1. Introduction
Global carbon dioxide emissions linked to man’s activeties amount to 30 billion tons per year, corresponding to 8.1 billion tons of carbon: 6.5 billion tons from burning fossil fuels and 1.6 billion tons from deforestation and agricultural practices. About half of it has been reabsorbed by vegetation and dissolved in the oceans, the latter causing acidification and potentially negative effects on marine life. The reminder has been accumulated in the atmosphere, where it causes climate change because carbon dioxide, a greenhouse gas, traps sun’s heat on the earth [1].
During the last ice ages carbon dioxide naturally accumulated in the oceans with releases during warmer time periods, however studying ice cores from the Antarctica by isotopic carbon analysis it could be shown that during the last 800,000 years there has never been as much as carbon dioxide in the atmosphere as today [2].
Currently, each year the ocean absorbs approximately 25% of all carbon dioxide we emit, ocean acidification has increased by 30% since the beginning of the Industrial revolution, i.e. ca. 250 years ago, and the rate of acidification will accelerate in the coming decades, i.e. further projections estimate an increase of acidification by 120 % until 2060. As carbon dioxide dissolves better at lower temperatures, cold oceans are hit harder by ocean acidification than warmer seas. Increasing temperatures by climate change might counteract ocean acidification to a small extent [3].
CCS (Carbon Capture and Storage) business comprises capture of CO2 at emission sources, transport by pipelines and ship to storage locations and injection into a suitable host rock geological formation, such as depleted natural gas—and oil fields, saline aquifers and unminable coal fields. After injection carbon dioxide accumulates in the rock pores while displacing other gas, oil and water. Rocks with high porosity and permeability, such as in sedimentary basins, such as sandstone (on land and in the ocean) represent most appropriate geological formations for CCS [1].
Carbon dioxide is transported and compressed as dense fluid and injected on site under pressure. During injection the surrounding water gets acidified. Long-term storage of carbon dioxide might lead to upwards movement and migration into other rock formations. Dissolution of and chemical reaction with minerals might change bioavailability of minerals and metals [1].
Monitoring of these processes is needed: operational (CO2 injection), safety and environmental (effects), society and financial. The EU Directive from 2008 on CCS demands monitoring. The following targets have to be monitored: plume imaging of the injection, cap-rock integrity (CO2 migration), well integrity (CO2 escape upwards to surface), migration to other rock layers [1].
Research is needed to study the effects of ocean acidification as an acute threat during carbon dioxide injection as well as chronic effects from long term or repeated lowdose releases and upwards migration of carbon dioxide on aquatic life. Apparently fish and shellfish larvae seem to be the most vulnerable taxa as well as corals [3]. Such scientific results will affect future monitoring requirements and schemes to protect sea-life.
Some laboratory studies already indicated severe effects of low pH (pH between 7.0 and 6.0) on marine benthic fauna recorded as decrease in survival and reproduction in crustaceans and polychaetes [4], increased shell abnormalities and decreased shell thickness/size in Abalone [5] and M. edulis [6], disrupted immune defense, such as impaired phagocytosis of pathogenous bacteria in Mytilus edulis under chronic exposure to pH 6.5 [7] and severe effects on blood pH and haemocyte functionality in M. edulis [8]. A two week exposure of the crab Necora puber to a pH range from pH 8.0 down to 6.0 showed a compensatory dissolution of carbonates from the exoskeleton in order to maintain acid base balance in body fluids [9], in contrary to the findings of Small et al. [10], who reported no net shell dissolution during 30 d of exposure to pH down to pH 6.7.
Hypercapnia (pCO2 ≥ 1000 μatm) had a narcotic effect causing lethargy in the sea star O. schayeri [11]. Narcotic effects of hypercapnia are well documented in insects and several invertebrates: whereas in some organisms hypercapnia induces an increase in the neurotransmitter adenosine, a nervous system depressor, in decapods, narcotic effect of hypercapnia appears to be due to accumulation of Mg+2 from dissolution of the exoskeleton to compensate for the acidosis [11].
Field biomonitoring studies showed changes in species composition at carbon dioxide dumping sites, such as decreases in molluscs and gammaridea abundances below pH 7 [12] and altered community function, such as affected nutrient cycling and bioturbation [13].
2. Aim
The aim of this investigation is to study potential acute behavioural responses to acidification by carbon dioxide injection in the common shrimp Crangon crangon under short term exposures to stepwise pH-decreases. It is intended to provide threshold values of response for these animals to inform the debate on environmental risks and could lead to future development of a field monitoring system.
3. Materials and Methods
3.1. Test Species
Crangon crangon (Crustacea, Decapoda) is a geographically widely spread edible marine shrimp with high abundances in the North Sea, North Atlantic and Mediterranean Sea [14]. The shrimp Crangon crangon is a key species of the coastal areas of the North Sea. It appears in high numbers and represents a significant food source for fishes and it is also an important predator on many invertebrates [15].
C. crangon shows tail-flip escape responses when exposed to a natural predator, the cod Gadus morhua, or an artificial stimulus. Shrimps escaped by rolling to their left or right during the initial tail-flip of a response, and thereafter swam on their side [16]. Similar escape/avoidance responses may be expected to chemical stressors, which might be used in biomonitoring systems.
Both ecological and economic considerations are the basis for the concern about potential negative effects of ocean acidification on this species. C. crangon tolerates a wide range of temperatures, salinities (17‰ - 32‰ [14]; 29‰ - 31‰ [17]) and substrates [14], as it also survives in coastal and estuarine regions in the North Sea and migrates seasonally from shallow to deeper regions. In spring-tide periods the shrimps are exposed to tidal salinity fluctuations ranging from 35‰ to 25‰ in summer and from 34‰ to 14‰ in winter [18]. Populations in the North Sea exhibit a diurnal activity rhythm with peaks in the night. Although being predators they do not actively hunt, rather wait for appropriate prey to come close. The shrimps use olfaction to detect their prey. During the day they often burrow in the upper sediment layers, indicating a rather inactive and passive behaviour.
3.2. Experimental Setup and Design
The acute acidification experiments were performed in a flow-through system, with constant renewal of fresh seawater filtered through sand (9˚C, 34‰), being distributed equally from a head tank to the 4 glass tanks (6 L, flow rate of 500 ml per minute per tank). Carbon dioxide was fed into a header tank via a regulator and monitoring system that maintained pH at the desired level (Figure 1).
The shrimps were collected from a local beach using a push net then returned to the laboratory where they were acclimated to the local water conditions (pH 8.1 PSU, 9˚C) for at least one day before use. Juvenile (2.0 - 3.5 cm) specimens were selected randomly for the experiments. Three subsequent pH-experiments as well as a control with four animals in each set were performed in reduced light conditions and during the same period of the day, following an identical scheme of stepwise pHdecreases with hourly decreases by 0.2 pH units between pH 7 down to pH 6.
Figure 1 shows CO2 lines going into both header tanks—in this experiment, it feeds into just one. In each tank one test chamber of the MFB is placed, with one animal in each test chamber.
Behaviour of the shrimps was recorded quantitatively and online with the Multispecies Freshwater Biomonitor (MFB) based on quadropole impedance conversion technique [19,20]. Shrimps (2.0 - 3.5 cm) were placed individually in cylindrical sensor chambers (5 cm length, 2 cm inner diameter) capped with screw lids holding nylon nets (0.2 mm) on both ends to allow flow-through of seawater. Previous experiments with Corophium volutator [21,22] as well as with Carcinus maenas [23] proved the MFB could function in brackish and fully marine waters.
3.3. Statistical Analysis
Statistical analysis was performed in SigmaPlot 12 using non-parametric statistics. Experiments with subsequent pH shifts (0.2 pH units) were analysed with repeated measures ANOVA on ranks to show differences between
individuals within one specific experiment and to show differences between the three subsequent experiments compared to the control at pH 8.05. Moreover, differrences in activity levels (% activity increase directly after versus directly before each pH shifts) were tested with ANOVA on ranks. All graphs were produced in SigmaPlot 12.
4. Results
4.1. Behavioural Pattern of C. crangon
As benthic species both adult and juvenile C. crangon are mostly resting on/in the sediment during the daytime. They show an endogenous rhythm that controls the majority of their activities to the hours of darkness. Deviation away from this pattern will leave them susceptible to the many predators that hunt them by sight. In the MFB three different types of behaviour could be discerned: 1) sporadic rapid short distance swimming (“shooting behaveiour”, Figure 2), i.e. single peaks of high amplitudes, 2) episodic undulation of the abdominal legs (“ventilation”), i.e. regular almost mono-frequent signals of low amplitudes (1 - 2 Hz) for time periods of ca. 5 - 10 seconds and c) resting or inactivity, i.e. no signal above the baseline.
4.2. Responses of C. crangon to Stepwise pH Downshifts
Stepwise pH-decreases between 7 and 6 PSU were applied to simulate possible leakage of CO2 from storage sites into seawater. Prior to the shifts the animals had been acclimated to the test chambers overnight. Stepwise pH-decrease caused immediate increase of activity by the animals followed by a return to the normal resting behaviour afterwards. These “activity waves” show that the animals react fast and in a very sensitive manner to subsequent pH-decreases of only 0.2 units per hour (Figures 3 and 4).
The observed activity pattern (Figure 3) was found identically in 3 subsequent experiments: directly after a pH-decrease locomotion (shooting behaviour) increased significantly, i.e. the animals sensed the changes in acidity and reacted with increased activity, indicating avoidance. This response lasted for about 30 minutes, i.e. the animals calmed down before the next pH-decrease.
The change in activity expressed as difference of activity after versus before the pH shift was plotted for all experiments pooled versus the pH intervals of pH shifts (Figure 5). It can be seen that the animals detect all pH shifts in a similar intensity of response, with no significant differences between a) the 3 experiments and b) pHsteps (ANOVA on ranks). Whereas 10 of the 12 tested animals responded within 10 Min. of pH downshift, 2

Figure 2. Different types of behaviour of C. crangon recorded by the MFB.

Figure 3. Overview over the activity of 4 C. crangon (2.0 - 3.5 cm) following the pH-decreases (pH 8 to pH 7.0 and further down in 0.2 units (marked with arrows) Three subsequent experiments and the control are presented.
responded later, i.e. after 20 - 30 Min.
5. Discussion
The animals reacted rapidly and sensitively to small pHdecreases with increased avoidance (shooting behaviour) within 10 - 20 Min. after start of pH-decrease. This pattern was constant and repeatable. The avoidance response might be comparable to the tail-flip behaviour, which has
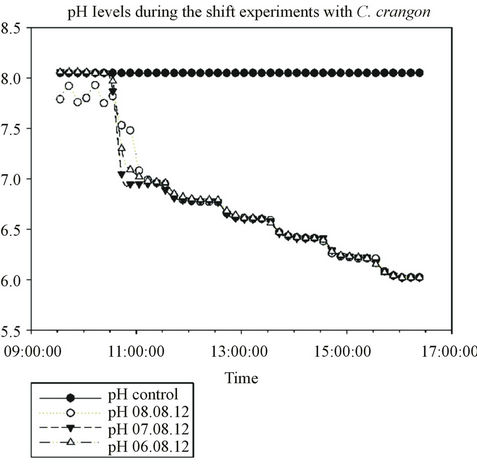
Figure 4. Stepwise pH-decrease (0.2 units) in 3 subsequent experiments compared to the control.

Figure 5. Degree of increase in avoidance (% activity) in Crangon crangon directly after pH decrease.
been described as predator avoidance before [16]. The whole data-series exhibited a series of “waves” of increasing activity due to hourly pH-downshifts, resembling a sinus function. Similar results have been reported earlier while studying the locomotory activity of Daphnia magna followed by repeated artificial light stimuli every 2 hours [24]. The observed sensitivity and rapidity of the responses of C. crangon indicate that CO2 leakage from carbon storage sites might have severe acute effects on shrimp behaviour. These effects might be reversible for some time as in our short term experiment, however repeated stress might weaken the fitness of the organisms in the long term. Moreover, avoidance responses expose the benthic shrimps to potential predators, as they leave their shelter. Lower fitness and increased predator vulnerability might lead to reduced population density. Also migration away from a region is possible.
The effects of CCS on the behaviour of marine invertebrates have barely been studied, therefore comparative literature is lacking. Most previous studies dealt with physiological changes due to pH decreases in marine mussels, some with crabs, however, behavioural studies are rare. The same applies to effects of acidfication in freshwater habitats (e.g. Herrmann et al. 1993).
Behavioural responses to pH-decreases (from circumneutral pH down to pH 3.5) during a period of 48 h were performed with Daphnia magna showing decreased locomotion and ventilation (pH 7 - 3.5), with a steep linear regression slope [25]. No avoidance response could be seen. This might be due to the difference in the behavioural response patterns of the planktonic daphnids compared to the benthic shrimps.
The decapod Atyaephyra desmaresti showed decreased activity under direct exposure to low pH-AMD (natural Acid Mine Drainage: acid pH combined with high metal ion levels) with the lowest observed response time (LORT) of 5 h at pH 5.0 [26]. In exposures to acid water without metal ions, stress behaviour of A. desmaresti consisted of decreasing activity and loss of diel rhythmicity at pH 4.4 within 48 h [27]. In these studies the freshwater shrimps did not have any time for a transient avoidance response, as they were exposed directly into very low pH causing directly irreversible toxic effects on behaviour and survival. Such a situation might happen in the sea in case of an accident during carbon injection procedure.
Freshwater crustaceans tolerate much lower pH levels than marine crustaceans, which might be due to differrences in ionregulation and osmoregulation. While freshwater organisms need to regulate their body to be highly hyperosmotic, marine organisms do have almost the same osmotic conditions in their body as in the surrounding sea water. Their regulation of the smaller differences might cause a more sensitive response to small pH changes. In freshwater habitats the critical pH for most invertebrates to maintain a viable population is around 5 - 5.5 [28], only some tolerant in-benthic insects or air-breathing insects can cope with lower pH-levels [26].
6. Acknowledgements
We kindly acknowledge the technical support by Sara Gerhardt and Stig Westerlund for constructing the pH-regulation system. The project was funded by NFR CLIMIT Nr. 215637 (Dr. Shaw Bamber).