Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2010, 3, 735-741 JBiSE doi:10.4236/jbise.2010.37098 Published Online July 2010 (http://www.SciRP.org/journal/jbise/). Published Online July 2010 in SciRes. http://www.scirp.org/journal/jbise Removal of hexavalent chromium by an aromatic alcohol Ankita Basu, Rumpa Saha, Jayashree Mandal, Sumanta Ghosh, Bidyut Saha* Bioremediation Lab, Department of Chemistry, the University of Burdwan, Golapbag, India; Email: b_saha31@rediffmail.com Received 17 May 2010; revised 21 May 2010; accepted 28 May 2010. ABSTRACT Cr(VI) is a widespread environmental contaminant and a known human carcinogen. Biosorption is a very common method to remove toxic Cr(VI) from industrial waste water. In biosorption Cr(VI) is re- duced to less toxic Cr(III) and adsorbed in biosor- bent as Cr(III). Effective biosorbents contain hydro- xy groups; it may be aliphatic or aromatic. Kinetics of reduction of Cr(VI) by an aromatic alcohol, benzyl alcohol, (which is an important volatile component of flowers of some night blooming plants) in micellar media have been studied spectrophotometrically. Micellar media is a probe to establish the mechanistic paths of reduction of Cr(VI) to Cr(III). Effects of electrolytes are studied to support the proposed reac- tion mechanism. Suitable surfactant & suitable con- centration of electrolyte enhance the biosorption pro- perty. Keywords: Biosorption; Carcinogen; Kinetics; Chro- mium (VI); Benzyl Alcohol; Non Functional Surfactants; Salt Effect 1. INTRODUCTION Chromate [Cr(VI)] compounds are widely used in in- dustry. Large amounts of toxic Cr(VI) are annually re- introduced into the environment through the discharge of chromium-containing industrial waste [1-4]. In the last few decades, the amount of chromium in aquatic and terrestrial eco-systems has increased a consequence of different human activities. Chromium is the new en- try, after lead, cadmium and mercury in the major toxic metal series. In the Hinkley (a small desert town in San Bernardino Country, USA) case hexavalent chromium was used by Pacific Gas and Electric Company (PG & E) in cooling systems to prevent pipes from rusting. The runoff of hexavalent chromium contaminated water on the PG & E property, seeped into the ground and con- taminated local water supplies. PG & E was required to compensate the plaintiffs $ 333 million, clean up the hexavalent chromium contamination, and stop using he- xavalent chromium in their operation this is the highest amount of compensation in metal toxicity history. Vari- ous methods used for removal of Cr (VI) ions include chemical reduction and precipitation, reverse osmosis, ion exchange and adsorption on activated carbon or similar material [5]. But all these methods suffer from severe constraints, such as incomplete metal removal, high reagent or energy requirements, generation of toxic sludge or other waste products that require safe disposal. Some of the treatment methods involve high operating and maintenance cost. The high cost of the chemical reagents and the problems of secondary pollution also make the above physico-chemical methods rather lim- ited in application. There is, therefore, a need for some alternative technique, which is efficient and cost-effec- tive. The process of heavy metal removal by biological mate- rials is known as biosorption and the biological materials used are called biosorbents. Various biosor- bents like bacteria, fungi, yeasts, agricultural by prod- ucts, industrial wastes, etc have been used for biosorp- tion. In this regard, considerable attention has been fo- cused in recent years upon the field of biosorption for the removal of heavy metal ions from aqueous solutions [6]. Recently it is established that for chromium (VI) biosorption, chromium (VI) is first reduced to chro- mium (III) and then it is adsorbed as chromium (III) in the biosorbent [7]. Understanding of mechanism of chromium (VI) reduction to chromium (III) by some alcohol is important in this context. In this respect ben- zyl alcohol is ideal one. Benzyl alcohol is a volatile component of flower of a night blooming plant Gaura drummondii [8] and strawberry leaves [9]. The present investigations have been carried out in micro-hetero- geneous systems to substantiate the proposed reaction mechanism as we carried out for other systems [10-17]. Effects of electrolytes are studied to support the pro- posed reaction mechanism. 2. THEORETICAL It is expected that the reduction of Cr(VI) by benzyl al-  A. Basu et al. / J. Biomedical Science and Engineering 3 (2010) 735-741 Copyright © 2010 SciRes. JBiSE 736 cohol in aqueous surfactant media is proceed through normal oxidation mechanism by chromate ester. Benzyl alcohol is oxidized to benzaldehyde and Cr(VI) is re- duced to Cr(IV). The over-all reaction can be written as: 3 PhCH2OH + 2HCrO4- + 8H+ → 3 PhCHO + 2Cr(III) + 8 H2O. 3. EXPERIMENTAL 3.1. Table for Materials and Reagents The materials and reagents are shown in Table 1. 3.2. Procedure and Kinetic Measurements Under the kinetic conditions, solutions of the oxidant and mixtures containing the known quantities of the sub- strate(s) (i.e, benzyl alcohol) (under the conditions [S]T >> [Cr(VI)]T), acid and the other necessary chemi- cals were separately thermostated (±0.1℃). The reaction was initiated by mixing the requisite amounts of the oxidant with the reaction mixture. It is assumed that zero time was taken when half of the required volume of the oxi- dant solution had been added. The progress of the reac- tion was followed by monitoring the decay of oxidant [Cr(VI)] at 415 nm at different time intervals (2 minutes) with a UV-VIS spectrophotometer [UV-2450 (SHIMA- DZU)]. Quartz cuvettes of path length 1cm were used. The observed pseudo-first-order rate con- stants [kobs(s-1)] were determined from the linear part of the plots of ln(A415) versus time(t). Reproducible results giving first-order plots (co-relation co-efficient, R2 ≥ 0.998) were obtained for each reaction run. A large ex- cess (≥ 15-fold) of reductant was used in all kinetic runs. No interference due to other species at 415 nm was veri- fied. Under the experimental conditions, the possibility of decomposition of the surfactants by Cr(VI) was inves- Table 1. Materials and reagents. Materials Brand 1. Benzyl alcohol AR, Merck, India 2. K2Cr2O7 AR, BDH, India 3. N-cetylpyridinium chloride (CPC)AR, SRL, India 4. Sodium dodecylsulphate (SDS) AR, SRL, India 5. TX-100 AR, SRL, India 6. NaCl AR, Merck, India 7. NH4Cl AR, Ranbaxy, India tigated and the rate of decomposition in this path was found to be kinetically negligible. 3.3. Product Analysis and Stoichiometry Under the kinetic condition benzyl alcohol is oxidized to benzaldehyde and estimation of the reaction products was carried by gravimetrically as 2, 4-dinitrophenyl hy- drazone [18]. In a typical experimental set, 10ml of 0.06 mol dm-3 Cr(VI) in 1.0 mol dm-3 H2SO4 was added to 40 ml of 0.2 mol dm-3 benzyl alcohol and the reaction was allowed to proceed to completion. Then the reaction mixture was added slowly with stirring to 60 ml of a saturated solution of 2,4-dinitrophenyl hydrazine in 2.0 mol dm-3 HCl. After storing for about 1hr in an ice-bath, the precipitate was collected weighed sintered glass cru- cible, washed with 2.0mol dm-3 HCl followed by water and dried to a constant weight at 100-105℃. The found ratio, [Cr(VI)]T/[Carbonyl compound]T ≈ 2/3 (from 3 independent determinations) supports the fol- lowing Stoichiometry. 3PhCH2OH + 2HCrO4- + 8H+→ 3 PhCHO + 2Cr(III) + 8 H2O. (1) 4. RESULTS AND DISCUSSION 4.1. Dependence on [Substrate]T i.e, [Benzyl Alcohol]T From the plot of kobs vs [benzyl alcohol]T, it is estab- lished that the path shows a first order dependency on [substrate]T i.e, [benzyl alcohol]T i.e., with increasing substrate concentration the rate of the reaction increases in a straight line manner. (Figure 1). So, kobs = ks[S]T The above first order dependence on [S]T also main- tained in the presence of surfactant like CPC, SDS, TX-100. 4.2. Dependence on [H+] The acid dependence was followed in aqueous HClO4 media at fixed Cr(VI) and [S]T. From the experimental fit (Figure 2), the observation is kobs = kH[H+]2 The above second order dependency is also main- tained in the presence of surfactant (e.g, SDS). 5. EFFECT OF SURFACTANTS 5.1. Effect of SDS Sodium dodecyl sulphate(SDS, a representative anionic surfactant) accelerate the reaction path. The plot of kobs vs [SDS]T [Figure 3] shows a continuous increase up to the concentration of SDS. 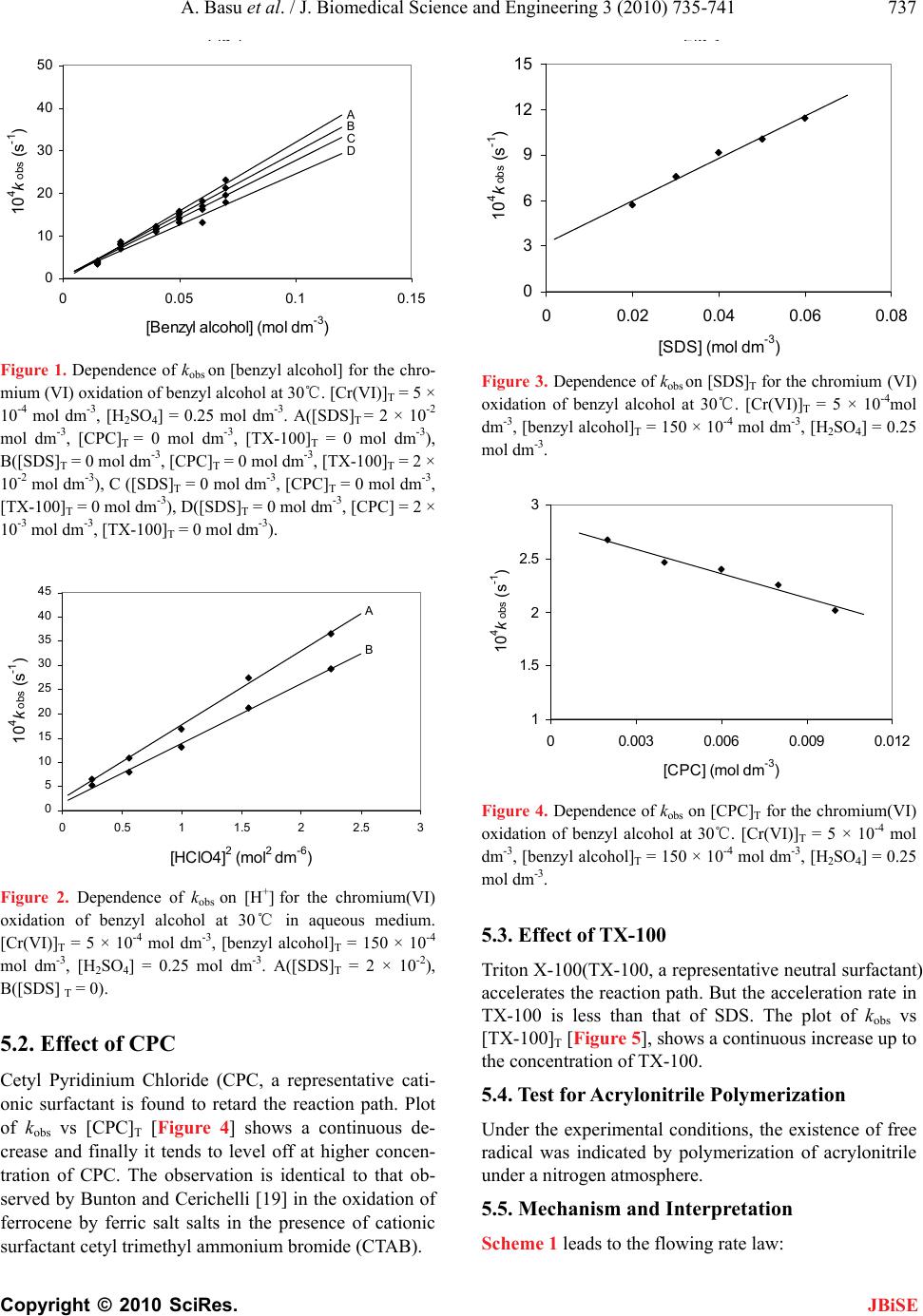 A. Basu et al. / J. Biomedical Science and Engineering 3 (2010) 735-741 Copyright © 2010 SciRes. JBiSE 737 Fig 1 0 10 20 30 40 50 00.05 0.1 0.15 [Benzyl alcohol] (mol dm -3 ) 10 4 k obs (s -1 ) A C D B Figure 1. Dependence of kobs on [benzyl alcohol] for the chro- mium (VI) oxidation of benzyl alcohol at 30℃. [Cr(VI)]T = 5 × 10-4 mol dm-3, [H2SO4] = 0.25 mol dm-3. A([SDS]T = 2 × 10-2 mol dm-3, [CPC]T = 0 mol dm-3, [TX-100]T = 0 mol dm-3), B([SDS]T = 0 mol dm-3, [CPC]T = 0 mol dm-3, [TX-100]T = 2 × 10-2 mol dm-3), C ([SDS]T = 0 mol dm-3, [CPC]T = 0 mol dm-3, [TX-100]T = 0 mol dm-3), D([SDS]T = 0 mol dm-3, [CPC] = 2 × 10-3 mol dm-3, [TX-100]T = 0 mol dm-3). 0 5 10 15 20 25 30 35 40 45 00.511.522.53 [HClO4 ] 2 (mol 2 dm -6 ) 10 4 k obs (s -1 ) A B Figure 2. Dependence of kobs on [H+] for the chromium(VI) oxidation of benzyl alcohol at 30℃ in aqueous medium. [Cr(VI)]T = 5 × 10-4 mol dm-3, [benzyl alcohol]T = 150 × 10-4 mol dm-3, [H2SO4] = 0.25 mol dm-3. A([SDS]T = 2 × 10-2), B([SDS] T = 0). 5.2. Effect of CPC Cetyl Pyridinium Chloride (CPC, a representative cati- onic surfactant is found to retard the reaction path. Plot of kobs vs [CPC]T [Figure 4] shows a continuous de- crease and finally it tends to level off at higher concen- tration of CPC. The observation is identical to that ob- served by Bunton and Cerichelli [19] in the oxidation of ferrocene by ferric salt salts in the presence of cationic surfactant cetyl trimethyl ammonium bromide (CTAB). Fig 3 0 3 6 9 12 15 0 0.020.040.060.08 [SDS] (mol dm -3 ) 10 4 k obs (s -1 ) Figure 3. Dependence of kobs on [SDS]T for the chromium (VI) oxidation of benzyl alcohol at 30℃. [Cr(VI)]T = 5 × 10-4mol dm-3, [benzyl alcohol]T = 150 × 10-4 mol dm-3, [H2SO4] = 0.25 mol dm-3. 1 1.5 2 2.5 3 00.003 0.006 0.009 0.012 [CPC] (mol dm -3 ) 10 4 k obs (s -1 ) Figure 4. Dependence of kobs on [CPC]T for the chromium(VI) oxidation of benzyl alcohol at 30℃. [Cr(VI)]T = 5 × 10-4 mol dm-3, [benzyl alcohol]T = 150 × 10-4 mol dm-3, [H2SO4] = 0.25 mol dm-3. 5.3. Effect of TX-100 Triton X-100(TX-100, a representative neutral surfactant) accelerates the reaction path. But the acceleration rate in TX-100 is less than that of SDS. The plot of kobs vs [TX-100]T [Figure 5], shows a continuous increase up to the concentration of TX-100. 5.4. Test for Acrylonitrile Polymerization Under the experimental conditions, the existence of free radical was indicated by polymerization of acrylonitrile under a nitrogen atmosphere. 5.5. Mechanism and Interpretation Scheme 1 leads to the flowing rate law: 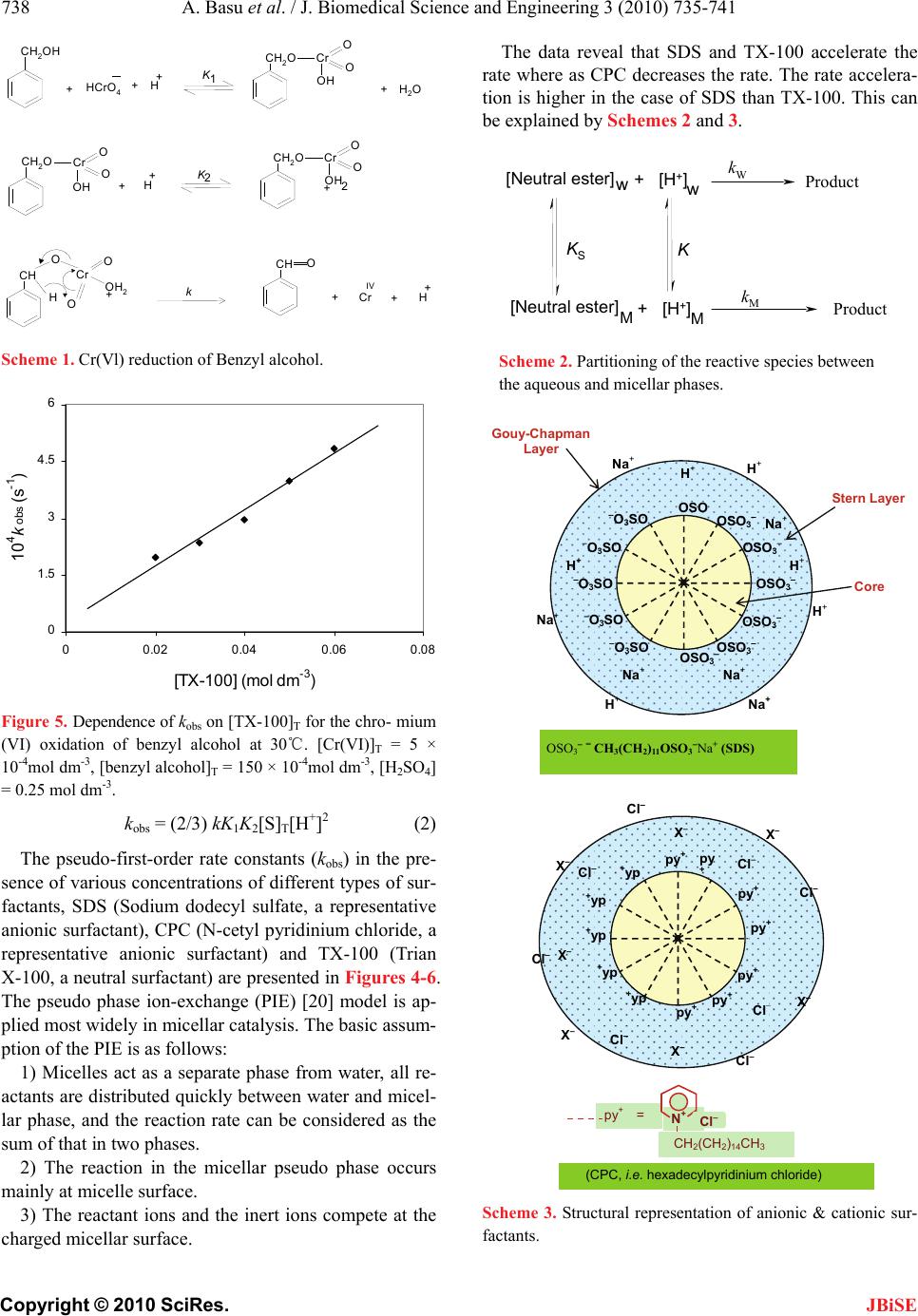 A. Basu et al. / J. Biomedical Science and Engineering 3 (2010) 735-741 Copyright © 2010 SciRes. JBiSE 738 CH2OH +HCrO4+H +K1 CH2O O Cr O OH CH2OO Cr O OH +H +K2 CH2O O Cr O OH2 CH O Cr O O OH2 Hk CH O +Cr IV +H + H2O + + + Scheme 1. Cr(Vl) reduction of Benzyl alcohol. 0 1.5 3 4.5 6 00.02 0.040.06 0.08 [TX-100] (mol dm-3) 104kobs (s-1) Figure 5. Dependence of kobs on [TX-100]T for the chro- mium (VI) oxidation of benzyl alcohol at 30℃. [Cr(VI)]T = 5 × 10-4mol dm-3, [benzyl alcohol]T = 150 × 10-4mol dm-3, [H2SO4] = 0.25 mol dm-3. kobs = (2/3) kK1K2[S]T[H+]2 (2) The pseudo-first-order rate constants (kobs) in the pre- sence of various concentrations of different types of sur- factants, SDS (Sodium dodecyl sulfate, a representative anionic surfactant), CPC (N-cetyl pyridinium chloride, a representative anionic surfactant) and TX-100 (Trian X-100, a neutral surfactant) are presented in Figures 4-6. The pseudo phase ion-exchange (PIE) [20] model is ap- plied most widely in micellar catalysis. The basic assum- ption of the PIE is as follows: 1) Micelles act as a separate phase from water, all re- actants are distributed quickly between water and micel- lar phase, and the reaction rate can be considered as the sum of that in two phases. 2) The reaction in the micellar pseudo phase occurs mainly at micelle surface. 3) The reactant ions and the inert ions compete at the charged micellar surface. The data reveal that SDS and TX-100 accelerate the rate where as CPC decreases the rate. The rate accelera- tion is higher in the case of SDS than TX-100. This can be explained by Schemes 2 and 3. kWProduct kMProduct [Neutral ester]+ [H+] [Neutral ester]+ [H+] K KS ww M M Scheme 2. Partitioning of the reactive species between the aqueous and micellar phases. OSO 3 O 3 SO OSO OSO 3 O 3 SO O 3 SO O 3 SO OSO 3 OSO 3 OSO 3 OSO 3 H + H + Na + Na + Na + H + Stern Laye r Gou y -Chapman Layer H + O 3 SONa + H + Core Na + Na + H + OSO 3 = CH 3 (CH 2 ) 11 OSO 3 Na + (SDS) py + Cl Cl Cl Cl py + py + py + + yp py + py + + yp + yp py + + yp + yp Cl Cl X Cl Cl X X X X X X N + CH 2 (CH 2 ) 14 CH 3 py + = Cl (CPC, i.e. hexadecylpyridinium chloride) Scheme 3. Structural representation of anionic & cationic sur- factants. 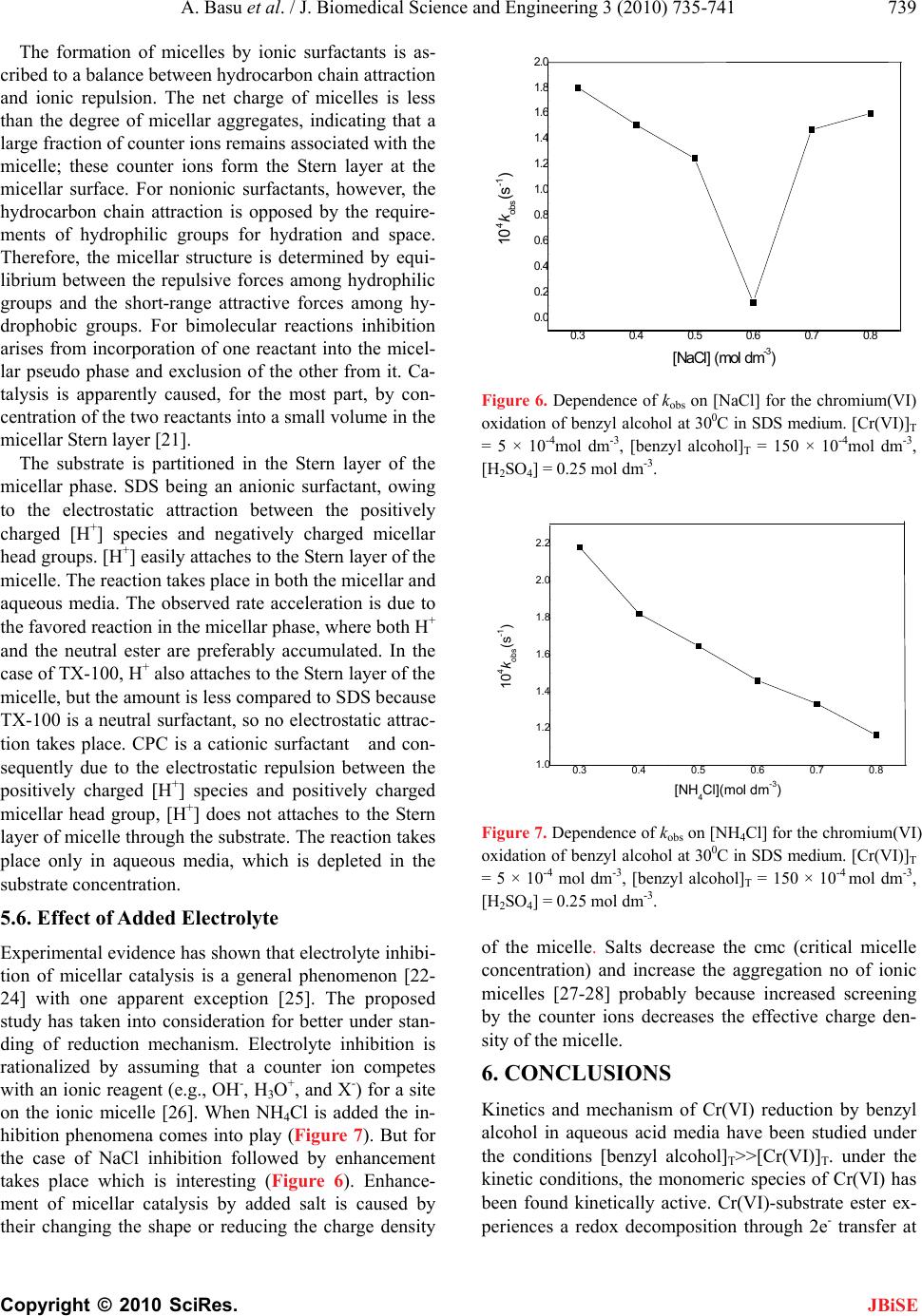 A. Basu et al. / J. Biomedical Science and Engineering 3 (2010) 735-741 Copyright © 2010 SciRes. JBiSE 739 The formation of micelles by ionic surfactants is as- cribed to a balance between hydrocarbon chain attraction and ionic repulsion. The net charge of micelles is less than the degree of micellar aggregates, indicating that a large fraction of counter ions remains associated with the micelle; these counter ions form the Stern layer at the micellar surface. For nonionic surfactants, however, the hydrocarbon chain attraction is opposed by the require- ments of hydrophilic groups for hydration and space. Therefore, the micellar structure is determined by equi- librium between the repulsive forces among hydrophilic groups and the short-range attractive forces among hy- drophobic groups. For bimolecular reactions inhibition arises from incorporation of one reactant into the micel- lar pseudo phase and exclusion of the other from it. Ca- talysis is apparently caused, for the most part, by con- centration of the two reactants into a small volume in the micellar Stern layer [21]. The substrate is partitioned in the Stern layer of the micellar phase. SDS being an anionic surfactant, owing to the electrostatic attraction between the positively charged [H+] species and negatively charged micellar head groups. [H+] easily attaches to the Stern layer of the micelle. The reaction takes place in both the micellar and aqueous media. The observed rate acceleration is due to the favored reaction in the micellar phase, where both H+ and the neutral ester are preferably accumulated. In the case of TX-100, H+ also attaches to the Stern layer of the micelle, but the amount is less compared to SDS because TX-100 is a neutral surfactant, so no electrostatic attrac- tion takes place. CPC is a cationic surfactant and con- sequently due to the electrostatic repulsion between the positively charged [H+] species and positively charged micellar head group, [H+] does not attaches to the Stern layer of micelle through the substrate. The reaction takes place only in aqueous media, which is depleted in the substrate concentration. 5.6. Effect of Added Electrolyte Experimental evidence has shown that electrolyte inhibi- tion of micellar catalysis is a general phenomenon [22- 24] with one apparent exception [25]. The proposed study has taken into consideration for better under stan- ding of reduction mechanism. Electrolyte inhibition is rationalized by assuming that a counter ion competes with an ionic reagent (e.g., OH-, H3O+, and X-) for a site on the ionic micelle [26]. When NH4Cl is added the in- hibition phenomena comes into play (Figure 7). But for the case of NaCl inhibition followed by enhancement takes place which is interesting (Figure 6). Enhance- ment of micellar catalysis by added salt is caused by their changing the shape or reducing the charge density 0.3 0.4 0.5 0.6 0.7 0.8 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0 Fig 6 10 4kobs (s-1) [NaCl] (mol dm-3) Figure 6. Dependence of kobs on [NaCl] for the chromium(VI) oxidation of benzyl alcohol at 300C in SDS medium. [Cr(VI)]T = 5 × 10-4mol dm-3, [benzyl alcohol]T = 150 × 10-4mol dm-3, [H2SO4] = 0.25 mol dm-3. 0.3 0.4 0.5 0.6 0.7 0.8 1.0 1.2 1.4 1.6 1.8 2.0 2.2 Fig 7 104kobs(s-1) [NH4Cl](mol dm-3) Figure 7. Dependence of kobs on [NH4Cl] for the chromium(VI) oxidation of benzyl alcohol at 300C in SDS medium. [Cr(VI)]T = 5 × 10-4 mol dm-3, [benzyl alcohol]T = 150 × 10-4 mol dm-3, [H2SO4] = 0.25 mol dm-3. of the micelle. Salts decrease the cmc (critical micelle concentration) and increase the aggregation no of ionic micelles [27-28] probably because increased screening by the counter ions decreases the effective charge den- sity of the micelle. 6. CONCLUSIONS Kinetics and mechanism of Cr(VI) reduction by benzyl alcohol in aqueous acid media have been studied under the conditions [benzyl alcohol]T>>[Cr(V I)]T. under the kinetic conditions, the monomeric species of Cr(VI) has been found kinetically active. Cr(VI)-substrate ester ex- periences a redox decomposition through 2e- transfer at 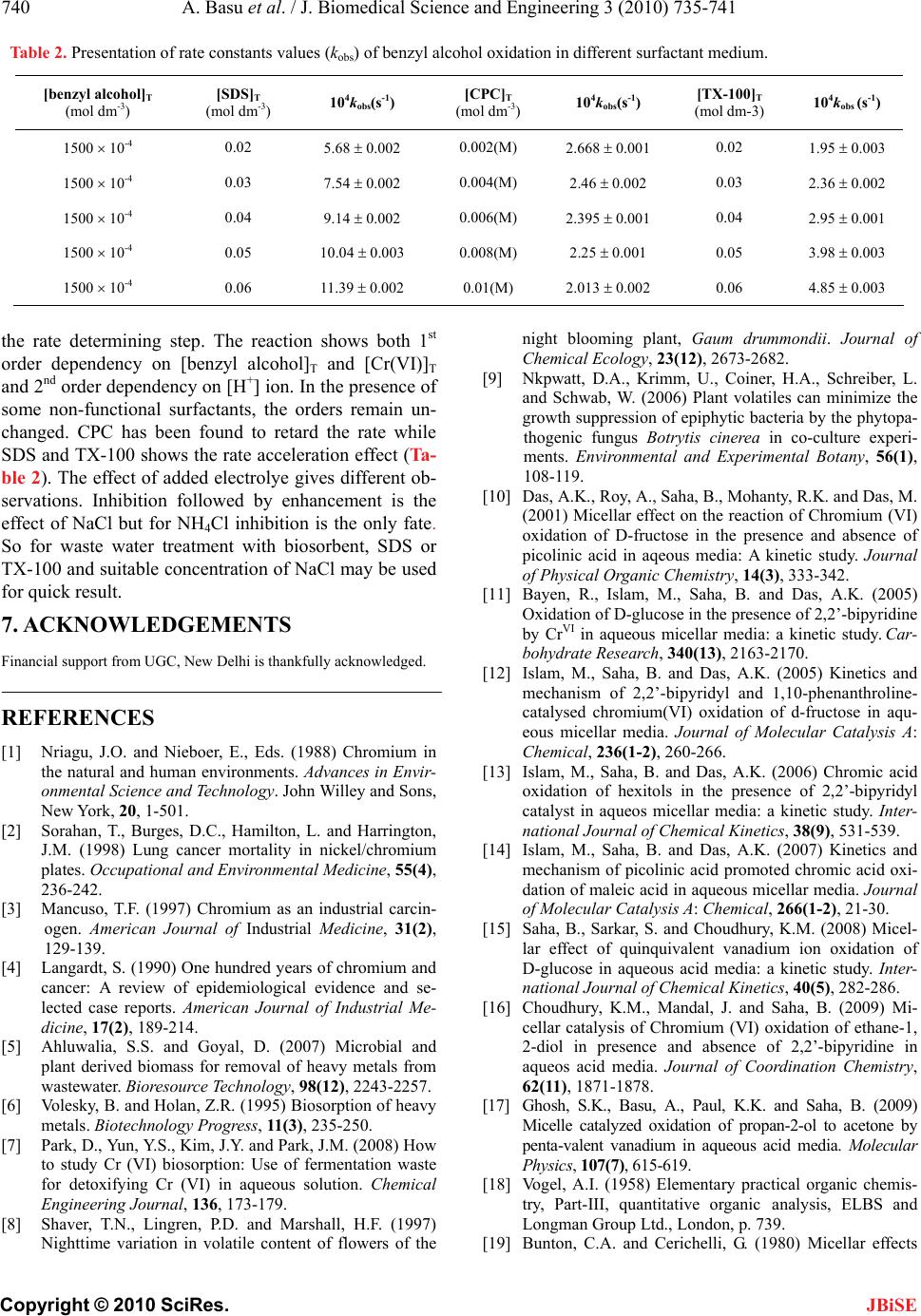 A. Basu et al. / J. Biomedical Science and Engineering 3 (2010) 735-741 Copyright © 2010 SciRes. JBiSE 740 Table 2. Presentation of rate constants values (kobs) of benzyl alcohol oxidation in different surfactant medium. [benzyl alcohol]T (mol dm-3) [SDS]T (mol dm-3) 104kobs(s-1) [CPC]T (mol dm-3) 104kobs(s-1) [TX-100]T (mol dm-3) 104kobs (s-1) 1500 10-4 0.02 5.68 0.002 0.002(M) 2.668 0.001 0.02 1.95 0.003 1500 10-4 0.03 7.54 0.002 0.004(M) 2.46 0.002 0.03 2.36 0.002 1500 10-4 0.04 9.14 0.002 0.006(M) 2.395 0.001 0.04 2.95 0.001 1500 10-4 0.05 10.04 0.003 0.008(M) 2.25 0.001 0.05 3.98 0.003 1500 10-4 0.06 11.39 0.002 0.01(M) 2.013 0.002 0.06 4.85 0.003 the rate determining step. The reaction shows both 1st order dependency on [benzyl alcohol]T and [Cr(VI)]T and 2nd order dependency on [H+] ion. In the presence of some non-functional surfactants, the orders remain un- changed. CPC has been found to retard the rate while SDS and TX-100 shows the rate acceleration effect (Ta- ble 2). The effect of added electrolye gives different ob- servations. Inhibition followed by enhancement is the effect of NaCl but for NH4Cl inhibition is the only fate. So for waste water treatment with biosorbent, SDS or TX-100 and suitable concentration of NaCl may be used for quick result. 7. ACKNOWLEDGEMENTS Financial support from UGC, New Delhi is thankfully acknowledged. REFERENCES [1] Nriagu, J.O. and Nieboer, E., Eds. (1988) Chromium in the natural and human environments. Advances in Envir- onmental Science and Technology. John Willey and Sons, New York, 20, 1-501. [2] Sorahan, T., Burges, D.C., Hamilton, L. and Harrington, J.M. (1998) Lung cancer mortality in nickel/chromium plates. Occupational and Environmental Medicine, 55(4), 236-242. [3] Mancuso, T.F. (1997) Chromium as an industrial carcin- ogen. American Journal of Industrial Medicine, 31(2), 129-139. [4] Langardt, S. (1990) One hundred years of chromium and cancer: A review of epidemiological evidence and se- lected case reports. American Journal of Industrial Me- dicine, 17(2), 189-214. [5] Ahluwalia, S.S. and Goyal, D. (2007) Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresource Technology, 98(12), 2243-2257. [6] Volesky, B. and Holan, Z.R. (1995) Biosorption of heavy metals. Biotechnology Progress, 11(3), 235-250. [7] Park, D., Yun, Y.S., Kim, J.Y. and Park, J.M. (2008) How to study Cr (VI) biosorption: Use of fermentation waste for detoxifying Cr (VI) in aqueous solution. Chemical Engineering Journal, 136, 173-179. [8] Shaver, T.N., Lingren, P.D. and Marshall, H.F. (1997) Nighttime variation in volatile content of flowers of the night blooming plant, Gaum drummondii. Journal of Chemical Ecology, 23(12), 2673-2682. [9] Nkpwatt, D.A., Krimm, U., Coiner, H.A., Schreiber, L. and Schwab, W. (2006) Plant volatiles can minimize the growth suppression of epiphytic bacteria by the phytopa- thogenic fungus Botrytis cinerea in co-culture experi- ments. Environmental and Experimental Botany, 56(1), 108-119. [10] Das, A.K., Roy, A., Saha, B., Mohanty, R.K. and Das, M. (2001) Micellar effect on the reaction of Chromium (VI) oxidation of D-fructose in the presence and absence of picolinic acid in aqeous media: A kinetic study. Journal of Physical Organic Chemistry, 14(3), 333-342. [11] Bayen, R., Islam, M., Saha, B. and Das, A.K. (2005) Oxidation of D-glucose in the presence of 2,2’-bipyridine by CrVI in aqueous micellar media: a kinetic study. Car- bohydrate Research, 340(13), 2163-2170. [12] Islam, M., Saha, B. and Das, A.K. (2005) Kinetics and mechanism of 2,2’-bipyridyl and 1,10-phenanthroline- catalysed chromium(VI) oxidation of d-fructose in aqu- eous micellar media. Journal of Molecular Catalysis A: Chemical, 236(1-2), 260-266. [13] Islam, M., Saha, B. and Das, A.K. (2006) Chromic acid oxidation of hexitols in the presence of 2,2’-bipyridyl catalyst in aqueos micellar media: a kinetic study. Inter- national Journal of Chemical Kinetics, 38(9), 531-539. [14] Islam, M., Saha, B. and Das, A.K. (2007) Kinetics and mechanism of picolinic acid promoted chromic acid oxi- dation of maleic acid in aqueous micellar media. Journal of Molecular Catalysis A: Chemical, 266(1-2), 21-30. [15] Saha, B., Sarkar, S. and Choudhury, K.M. (2008) Micel- lar effect of quinquivalent vanadium ion oxidation of D-glucose in aqueous acid media: a kinetic study. Inter- national Journal of Chemical Kinetics, 40(5), 282-286. [16] Choudhury, K.M., Mandal, J. and Saha, B. (2009) Mi- cellar catalysis of Chromium (VI) oxidation of ethane-1, 2-diol in presence and absence of 2,2’-bipyridine in aqueos acid media. Journal of Coordination Chemistry, 62(11), 1871-1878. [17] Ghosh, S.K., Basu, A., Paul, K.K. and Saha, B. (2009) Micelle catalyzed oxidation of propan-2-ol to acetone by penta-valent vanadium in aqueous acid media. Molecular Physics, 107(7), 615-619. [18] Vogel, A.I. (1958) Elementary practical organic chemis- try, Part-III, quantitative organic analysis, ELBS and Longman Group Ltd., London, p. 739. [19] Bunton, C.A. and Cerichelli, G. (1980) Micellar effects  A. Basu et al. / J. Biomedical Science and Engineering 3 (2010) 735-741 Copyright © 2010 SciRes. JBiSE 741 upon electron transfer from ferrocenes. International Journal of Chemical Kinetics, 12(8), 519-533. [20] Menger, F.M. and Portnoy, C.E. (1967) Chemistry of rea- ctions proceeding inside molecular aggregates. Journal of the American Chemical Society, 89(18), 4698- 4703. [21] Bunton, C.A. (1979) Reaction kinetics in aqueous surfac- tant solutions. Catalysis Reviews - Science and Engineer- ing, 20(1), 1-56. [22] Morawetz, H. (1969) Catalysis and inhibition in solu- tions of synthetic polymers and in micellar solutions. Advances in Catalysis & Related Subjects, 20, 341-371. [23] Cordes, E.M. and Dunlop, R.B. (1969) Kinetics of or- ganic reactions in micellar systems. Accounts of Chemi- cal Research, 2(11), 329-337. [24] Fendler, E.J. and Fendler, J.H. (1971) Micellar catalysis in organic reactions: Kinetic and mechanistic implica- tions. Advances in Physical Organic chemistry, 76, 271- 406. [25] Bunton, C.A., Minch, M. and Sepulveda, L. (1971) En- hancement of micellar catalysis by added electrolyte. Journal of Physical Chemistry, 76(2), 2707-2709. [26] Das, A.K. (2004) Micellar effect on the kinetics and mechanism of chromium (VI) oxidation of organic sub- strates. Coordination Chemistry Review, 248(1-2), 81-99. [27] Mysels, K.J. and Princen, L.H. (1957) Light scattering by ideal colloidal electrolyte. Journal of Colloid Science, 12(6), 594-605. [28] Shinoda, K. (1955) The critical micellar concentrations in aqueous solutions of potassium alkyl malonates. Jour- nal of Physical Chemistry, 59(5), 432-435. |

