Soluble Interleukin 2 Receptor-Alpha (sIL-2Rα) in the Peripheral Blood of Dogs—Comparison of Malignant Neoplasia with Other Diseases ()
1. Introduction
Mature IL-2 molecule binds to the IL-2 receptor consisting of three subunits (α, β and γ). Both β-(CD 122) and γ-subunit (CD 132) are already present on the cell surface. The α-subunit is expressed when a specific antigen activates the target cell. Only when all three subunits are present on the cell binding affinity of IL-2 is high enough to bind to the receptor [1]. Expression of IL-2-receptor molecules is increased in activated T-cells. Furthermore, a soluble form of the receptor is released from the cell (sIL-2Rα). The soluble form of the IL-2 receptor consists of the α-chain of the membrane-tied receptor.
T-lymphocytes play a central role in the immune system. They maintain cellular immunity, especially against intracellular pathogens (viruses, bacteria, parasites). The binding of IL-2 on the cell surface triggers the main signal for T-lymphocyte activation. For the quantification of lymphocytic activation in humans the soluble IL-2 receptor alpha is used.
So sIL-2Rα became an established marker for the monitoring of graft rejection after organ transplantation, especially regarding kidneys, hearts and livers: increased levels appear in case of graft rejection by the organ receiver [2-5], because those graft versus host reactions go along with T-lymphocyte activation.
In human medicine, sIL-2Rα is also an already established marker for different diseases. In humans sIL-2Rα is known to be expressed mainly by lymphoid cancer cells. A retrospective study from 2008 showed that expression of sIL-2R and its soluble form increased in most haematological malignancies, including different types of leukaemias and lymphomas [6]. Furthermore both lymphoid and non-lymphoid tumor cells express more sIL- 2Rα. They include malignant melanoma, carcinomas of the kidney and different tumors of the head, neck an oesophagus as well as lung cancer [6,7]. There is a strong correlation between increased serum levels of sIL-2Rα and the severity of disease in patients with a pulmonary sarcoidosis [8]. Since this correlation was found sIL-2Rα is used routinely as a marker for the diagnose and progression of sarcoidosis. In 2011 a study was made to find prognostic factors for B-cell lymphoma in humans. Increased serum levels of sIL-2Rα were determined as very important prognostic factors for this disease [9].
Increased sIL-2Rα serum levels were also found in patients with nasopharyngeal carcinomas [10], renal cell carcinomas [11] and autoimmune chronic active hepatitis [12]. One study demonstrated that dysregulated expression of IL-2 receptors in canine lymphoid and haematopoietic malignancies could be used as a model for human cancer [13]. Another study was made on human patients with systemic lupus erythematodes [14] which showed that in SLE patient sIL-2Rα levels in serum and urine were increased.
So far no studies were made regarding stability and storage of sIL-2Rα in canine blood samples. Besides there are neither studies to determine reference ranges for serum levels of sIL-2Rα in healthy dogs.
To find out if the disease goes along with inflammation we measured the number of leukocytes in peripheral blood of the dogs tested on sIL-2Rα serum levels. It was interesting to see if increased serum levels of sIL-2Rα are correlated with leukocytosis in any way. Within the first 6 to 12 hours of inflammatory reaction leukocyte count increases and mature, so-called segmented neutrophil granulocytes are set free by the bone marrow. Bacterial infections, tumors and many other different diseases can lead to inflammation, too.
The aim of this study was to measure serum levels of sIL- 2Rα in dogs and to find evidence if serum levels of sIL- 2Rα in dogs are correlated with leukocytosis to find out if sIL-2Rα was a possible marker for inflammatory diseases. Furthermore we analyzed if serum levels of sIL- 2Rα differ in cases of different non-tumorous and tumorous diseases compared to dogs with malignant tumors. Based on the results of human studies we assumed that sIL-2Rα may be increased in serum of dogs with tumors, especially in those with malignancies.
2. Material and Methods
2.1. Animals
Altogether serum levels of 36 dogs were measured (nonneoplastic diseases and benign neoplasia n = 20, malignnant neoplasia n = 16). The control group consisted of 12 healthy individuals. 22 dogs were bitches (10 with malignant neoplasia) and 14 male dogs (6 with malignant neoplasia). 16 individuals were crossbreed while 20 were breed dogs, most frequent breed were German shepherd (3) and boxer (3). The study was made according to the German animal welfare act.
Serum was isolated and aliquoted four times. The first time point was set to the time right after the isolation. The remaining three aliquots were frozen down immediately at ≤−20˚ Celsius. After 3, 6 and 9 weeks concentrations of sIL2-Rα were measured.
2.2. ELISA
We used an ELISA developed for the quantitative determination of human sIL-2Rα concentrations in serum (Quantikine® ELISA, catalog number DR2A00, R&D Systems, Minnesota/USA). The genetic sequence homology of 77% regarding human and canine sIL-2Rα made it possible to use a human ELISA to measure serum levels of sIL-2Rα in canine blood samples.
ELISA technique is based on monoclonal antibody specific for sIL-2Rα that has been pre-coated onto a microplate. The measurement of sIL-2Rα was according to manufactures recommendation. To determine the optical density of each well a microplate reader set to 450 nm wavelength was used.
The dogs tested for serum levels of sIL-2Rα were patients of the Small Animal Clinic of Georg-August-University in Goettingen/Germany. Depending on their diseases diagnosis was found by general examination, blood and urine tests, X-ray, ultrasound diagnostics, computed tomography, endoscopy, diagnostic laparoscopy and histopathology. In single patients who suffered from different diseases simultaneously the most severe diagnose was mentioned in this study.
2.3. Statistics
The statistical analysis was performed with the SPSSstatistics program (version 21, IBM, NY/USA). For purposes of analysis, dogs with sIL-2Rα values ≤ 0 pg/ml were set at 0 pg/ml. The dogs with diseases were divided into two groups: dogs with non-malignant diseases and dogs with malignancies. Furthermore it was tested if there was a correlation between sIL-2Rα serum levels and the leukocyte count (reference range: 6 - 12 K/μl, all values >12 K/μl are a so called leukocytosis).
Because both concentrations of sIL-2Rα and the number of leukocytes were not normally distributed, nonparametric methods were used for statistical analysis.
To compare the sIL-2Rα concentrations between patients with and without malignancies the Mann-Whitney U test was used. For the comparison of sIL-2Rα in serum and leukocyte count Mann-Whitney U test was used, too. In all cases a p-value < 0.05 was considered statistically significant.
3. Results
3.1. Storage
Our analyses showed that sIL2-Rα in serum can be stored at ≤ −20˚ Celsius for at least nine weeks. Even after that time there was no obvious decrease of sIL2-Rα in the samples.
3.2. sIL-2Rα in Healthy Dogs
Significantly higher serum levels of sIL-2Rα were found in patients suffering from disease compared to those of healthy controls (U-test p < 0.001).
The healthy control group consisted of 12 dogs. 7 dogs showed detectable amounts of sIL-2Rα in serum, but only in low concentration ranges (0.25 - 9 pg/ml).
3.3. sIL-2Rα in Dogs with Different Diseases
sIL-2Rα serum concentrations were measured in 36 dogs with different non-neoplastic diseases and benign neoplasia (20) and malignant neoplasia (16) (Table 1). Three out of the 14 male dogs showed no detectable sIL-2R in serum (21.4%), and so did 4 of the 22 bitches (18.1%).


Table 1. Serum levels of sIL-2Rα in dogs with different malignant and non-malignant diseases (neg = concentration below detection limit). Numbers 1 - 20 different non-neoplastic diseases and benign neoplasia, 21 - 36 malignant neoplasia.
The age of the dogs in the malignancy group lay a little higher than in the non-neoplastic/benign tumor group, in detail between 5 and 13 years in the malignant neoplasiagroup (median 10.5, mean 10.1) and between 3 and 11 years in the non-neoplastic/benign tumor group (median 9.5, mean 7.6).
The levels of 29 dogs with detectable sIL-2Rα concentrations were between 5.4 - 856.0 pg/ml. Median was 58.0 pg/ml in the group of non-neoplastic diseases/benign tumors (total range 5.4 - 865.0 pg/ml) compared to a median of 37.3 pg/ml in the malignant neoplasia group (7.0 - 71.8 pg/ml) (Figures 1-4). The overall mean in the non-malignant group (112.2 pg/ml) lay appreciably over the one in the malignant neoplasia group (39.1 pg/ml) (Figures 3 and 4).
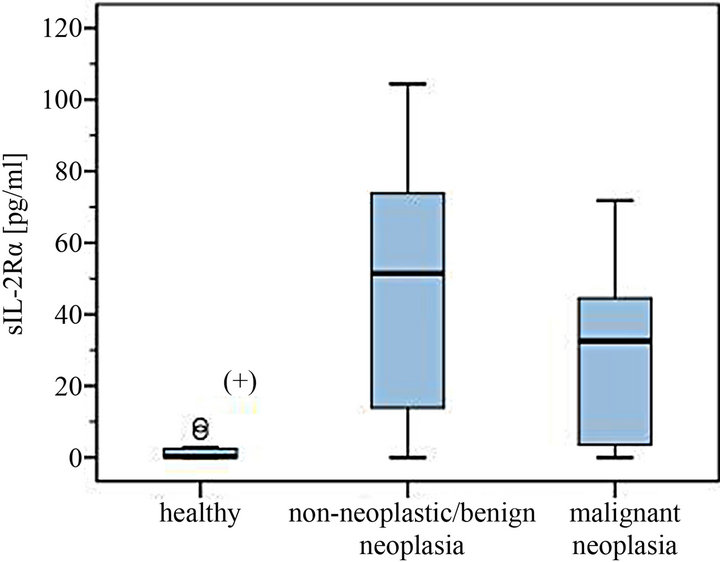
Figure 1. Serum levels of sIL-2Rα in dogs with different non-neoplastic diseases/benign tumors and malignant neoplasia compared to healthy controls. (+) Significantly lower serum levels in the healthy control group.

Figure 2. Serum levels of sIL-2Rα in dogs with different non-neoplastic diseases or benign tumors and malignant neoplasia compared to healthy controls (with statistical outliers in the non-neoplastic/benign tumor group). (+) Significantly lower serum levels in the healthy control group.
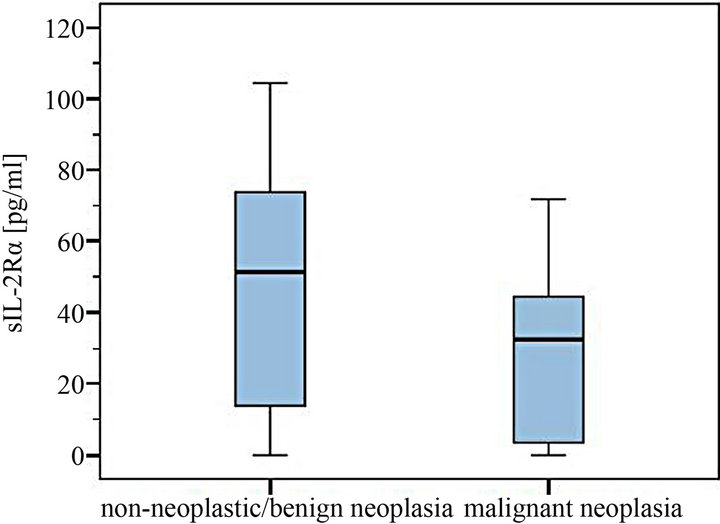
Figure 3. Comparison of sIL-2Rα serum levels in dogs with and without malignant neoplasia.
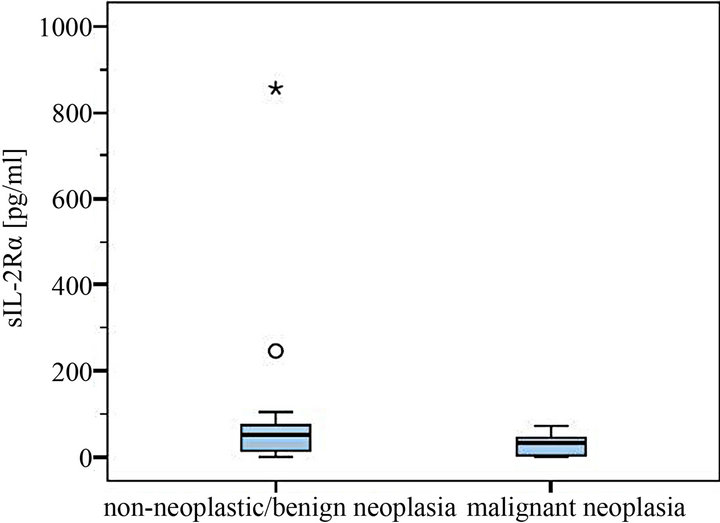
Figure 4. Comparison of sIL-2Rα serum levels in dogs with and without malignant neoplasia (with statistical outliers in the non-neoplastic/benign tumor group).
In the malignant neoplasia group 3 dogs were tested negatively on sIL-2Rα, while in the other group 4 dogs had no detectable amount of sIL-2Rα in serum.
Highest serum levels of sIL-2Rα were all found in the malignant neoplasia group, in detail in a dog with leishmaniasis (856 pg/ml), one with a purulent rhinitis (246 pg/ml) and one with liver cirrhosis (104.4 pg/ml) (Figures 2 and 4). The highest serum level in the malignnant neoplasia group had one dog with a malignant lymphoma. Its serum level of 71.8 pg/ml lay clearly below the highest serum levels measured in the non-neoplastic/benign tumor group.
Serum levels of sIL-2Rα compared to the number of leukocytes in peripheral blood 15 out of the group of 29 dogs tested positively on sIL-2Rα showed a leukocytosis (leukocyte count >12 K/μl) in peripheral blood. Together with the 7 dogs that had no detectable amount of sIL-2Rα in serum (3 out of 7 with a leukocytosis) a correlation was found between sIL-2Rα serum levels and the number of leukocytes (Mann-Whitney-U-test p < 0.001).
When we look at the two different disease groups it is noticeable that in always both groups a correlation was found between serum levels of sIL-2Rα and the leukocyte count (Mann-Whitney-U-test p < 0.05).
Furthermore we tested two different groups of the patients suffering from disease regarding the amount of sIL-2Rα and the number of leukocytes. In the non-malignancy group 17 out of the 20 dogs were tested positively on sIL-2Rα in serum. 9 of them had a leukocytosis in peripheral blood.
In the malignant neoplasia group 12 out of 16 dogs had detectable amounts of sIL-2Rα in blood while 6 of them showed a leukocytosis.
4. Discussion
Many diseases lead to immune activation with increased serum levels of sIL2-Rα. Both non-tumorous and tumorous diseases may go along with immune activation and inflammation. Data of many human studies suggest that depending on the character of the disease and the stage of inflammation sIL2-Rα levels in peripheral blood may increase stronger following tumor growth as they do in any other disease, especially in malignant neoplasia [6, 9,15-17].
As mentioned above so far no studies were made to detect stability of sIL-2Rα in stored canine blood samples, also no studies were made to determine reference ranges of sIL-2Rα in healthy dogs. Results of our study assume that healthy dogs show very low serum levels but in case of inflammatory diseases the amount of measureable sIL-2Rα increases. The absolute amount of detectable sIL-2Rα in canine serum samples is appreciably lower than in most of the human studies. In most of the studies ranges of sIL-2Rα levels in serum in healthy controls were < 2000 pg/ml, based on the techniques and reference ranges of the different laboratories.
Whenever patients suffering from disease showed increased amounts of sIL-2Rα, levels were clearly higher than those of the healthy controls, for example in studies on patients with different tumors, autoimmune diseases and visceral leishmaniosis [6,9,18,19].
Looking at our results there is a remarkable difference between serum levels in dogs with non-neoplastic disease and benign tumors compared to malignant neoplasia. The separation of the dogs into two groups was made according to the disease’s character. In malignant diseases an early diagnosis enhances the chances for a successful therapy and may so lead to a better prognosis. Some retrospective studies showed that cancer is one of the most important natural death causes of dogs. Studies of lifetime risk have suggested a wide range in estimates of deaths due to cancer. Death rates went from 3% of deaths in military working German shepherds [20] to 27% of dogs from a German study [21]. In one study from the UK 16% of deaths were attributed to cancer, twice as many as to heart disease [22].
In our study highest serum levels were found in the group of non-neoplastic diseases and benign tumors that went along with inflammation. The absolute amounts of the patients with higher serum levels lay appreciably over those measured in the dogs with malignant neoplasia.
It is an interesting fact that sIL2-Rα is an established marker in human medicine for diagnosis and progression of many tumorous diseases [6,8,9,23], but does not have huge relevance as a marker for inflammation in non-tumorous diseases. There are two exceptions: rheumatoid arthritis [24] and human visceral leishmaniasis [18]. In human leishmaniasis increased serum levels of sIL-2Rα were correlated with severity of disease and returned to the normal range during successful chemotherapy. In our study absolute highest amount of detectable sIL-2Rα (856.0 pg/ml) and also the fourth-highest amount (104.25 pg/ml) were also found in dogs with leishmaniasis. Both serum levels were higher than those in the malignancy group. Leishmaniasis is rare in dogs in Germany, so it could be interesting to make further studies on dogs in countries where leishmaniasis appears to be a bigger problem.
Three dogs with diabetes mellitus were tested positively on sIL-2Rα with similar serum levels between 22.2 and 45.0 pg/ml. Actually the character of type 1 and type 2 diabetes mellitus as chronic inflammatory diseases with T-cell-mediated immune activation is discussed [25]. Type 1 diabetes mellitus is the main type of diabetes in dogs. It goes along with a loss of insulin-producing β-cells of the pancreas. Early stages of the disease are based on the secretion of high levels of pro-inflammatory cytokines. Those effects trigger the fatal destruction process of β- cells. There are approaches of anti-inflammatory interventions in treating type 1 diabetes mellitus in humans [26]. The three dogs with diabetes mellitus in our study were tested positively on sIL-2Rα with similar serum levels (22.2 - 45.0 pg/ml). One of the dogs (45.0 pg/ml) showed clinical signs of acute renal failure and another one was suspected to have a pancreatitis (29.8 pg/ml). These data suggest that in dogs before receiving therapy to acute exacerbation of diabetes reactions of the immune system lead to increased sIL-2Rα. We can’t be certain that only diabetes leads to increased sIL-2Rα because two dogs also had other clinical signs following final stages of diabetes that could have the same effect, too.
Two of the dogs diagnosed with chronic renal failure showed similar serum levels of 58.0 and 63.8 pg/ml, while one of them had no detectable sIL-2Rα in serum. All three dogs had severe clinical symptoms and were euthanized within a couple of days after beginning of their treatment. In human studies it was shown that chronic renal failure is a major cause for T-lymphocyte activation, which leads to increased expression of sIL-2Rα [27,28]. Serum levels of sIL-2Rα were significantly higher in patients with chronic renal failure compared to healthy controls [29]. Because early diagnosis and proper treatment of chronic renal failure can lead to a longer survival time maybe sIL-2Rα could become a prognostic marker together with the already established diagnostic techniques.
Two of the dogs in the malignant neoplasia group were diagnosed with malignant lymphoma. In contrast to human medicine, where sIL-2Rα is an already established marker for malignant lymphoma [9,16,23], we could not find evidence that sIL-2Rα is a useful marker for detecting malignant lymphoma in dogs. Although there were only two dogs with malignant lymphoma it is noticeable that only one of the dogs showed detectable sIL-2Rα in serum: it was the highest serum level of the dogs in the malignant neoplasia group (71.8 pg/ml) which lay remarkably lower than the highest serum levels in the nonneoplastic/benign tumor group. But in addition it is an interesting fact that the malignant lymphoma patient with the reduced general condition and severe clinical symptoms was the one that had no detectable sIL-2Rα in serum.
It is interesting that all three German shepherds belonged to the malignant neoplasia group and showed highly malignant tumors with poor prognosis (renal adenocarcinoma, haemangiosarcoma of the spleen, malignnant prostate tumor with liver metastases). Serum levels of sIL-2Rα were ranged in relatively low amounts (10.2 - 30.0 pg/ml) compared to both the rest of the same group and the non-neoplastic/benign tumor group.
To find out if the disease goes along with inflammation we measured the number of leukocytes in peripheral blood of the dogs tested on sIL-2Rα serum levels. It was interesting to see if increased serum levels of sIL-2Rα are correlated with leukocytosis in any way. Within the first 6 to 12 hours of inflammatory reaction leukocyte count increases and mature, so-called segmented neutrophil granulocytes are set free by the bone marrow. Bacterial infections, tumors and many other different diseases can lead to inflammation, too. The number of leukocytes in peripheral blood is an already established marker for inflammation both in human and veterinary medicine since decades. Beside new markers for the grade of inflammation like C-reactive protein it was interesting to measure serum levels of sIL-2Rα to see if there was a correlation between those levels and the number of leukocytes.
Our data suggest that in dogs sIL2-Rα is increased in acute inflammation whether in non-neoplastic and benign disease or in malignant neoplasia. Results show a tendency that sIL-2Rα in dogs seems to be increased rather in non-neoplastic and benign diseases going along with inflammation.
In our study no evidence was found that malignant tumors automatically lead to significantly increased sIL- 2Rα serum levels. This result noticeably differs from many of the human studies. Furthermore the results of our study show that there is a correlation between increased levels of sIL-2Rα and the number of leukocytes in peripheral blood, and this correlation obviously does not depend on the character of the disease: correlation was found in both groups, whether non-neoplastic/benign tumor or malignant neoplasia.
The results of this study assume that sIL-2Rα serum levels could be used as a pro-inflammatory diagnostic marker associated with different diseases. High serum levels of sIL-2Rα in dogs seem to be related rather to non-neoplastic disease or benign tumors than to malignant neoplasia, though immune response in many malignant diseases also leads to increased sIL-2Rα serum levels. Although there was no statistical significance found, there is an obvious tendency that sIL-2Rα could be a helpful marker for the differentiation between non-neoplastic disease/benign tumor and malignant neoplasia. Further studies on a larger group of patients should follow to approve these results. Maybe another correlation can be found, for example between high sIL-2Rα serum levels and an increased concentration of pro-inflammatory markers like C-reactive protein (CRP) in blood, which was not part of this study.
As a result sIL-2Rα could become a useful diagnostic parameter for the detection of the moment and the degree of inflammatory response in the body.
Altogether measured amounts of sIL-2Rα even in dogs suffering from the disease were noticeably lower than in human patients. Possibly a specific ELISA for the detection of canine sIL-2Rα could lead to better results regarding the quantity of measurable serum levels. Though the sequence homology of 77% between human and canine sIL-2Rα made it possible to use a human ELISA for detecting canine sIL-Rα in serum samples, the absolute amounts of sIL-2Rα could have been even higher than our results suggest.
Further studies should approve that sIL-2Rα serum levels in dogs are significantly higher in diseases not caused by malignant neoplasia. sIL-2Rα measurements in serum could become a marker for the differentiation if inflammation is caused by a malignant tumor or not. Interestingly, this would stay in contrast to many studies implemented in human medicine. In addition to that positive results of studies on a larger group of patients may give a useful hint if sIL-2Rα can be used for the differentiation if inflammation is caused by a tumor or not, especially because both diagnosing and treating tumorous diseases becomes more and more important in small animal medicine.
NOTES