and few studies has been carried out on the photoluminescence properties of nanostructured materials. As we know, the nanostructured materials have different and unique properties which are reported in bulk, since in this sizes range, the electronic states are disturbed by changing its optical properties. In this way, the study of the luminescent properties in relation to the size of the nanostructures is essential, in order to improve the efficiency of the luminescent materials. There are different synthesis methods to obtain nanostructured oxides, which include chemical synthesis, solgel method, among others [11,12]. The solvotermal route is an efficient and inexpensive method. It’s use is necessary in the preparation of those oxides with high supersaturation values that precipitate in nanocrystalline or amorphous structures [13, 14]. Hydrothermal methodology allows to control the size, the morphology, the crystalline phase and the chemical composition of the reaction products; to achieve dispersed and uniform nanoparticles, through the variation of parameters such as temperature, pressure, processing time, concentration and acidity (pH) of the initial solutions. The control in the particle size results from the processes of coarsening and redisolucion-recristalizacion that take place under conditions of high pressure and temperature. One promising approaches to generating white light in solid state devices is the use of a LED (Light Emitting Diode) that emits UV light in the wavelength range λ = 360 - 400 nm in combination with good green and red emitting materials. Many efforts are being made in order to develop new phosphors that emit a more intense component in the red region. Metal oxides are promising phosphor materials because of their wide band gap, low absorbance in the visible region and their chemical and thermal stabilities. Among these oxides, the HfO2:Eu3+ (red emission) offers an excellent possibility for white LED applications, because, in addition to the oxides advantages, it has an intense absorption band at λ = 395 nm, which matches with the emission spectrum of GaN-based LEDs. In this research, the photoluminescence and structural properties of hafnium oxide doped with europium ions (HfO2:Eu3+) as a function of the hydrothermal reaction time, are reported.
2. Experimental Details
The synthesis of HfO2 doped with Eu3+ ions is based on hydrothermal route [15-18]. An aqueous solution of 0.01 M of HfCl4 (alfa Aesar 99%), using deionized water with subsequently addition of EuCl3·6H2O (Sigma Aldrich 99.9%) was prepared. The Eu3+ concentration was varied (0, 1, 3, 5 and 10 atomic percent), having been found that the optimal concentration correspond at 3 atomic percent in relation to hafnium content. NH4OH was added drop by drop to solution, under constant magnetic stirring for 1 hour; the obtained suspension was placed into a teflon vessel inside the stainless steel autoclave (35 ml) and subjected to hydrothermal treatment at 120˚C, under autogenous pressure at reaction times of 24, 40, 52 and 72 hours; after the autoclave was allowed to cool down to room temperature. The resulting precipitate was repeatedly washed with deionized water, using a centrifuge to remove excess of Cl– ions and NH4OH. Finally the precipitate was dried at 80˚C for 24 hours.
The crystalline characteristics of the HfO2 powders doped with Eu3+ ions were analyzed by X ray diffraction (XRD) in a diffractometer D8-Advance-Bruker with wavelength radiation of 1.5406 Å (Cu Ka). The size and morphology of the synthesized nanoparticles were studied in a transmission electron microscope (TEM) JEOL-JEM 1010. Also a high resolution transmission electron microscope (HRTEM) was used to analyze the structure of the powders. The chemical analysis of the elements present in the precipitates was carried out by Energy Dispersive Spectroscopy (EDS) using a LINK ISIS 300 analyzer. Finally, the photoluminescence excitation and emission spectra were obtained using a Flouro Max-P Jobin Yvon Horiba spectrofluorimeter. All photoluminescence spectra were obtained at room temperature.
3. Results and Discussion
Figure 1 shows the XRD diffraction patterns for the samples obtained with hydrothermal treatment of 24, 52 and 72 hours, processed at 120˚C. The diffraction pattern for the sample with 40 hours of hydrothermal treatment is not included because it is similar to those processed at 24 hours.
These diffraction patterns show that at reaction times less than 40 hours the material presents an amorphous structure, however at long reaction time, the patterns showed peaks well defined which present series of Bragg reflections corresponding to the hafnium oxide monoclinic phase (reference JCPDS-ICDD # 34-0104). These diffraction patterns show no significant alterations in the position of the peaks with increasing time of hydrothermal treatment. It can be seen that the maximum half width (FWHM) of the diffraction peaks are narrow, while the intensities are increasing, suggesting an increase in the crystals size and improved crystal quality of the HfO2 particles as a function of the treatment time.
Table 1 shows the lattice parameters and the unit cell volume calculated from XRD measurements for doped (with 3% of Eu3+ ions) and undoped HfO2 powders, synthesized with 72 hours of hydrothermal treatment at temperature of 120˚C. Here it can be seen that the volume of the unit cell of HfO2 doped with Eu3+ is higher than the intrinsic, which suggests that the ions Eu3+ are

Figure 1. X ray diffraction patterns of doped HfO2:Eu3+ powders, as a function of the reaction time.
Table 1. Lattice parameters and unit cell volume of intrinsic and doped HfO2:Eu3+, synthesized at 120˚C during 72 hours of hydrothermal treatment).
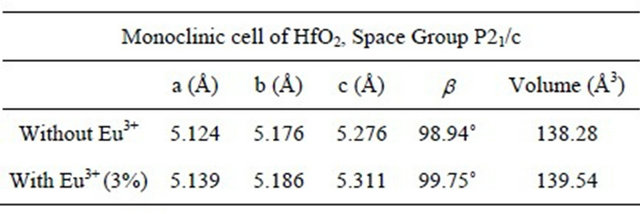
within the network may be replacing to the ions hafnium, generating an increase in the volume, because the ionic radius of Eu3+ (1.01 Å) is larger than the Hf (0.76 Å).
Figure 2 shows the results obtained by Energy Dispersive Spectroscopy (EDS) of the elements present in the synthesized materials.
The oxygen, hafnium and europium quantities are in atomic percent as a function of time of hydrothermal treatment. The graph shows that with increasing reaction time, the composition of the synthesized material is closer to the ideal stoichiometry of hafnium oxide. It is expected that the amorphous phase contains a high concentration of water in the form of OH– groups which are condensed as the crystallization of the material progresses. This could be related to the higher crystallinity obtained by the material with increased time of hydrothermal process. Furthermore, with respect to the amount of dopant, although variations in their amounts, these are small and could say that virtually remain constant, which corresponds to the fact that the amount of Eu3+ was kept constant at 3 atomic percent for all synthesized samples.
Figure 3 shows HRTEM images of HfO2:Eu3+, with a) 24 hours and b) 72 hours of hydrothermal treatment. Figure 3(a) shows that in the material synthesized during 24 hours of hydrothermal treatment small crystalline regions exist, however, the material is essentially amorphous.
The FFT (Fast Fourier Transform) of the image is contained in the upper left corner, showing the characteristic pattern for an amorphous material. On the other hand, Figure 3(b) shows the image of the synthesized material during 72 hours of hydrothermal treatment, which clearly shows the coexistence of well crystallized particles and amorphous material which is in perfect agreement with the X ray diffraction results. With increasing treatment time to 72 hours (Figure 4(a)) the formation of agglomerates of particles with a preferential growth and adopting the prismatic morphology typical of monoclinic crystals with space group P21/c can be observed. The angles formed by the surfaces of the agglomerates coincide in value with the angles between the principal axes of the monoclinic structure (99.18˚ and 80.82˚). Detailed observation at higher magnifications of
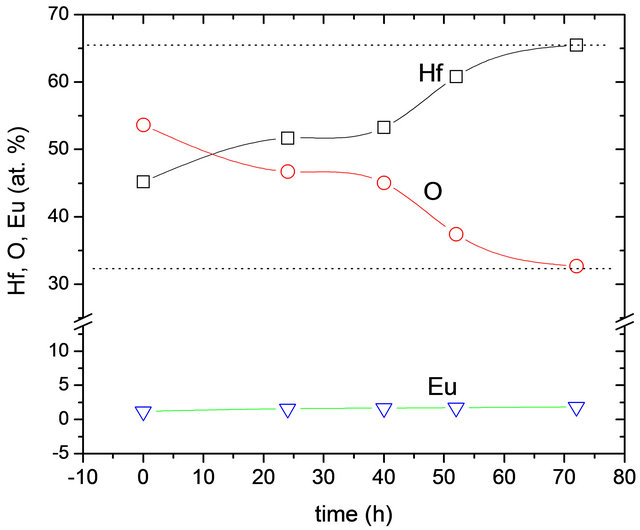
Figure 2. Hafnium (Hf), Oxygen (O) and Europium (Eu) content as a function of the hydrothermal reaction time. The dotted lines correspond with theoretical values expected for HfO2.
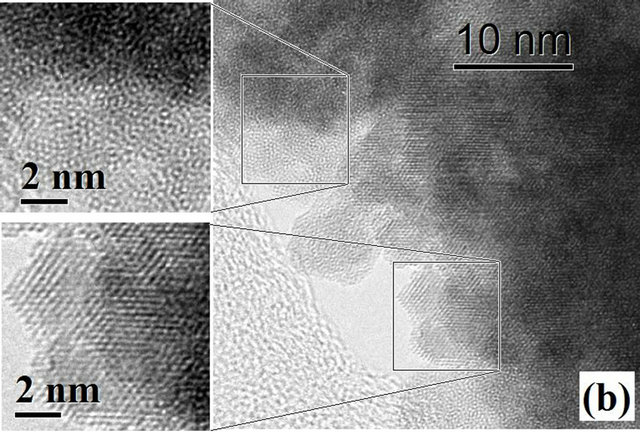
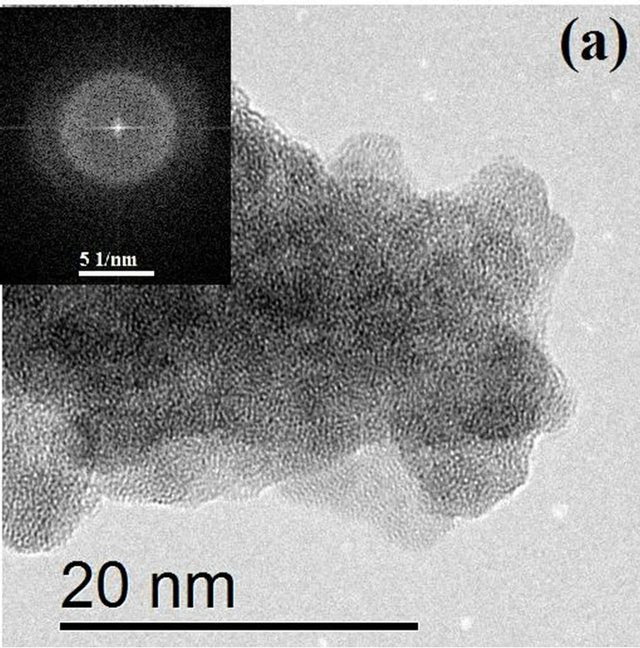
Figure 3. HRTEM images of HfO2:Eu3 + at 120˚C for (a) 24 hours and (b) 72 hours of hydrothermal treatment.

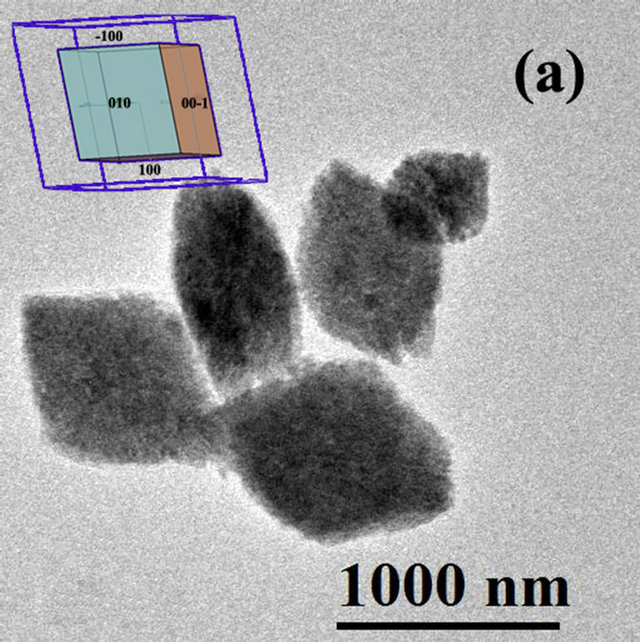
Figure 4. HRTEM Image of HfO2:Eu3 + prepared at 120˚C for 72 hours of hydrothermal treatment at different magnifications, (a) agglomerate particles with prismatic morphology, the insert corresponds to the shape expected for HfO2 with monoclinic structure; (b) edge of an agglomerate showing the binding of various nanoparticles.
these agglomerates as well as the FFT of the image in Figure 4(b) shows a clear preferential orientation in the particles, which explains the tendency of the morphology of the agglomerates. The common zone axis to the observation field (orthogonal to the image) is the direction (0 - 11).
From these results we can see the formation of the system as a gradual process of crystallization within the amorphous phase which involves nucleation and growth followed by preferential agglomeration (oriented attachment). Figure 5 shows the photoluminescence excitation spectrum for HfO2:Eu3+ nanoparticles synthesized at 120˚C during 72 hours. This spectrum was obtained with a fixed emission at λ = 613 nm. In this spectrum is possible to observe seven main bands centred at 265, 300, 320, 364, 384, 395 and 416 nm.
The spectrum shows excitation lines at longer wavelengths assigned as follows: 7F0 → 5D4 (364 nm), 7F0 → 5L7 (384 nm), 7F0 → 5L6 (395nm), 7F0 → 5D3 (416 nm), which corresponds to the absorption of the Eu3+ ions. An efficient energy transfer from the HfO2 host lattice to the Eu3+ activator ions is confirmed by the photoluminescence excitation spectrum. A broad excitation band centered at λ = 265 nm can be observed and could be attrib-

Figure 5. Excitation spectrum HfO2:Eu3 + at 120˚C for different hydrothermal treatment times. The wavelength of the exciting was 395 nm.
uted to band to band transitions of the host lattice. Probably this broad band is also related with 4 f - 5 d charge transfer transitions. This contributes to enhance the characteristic photoluminescence emission intensity of the Eu3+ ions. We choose 395 nm wavelength radiation to excite the powders studied in this investigation. Figure 6 shows the photoluminescence emission spectra of HfO2:Eu3+ nanoparticles with variations of the hydrothermal reaction time, these powders show intense red emissions under long UV excitation; an excitation wavelength of 395 nm was used.
The main characteristics of these spectra are the sharp emission lines originated within the intra-shell transitions from the 5D0,1,2,3, excited levels to the 7FJ ground states of Eu3+. In this case, the emission lines correspond to electronic transitions 5D0 → 7FJ (J = 0 → 4). The observed five peaks centered at 580, 593, 613, 653 and 702 nm are assigned to transitions 5D0 → 7F0, 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F3, and 5D0 → 7F4, respectively. The

Figure 6. Emission intensity PL of HfO2:Eu3 + at 120˚C, with variations of the hydrothermal treatment time.
intense emission peak centered at 613 nm corresponding to the transition 5D0 → 7F2 it is forced by an electric dipole mechanism, which is responsible for the characteristic red emission of Eu3+ ion. The hypersensitive dominant transition 5D0 → 7F2, could be attributed to the fact that Eu3+ ions are located on sites with lack of inversion symmetry. As can be seen, the tendency of the photoluminescence intensity rises with increasing treatment time, reaching a maximum at 72 hours. The photoluminescence emission intensity always rises in accordance with the treatment time increases. A better crystallization of the films as the treatment time rises is probably responsible of this behavior. These effects produce a better distribution and incorporation of the Eu ions into the host crystalline matrix, given as a result an increment in the photoluminescence emission intensity.
4. Conclusion
HfO2 doped with Eu3+ ions was synthesized in its stable monoclinic phase via hydrothermal route from chlorides as raw reagents. Nanocrystals with size lower than 10 nm were observed in the synthesized material at 120˚C during 72 hours of hydrothermal treatment. HfO2:Eu3+ nanocrystals showed a strong red photoluminescence emission that could easily be appreciated at naked eye in normal room light, when excited with a 4 watts UVmercury lamp (254 nm and 366 nm). It was observed that the photoluminescence response is dependent on the reaction time, being obtained the best response at 72 hours of hydrothermal treatment. Also, it was determinate that the best photoluminescence emission intensity was reached for a 3 atomic percent of Eu3+ ions. Based on its photoluminescence emission properties, and photo-excitation characteristics, this oxide is an excellent candidate as the red emitter in white-light solid state lamps. In addition, it was confirmed that hafnium oxide is an adequate host matrix for rare earth ions, as active centers to cause strong photoluminescence emissions.
5. Acknowledgements
The authors thank Dr. Carlos Otero his suggestions and comments and M. en C. Zacarias Rivera García for the technical support provided. They also thank the National Council for Science and Technology in México (CONACyT) and SIP-IPN for the financial support through the programme No. 20120623.