1. Introduction
Malignant triton tumor (MTT) is a variant of malignant peripheral nerve sheath tumor (MPNST) with rhabdomyosarcomatous differentiation. It is a rare tumor constituting 5% of all MPNSTs [1], and about two-thirds of MTT arise in patients with neurofibromatosis type 1 (NF1) [2].
Computed tomography (CT) and magnetic resonance imaging (MRI) are useful for detecting the localization and delineating the extent of MPNST including MTT, and 2-[F-18]-fluoro-2-deoxy-D-glucose (FDG) accumulation reflects their biological activities.
To date, FDG-positron emission tomography (FDGPET) findings of MTT have been demonstrated in only three reports [3-5], to the best of our knowledge. Although several studies reported that FDG-PET was a sensitive and specific tool for distinguishing MPNST from benign peripheral nerve sheath tumor [5-10], there has been very little discussion about the details of the radiological features of MTTs.
We present the case of a patient who was found to have an MTT arising in the abdominal wall, and we report the imaging findings obtained by CT, MRI, and FDG-PET/CT.
2. Case Report
A 54-year-old male was admitted to our hospital after a 2-year history of an enlarging right upper abdominal wall tumor. He had no pain or other symptoms and no family history of NF1. A tumor about 10 cm in dia. was palpable on the right side of the lower chest to the upper abdomen. There was no skin reddening, skin ulcer, bleeding or tenderness. The blood examination results included a white blood cell count of 8720/µl and C-relative protein level of 1.27 mg/dl, indicating the existence of slight inflammation. Total cholesterol (284 mg/dl) was slightly elevated but all other serological data were normal, including liver and renal functions.
On non-contrast enhanced CT, the tumor (7.5 × 6.5 × 11.5 cm) was found at the right abdominal wall, destroying the patient’s right 11th rib and extending outward from the body. The tumor presented slightly lower and heterogeneous attenuation, and it consisted of roughly two components: the lateral component, presenting nearly equal density to the surrounding muscles, and the medial component, presenting relatively low attenuation. Several broken bone fragments were seen inside the tumor, and invasion into the intercostal and latissimus dorsi muscles was suggested. Multiple metastases were also found in the bilateral lungs (Figure 1).
The FDG uptake in the lateral component of the tumor was much higher than that of the medial part, and the maximum standardized uptake value (SUVmax) of the lateral and medial components of the tumor were 7.1 and 2.0, respectively. The SUVmax of the multiple lung nodules was less than 1.8 (Figure 2).
The MRI examination of the tumor showed heterogeneous higher signal intensity on T2-weighted images and lower signal intensity on T1-weighted images compared to the adjacent muscles. A gadolinium-enhanced fatsaturated T1-weighted image showed a heterogeneous marginal enhancement of the tumor. The diffusionweighted image appeared to be higher intensity in the lateral component compared to the medial component, and the lateral part of the tumor showed a lower apparent
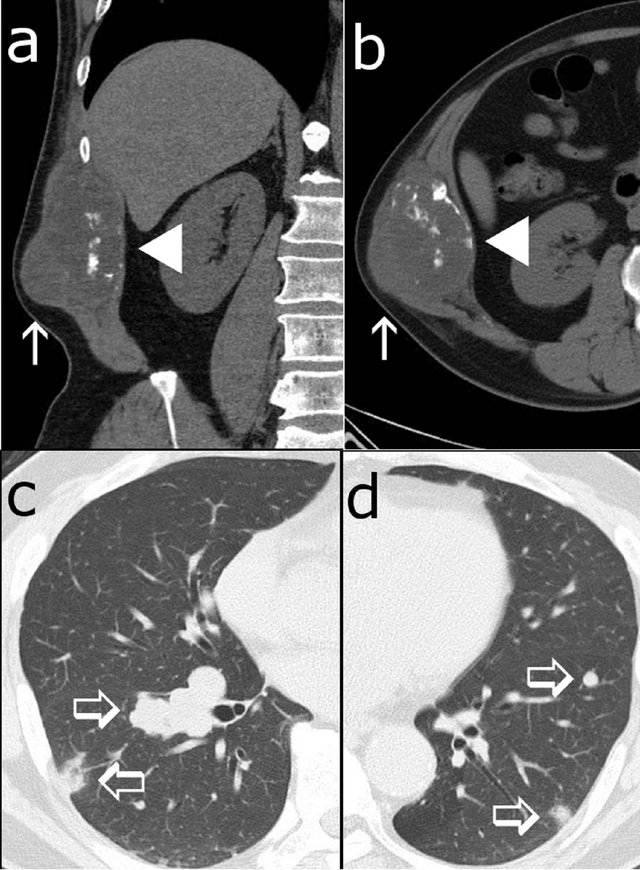
Figure 1. Non-contrast enhanced CT scans show a 7.5 × 6.5 × 11.5 cm tumor at the right abdominal wall of the patient, a 54-year-old male ((a), (b)). The lateral component of the tumor presented nearly equally but heterogeneous density to surrounding muscles (arrow), and the medial component presented slightly low attenuation with broken bone fragments (arrow head). Multiple metastases were found in the bilateral lungs ((c), (d); open arrows).
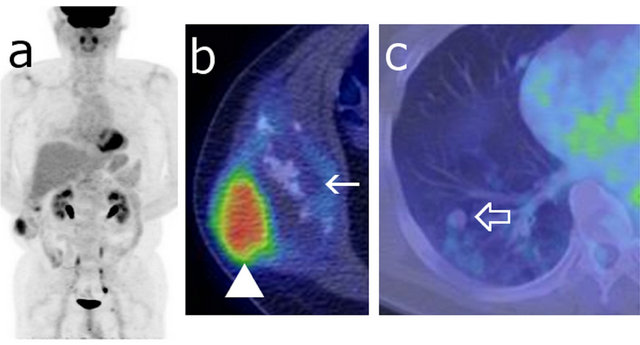
Figure 2. Maximum intensity projection (MIP) image (a) and fusion images ((b), (c)) of FDG-PET/CT showing the right abdominal wall tumor. The SUVmax of the tumor was measured as 7.1 at the lateral part of the tumor ((b) arrow head) and 2.0 at the medial component ((b) arrow). The FDG accumulation of the multiple lung metastases was weak (SUVmax < 1.8) ((c) open arrow).
diffusion coefficient compared to the medial component (Figure 3).
A biopsy was performed after the imaging examinations. The specimen of the tumor’s lateral component showed cellular proliferation of spindle-shaped cells and less cellular myxoid stroma, accompanied by small slitlike vessels and chronic inflammatory infiltration. Scattered oval to short spindle-shaped cells with granular eosinophilic cytoplasm were also observed. With an immunostaining procedure, the spindle-shaped cells with eosinophilic cytoplasm were positive for desmin and myogenin and very focally positive for S-100 protein, but negative for alpha-SMA. The MIB-1 labeling index was 60% (Figure 4). These histological findings suggested MPNST with rhabdomyomatous differentiation, which is known as MTT.
Because the tumor invaded adjacent muscles and multiple lung metastases were found, chemotherapy rather than surgical tumor resection was selected for treatment. Although 100 mg/body of adriamycin was administered, the tumor grew to 97 × 71 × 180 mm and the multiple lung metastases were much deteriorated. Three months after his admission, the patient died from respiratory failure caused by pneumonia.
3. Discussion
MTT is a very rare and highly progressive variant of MPNST with rhabdomyomatous differentiation. The first reported MTT case was described by Masson in 1932 [11]. Malignant triton tumor is named after the Triton salamander, which is able to regenerate limbs consisting of muscle, bone and nerve tissue if the cut end of the sciatic nerve is implanted into the soft tissue of its back. This regeneration was thought to be similar to the differentiation of muscular cell lines by malignant neural elements, as originally thought to occur in MTT [1,12].
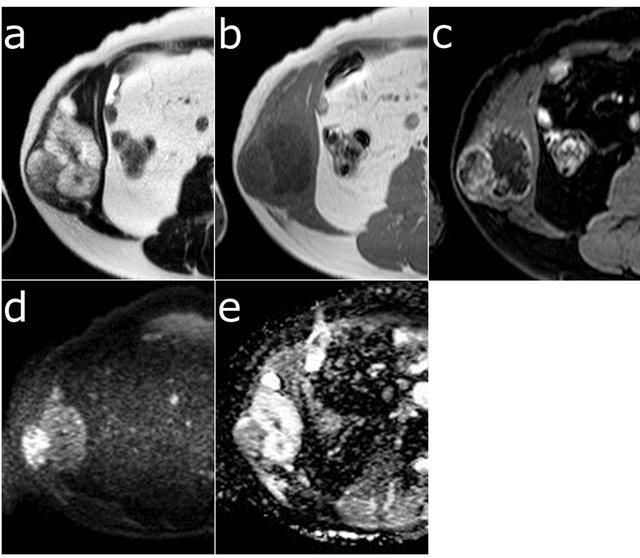
Figure 3. Axial MRI of the tumor showing heterogeneous high signal intensity on T2-weighted images (a) and low signal intensity on T1-weighted images (b). Gadoliniumenhanced fat-saturated T1-weighted image showed heterogeneous marginal enhancement of the tumor (c). The lateral component of the tumor, corresponding to high FDG accumulation, showed higher intensity on the diffusionweighted image (d) and a lower apparent diffusion coefficient (e) compared to the medial component.
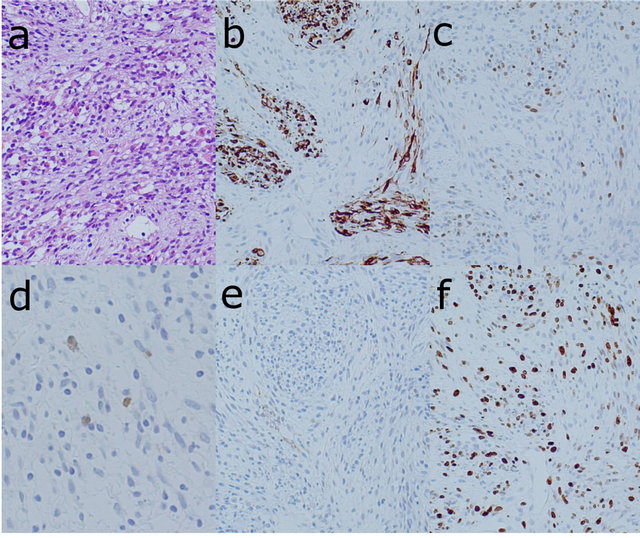
Figure 4. Photomicrographs of the specimen biopsied from the lateral component of the tumor showing the cellular proliferation of spindle-shaped cells with small slit-like vessels and chronic inflammatory infiltration and less cellular myxoid stroma ((a) HE ×100). Immunohistochemical stain of desmin ((b) ×100) and myogenin ((c) ×100) indicated the rhabdomyosarcomatous component of the tumor. Immunohistochemical stain of S-100 protein ((d) ×200) showed that the spindle-shaped cells with eosinophilic cytoplasm were very focally positive, which indicates nerve sheath differentiation. No staining of alpha-SMA ((e) ×100) indicated that there was no smooth muscle component in the tumor. The MIB-1 index was calculated as 60% from the staining with Ki-67 ((f) ×100).
Woodruff et al. reported that 57% of MTT patient had NF1 [2]. MTTs in patients with NF1, have shown a male predominance, young age (20 to 30 years old), and common presentation in the head and neck region. In contrast, MTT patients without NF1 show a female predominance, older age (30 to 50 years old), and tumors frequently located on the trunk, which agree with our case except for the gender [13,14].
Woodruff et al. defined MTT with the following histopathological criteria: 1) the neoplasm is connected to peripheral nerves or occurs in patients with NF1; 2) most of the neoplasm consists of Schwann cells; and 3) the neoplasm contains rhabdomyoblasts [15]. The biopsy specimen from our patient meets these criteria and was diagnosed as MTT.
MTT commonly presents heterogeneous CT attenuation and MR signal intensity because this tumor often contains necrosis, hemorrhage, degeneration, or cystic change. The contrast enhancement pattern of MTTs is also frequently heterogeneous [15]. These radiologic features are surely helpful for the diagnosis of MTT, but CT and MRI cannot reliably differentiate MTT from benign tumors, because the same features may also be seen in benign nerve sheath tumors [6,16,17]. Several studies demonstrated that FDG-PET was a sensitive and specific tool for distinguishing MPNST including MTT from benign peripheral nerve sheath tumors. The suggested cutoff values of SUVmax for the differentiation between malignant and benign peripheral nerve sheath tumor are 2.5 - 6.1, which is a relatively wide-range with some degree of overlap [6,8-10]. To the best of our knowledge, three reports that include FDG-PET findings of MTT have been published [3-5].
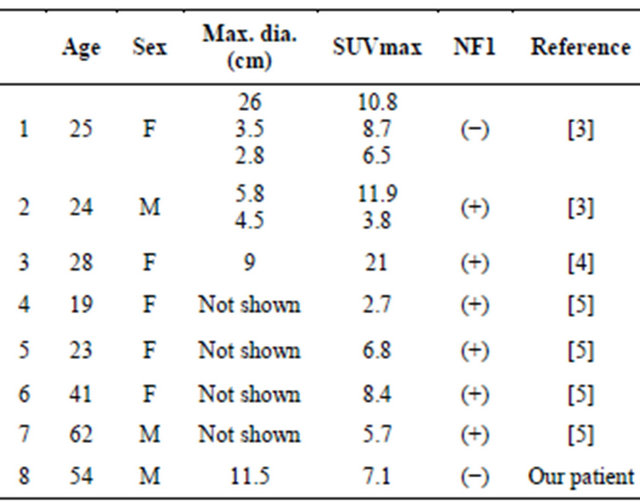
Table 1 summarizes the characteristics of the seven MTT patients in the previous three reports and those of our patient, all of whom underwent FDG-PET or FDGPET/CT (males 3, females 5, age: 19 - 62, median 26.5). There were total of 11 lesions in these eight patients, and the SUVmax of the lesions ranged from 2.7 to 11.9. Two patients did not have a history of NF1. Although SUVmax is affected by many factors including lesion size, applied FDG dose, and the time between the administration of FDG and the data acquisition, the intensity of FDG accumulation in these MTTs seems to be high, as is the case for MPNST [5]. It may be difficult to distinguish MTT from MPNST, even though FDG accumulation would be an indicative factor for the differentiation of MTT and benign peripheral nerve sheath tumor.
In our patient, we concluded that the lateral component of the tumor—which showed high FDG uptake—reflected the histopathological findings of cellular proliferation of spindle-shaped cells and less cellular myxoid stroma. The medial component of the tumor—which showed low FDG uptake—seemed to correspond to the myxoid tissue considering the MRI findings, but a histological verificaTable 1. Reported MTT patients who underwent FDG-PET or FDG-PET/CT.
tion of this was not obtained. Benz et al. suggested that the expression level of hypoxia-inducible factor-1α (HIF- 1α) might also contribute to different glucose metabolic phenotypes in FDG-PET imaging [10]. However, the detailed mechanism underlying the FDG accumulation in this type of tumor has not been established. HIF-1α was not measured in our patient.
Complete surgical resection with a wide margin and adjuvant radiotherapy are generally accepted in order to obtain the best outcome for an MTT. Systemic chemotherapy is selected if there is evidence of metastatic disease. The survival rates of patients with MTT are much lower than those of patients with MPNST. The reported crude 2- and 5-year survival rates of patients with MTT and MPNST are 15% and 11%, versus 57% and 39%, respectively [18,19].
4. Conclusions
Our patient’s MTT showed high FDG accumulation. In the light of the histopathological findings, the high FDG uptake seemed to correlate with the proliferation of spindle-shaped cells and less cellular myxoid stroma.
Although it is still difficult to distinguish MTT from MPNST, FDG-PET/CT can be a helpful tool for differentiation between MTT from benign peripheral nerve sheath tumor.