1. Introduction
Tto1 is one of the few active retrotransposons in tobacco. The sequence analysis suggests that Tto1 carries all functions required for autonomous transposition through reverse transcription. The copy number of Tto1 increases 10-fold during protoplast culture, regenerated plants from tissue cultures [1]. There are reports for the increase of retrotransposition during tissue culture and regenerated plants with other retrotransposons in barley [2,3]. Tto1 transposition is regulated mainly at the transcriptional level. It is shown that expression of Tto1 can be induced in leaves of tobacco by wounding stress [4]. Tto1 has been implicated in the expression of phenylpropanoid synthetic genes in response to defense-related stresses [5]. Tto1 is also re-activated by the implantation of the lowenergy ion beam ion [6].
In crop production, the use of bacterial genes has created new approaches for herbicide tolerance [7]. Bacterial dehalogenases cleave carbon-halogen bonds with different stereo-configurations. Three dehalogenases are produced by Rhizobium sp. collectively known as dehD, dehL and dehE. The dehE from Rhizobium sp. RC1 is non-stereo specific dehalogenase that can act on D-2- chloropropionate (D-2-CP), L-2-chloropropionate (L-2- CP), 2,2-dichloropropionate (2,2-DCP)-Dalapon, trichloroacetate (TCA), dichloroacetate (DCA) and monochloroacetate (MCA). The dehE is further characterised [8,9]. Dalapon is a chemical with herbicide properties only when active ingredient of 2,2-DCP is present. It is used selectively to control weed grasses [10,11]. The high concentration of Dalapon may kill desired plant that lacks of herbicide tolerance. To improve the Dalapon resistance level, production of transgenic plants is needed. Our group developed transgenic tobacco plant via A. tumefaciens introduction and expression of the herbicidetolerance gene, dehE [12].
Several molecular marker systems based on retrotransposons have been developed. All rely on the principle that a joint is formed, during retrotransposon integration, between genomic DNA and the retrotransposon. These joints may be detected by amplification between a primer corresponding to the retrotransposon and a primer matching a nearby motif in the genome [13]. The interretrotransposon amplified polymorphism (IRAP) method [14] detects retrotransposon insertional polymorphisms by amplifying the portion of DNA between two retroelements. It uses one or two primers pointing outwards from an LTR and amplifies the tract of DNA between two nearby retroelements. IRAP can be carried out with a single primer matching either the 5’ or 3’ end of the LTR but oriented away from the LTR itself, or with two primers.
There are very limited reports on transgenic plants in terms of genomic variations [15]. In the present research, we aimed to analyse transgenic tobacco plants carrying herbicide-tolerance gene, dehE gene in terms of Tto1 retrotransposition using IRAP technique.
2. Materials and Methods
2.1. Plant Material, Gene Construct and Transformation
N. tabacum cv. TAPM24 plants transformed with pCAMBIA1301a vector were tested whether they carry dehE gene or not. In addition, expression of dehE gene was determined by qPCR with dehE specific primers [12]. To analyses Tto1 retrotransposon caused genetic variations, 5 transgenic plants were randomly selected and used for DNA isolation. DNA isolations were performed from leaves by TRI Reagent® (Sigma, T9424) according to the manufacturer’s instructions. We also isolated DNA from 3 non-transformed tobacco plants and used them as control. Spectrophotometric assays were conducted to determine the quantity and quality of the isolated DNA.
2.2. Inter-Retrotransposon Amplified Polymorphism (IRAP) PCR
IRAP was performed with one primer (5’-TCCGCTGTGCAGTAGTGTTTAGTG-3’) designed from LTRsequences of Tto1 retrotransposon [16]. Amplification were carried out in 20 μl reaction volume containing 10 μl 2× Sapphire Enzyme Mix (Takara, RR350), 2 μl primer (10 pmol), 2 μl template genomic DNA (1 ng/μl) and 6 μl dH2O. The amplification conditions were one initial denaturation step at 94˚C for 3 min followed by 35 cycles at 94˚C (20 s), 55 (20 s) and 72˚C (1 min); the reactions were completed by a final extension step at 72˚C for 10 min.
2.3. Evaluation of PCR Products
Ten-μl IRAP-PCR products were loaded to 6% nondenaturing polyacrylamide (29:1 Acrylamide:Bis) gel and run at 150 V for 5 h in 1× TBE buffer. Gel were stained in 1× TBE buffer containing ethidium bromide for 15 minutes and scored visually. Both homomorphic and polymorphic bands were scored and similarity index was calculated using Jaccard’s coefficient [17].
3. Results and Discussion
In this study, IRAP marker technique was applied to 5 transgenic tobacco plants, transformed with dehE gene. In addition, we used 3 non-transformed control plants to find out whether there is a naturally occurred polymorphism or not between individuals. As result of IRAPPCR, we did not detect any polymorphic band among control plants (Figure 1, line C1-3). Hence, we conclude that Tto1 retrotransposon do not cause natural polymorphism.
The IRAP results of 5 dehE transformed tobacco plants showed that all samples have different band patterns (Figure 1, line T1-5). Although there is no polymorphism between control plants, transgenic lines showed 0% - 20% (Table 1). These polymorphism rates proved that dehE gene transformation could be caused retrotransposition events.
T1-5 indicate transgenic plants; C1-3 indicate control plants; NC indicates negative control; first and tenth lines are marker (arrows indicate some polymorphic and missing bands).
As shown Figure 1, all the transgenic lines have different band patterns. First transgenic plant has two novel bands (arrows a) while T5 missed a band (arrow b).
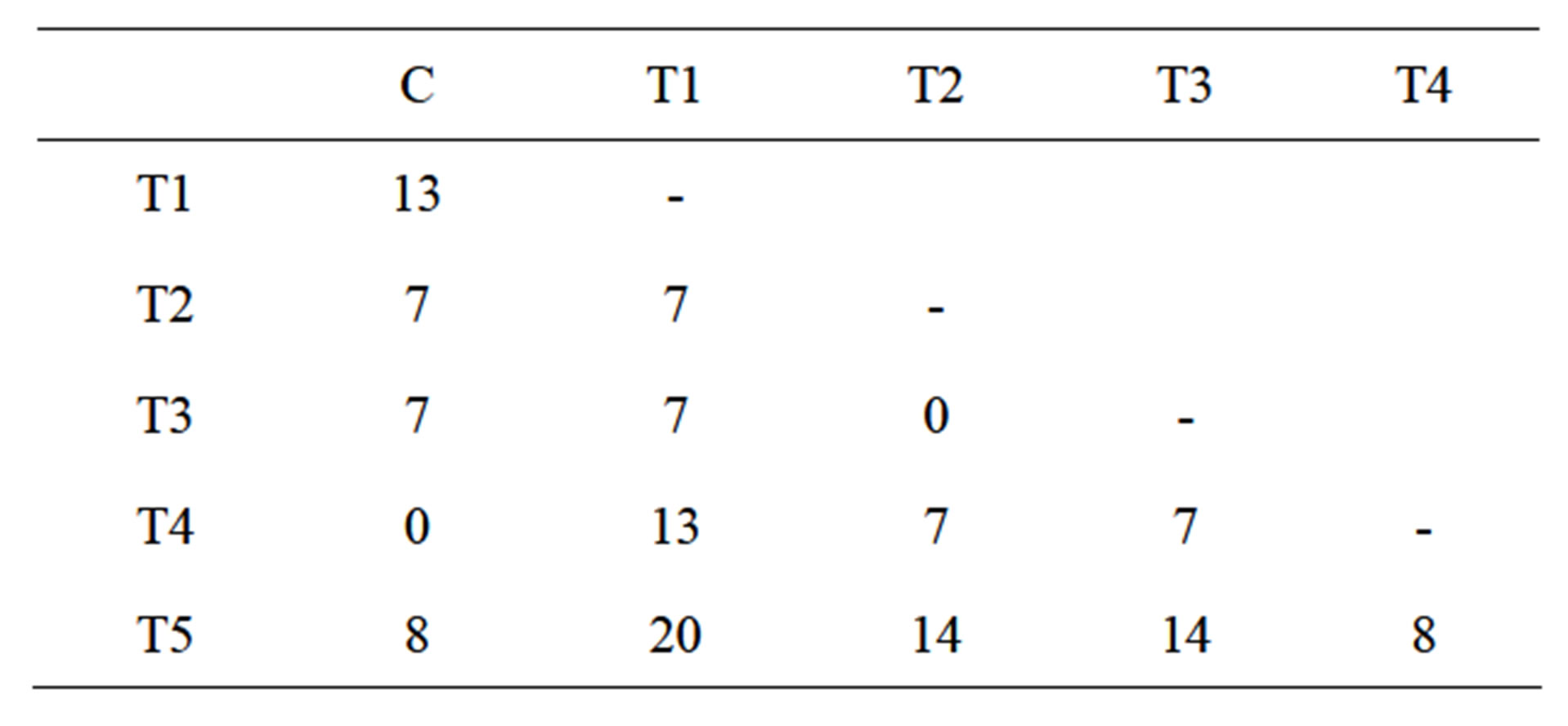
Table 1. IRAP polymorphism percentage between transgenic and control plants. T1-5 indicate transgenic plants; C indicates control plant.
Both T2 and T3 showed polymorphic patterns compared with control plants although they were homomorphic with each other. On the other hand, T4 line has identical band patterns with control plants. This results show that transformation of tobacco plants might cause retrotransposon movement and this effect is different for every individual.
Campbell et al. [18] used IRAP technique to determine somaclonal variation in regenerated plantlets from barley calli cultures. They obtained novel non-parental bands during analysis of DNA polymorphisms in parental controls and regenerants. They stated that retrotransposon-based marker systems, such as IRAP, based on retrotransposons such as BARE1, are valuable tools for the characterization of mutations that arise during tissue culture. Muhammad and Othman [19] studied banana somaclones with Random Amplified Polymorphic DNA (RAPD) and IRAP markers. They observed that RAPD markers were more polymorphic than IRAP markers [15]. IRAP was also recently used to study genetic diversity of cultivated flax (Linum usitatissimum L.) germplasm [20]. Retrotransposons show unique patterns of environmental and/or developmental regulation, e.g. tobacco Tnt1 and barley BARE1 copies were detected in roots and leaves, respectively [21,22]. Many retrotransposons were showed to be activated by biotic and abiotic stresses [23]. Copy numbers of tobacco Tto1 [1] and rice Tos17 [24] elements increased during tissue culture procedures. In our previous studies on barley tissue cultures we also observed retrotransposon movement increase in relation to the callus age and regenerated plantlets [2,3]. Although 10-fold increase in copy number of Tto1 during protoplast culture and regenerated plants from tobacco tissue cultures [1], this is the first report on transgenic lines in terms of retrotransposon movements.
4. Conclusion
In this study, retrotransposon integration events in transgenic tobacco plants were investigated. 0% - 20% polymorphisms were observed between transgenic individuals and controls. It was clear that some retrotransposition events occured during transformation. However, sequence characterization of the regions, which exhibit polymorphism, is crucial for the exploration of transformation development and retrotransposition.
5. Acknowledgements
Authors would like to thank The Malaysian Government for partly sponsor the work under research grant schemes FRGS 4F008 and GUP (QJ130000.7135.00H34 and Istanbul University, Molecular Biology and Genetics Department).
NOTES