Evaluation of Reactive Oxygen Species (ROS) Generated on the Surface of Copper Using Chemiluminesence ()
1. Introduction
Copper and its alloys have been widely recognized from ancient to modern civilized societies as natural antibacterial materials [1] , and they had been used as such as a water vessel, the building parts of shrine or temples, or a balustrade of bridge in Japan. Ancient civilizations utilized these antimicrobial properties long before the discovery of bacteria in modern civilization. Recently, the mechanism of antibacterial activity of copper and the related alloy has been much attracted again due to the worldwide disaster of Covid-19 [2] . Mainly 4 types of copper’s antibacterial activities have been proposed [3] [4] ; 1) bacteriolysis, i.e., membrane breakdown; 2) membrane breakdown under stress; 3) DNA damages caused by reactive oxygen species (ROS) generated on the copper surface; 4) devolution and disaggregation of genome and or plasmid. These killing processes are multifaced with the main mechanism of bactericidal activity generated by ROS, which irreversibly brings damage of membranes and DNA chain in cell.
As far as oxygen concerned, there are 4 kinds of ROS [5] , such as hydroxyl radical ·OH, hydrogen peroxide H2O2, superoxide anion
and singlet oxygen 1O2. Especially, ·OH reveals strong antibacterial activity due to its high reactivity which originates from high standard redox potential Eo = 2.38 V, the second highest followed by active fluorine Eo = 2.85 V [6] . Reactive oxygen species are generated on the surface of metal copper and metal oxides such as zinc and titanium oxides too. The present authors have been publishing some papers of the antibacterial activity of ZnO [7] [8] [9] anatase TiO2 [10] , which activity can be sustained even in a dark condition (i.e., no sunlight). And further, a paper of anatase TiO2 added Cu powders with improved antibiotic properties has been published recently [11] . Microscopically, the surface of metal copper is covered with very thin film of cuprous oxide Cu2O and cupric oxide Cu2O [12] . Therefore, ROS could be stated that its generation originates at the metal oxide surfaces [13] .
Up to now, many ROS detection methods have been proposed [14] ; electron spin resonance (ESR), fluorescence (FL), spectrophotometry, HPLC coupled with UV detection and chemiluminescence (CL). Among them, CL method has attracted much attention because of its unique advantages such as high sensitivity, instantaneity and simplicity of operation [15] . The detecting sensitivity much depends on the luminescence agents. Among emission agents, luminol is the most popular and has high sensitivity, therefore this agent is always used in a crime investigation. However, luminol has disadvantage, i.e., this emission is limited by pH value of aqueous solution which contains antibacterial substance. It is reported that the best pH value is about 11.0 [16] . Furthermore, identification of ROS can be performed by selecting the suitable scavengers for each ROS in CL measurement. Many reports concerning about scavengers used in CL measurement have been published [17] [18] : 1). alcohol [19] , mannitol, potassium formate, ascorbic acid, DMSO, and phthalhydrazide [20] [21] for hydroxyl radical ·OH, 2) riboflavin [22] [23] , pyruvic acid [17] [24] for hydrogen peroxide H2O2, 3) ((2-Methyl-6-(4-methoxyphenyl) imidazo [1,2-a] pyrazin-3(7H)-one, C14H13N3O2, in simple terms as abbreviated, “MPEC” [25] and nitro blue tetrazolium (in simple terms, “nbt”) [17] [18] [26] for superoxide anion
, and 4) 2,5-dimethylfuran, sodium azide “NaN3” [17] , MnTBAP (manganese(III)-tetrakis (4-benzoic acid) for singlet oxygen 1O2. However, there are some features in each scavenger, for examples, its sensitivity, a combination of emission reagents, and pH dependence of efficiency. For examples, phthalhydrazide and riboflavin were incorrect deemed in this study, furthermore, n-butanol, which was reported to be the suitable scavenger for detecting hydroxyl radical, has been found to not suitable for CL method recently in our investigation. In our previous study, antibacterial activity of ZnO, anatase TiO2 and Cu powders have been investigated to understand its mechanism and improve the antimicrobial properties, available even in a dark condition for the former two.
Purpose of this study is to inquire what kind of and how amount of ROS are generated on the surface of metal copper, using the combination of light emission reagent and scavengers in CL measurement, and by observation of the outermost surface by TEM and identification of crystalline phases by thin-film XRD.
2. Experimental Procedure
2.1. Preparation of Bulk Cu Plates
Oxygen-free copper (99.99% purity, C1020) plate, a square shape in size 25 × 25 × 1.0 mm3 (Kikukawa Industry Co., Ltd, Tokyo, Japan), were used as starting material. These Cu plates, after wiping their surfaces with ethanol, were heated in air at 373, 473, 523, 573, 623, 673 K for 4.2 × 102 s; heat treatment was performed in quick heating and cooling.
2.2. Evaluation
2.2.1. Chemiluminescence (CL) Intensity Measurement and Evaluation of Reactive Oxygen Species (ROS)
Chemiluminescence (CL) of Cu plate in a 2.5 × 10−7 m3 (0.25 mL) aqueous luminol solution with a concentration of 5.0 × 10−1 - 5.0 × 10−6 mol·m−3 (5.0 × 10−4 - 5.0 × 10−9 mol·L−1) mixed with 4.0 × 10−6 m3 (4.0 mL) carbonic acid buffer solution (NaOH/NaHCO3, pH = 10.8 - 10.9) [27] was observed under dark conditions using a CL detector (CLA-FS3, Tohoku Electronic Industrial Co., Ltd., Sendai, Japan). After dropping the luminol solution in a 6.0 × 101 s’ warming up of the detector, the intensity of CL was integrated between 6.1 × 101 - 6.0 × 102 s. The obtained summation of CL intensity for 5.4 × 102 s (9 min), SCL (9 min), which was calculated as follows: at first estimate summation of the background noise, SCL(BG), from 0 to 6.0 × 101 s, and then summarize the CL intensity from 6.1 × 101 to 6.0 × 102 s, SCL (total 9 min), finally, true SCL (9 min) was determined to be as true SCL (9 min) = SCL (total 9 min) – SCL (BG) × 9. This true SCL (9 min), in simple terms, SCL (9 min) value was utilized to evaluate the amount of ROS generated from the surface of Cu plate.
Among 4 kind of ROS, such as ·OH, H2O2,
and 1O2, scavengers, 2-propanol (“2-pro”, for ·OH, Nacalai Tesque Chemicals, Kyoto, Japan) [28] [29] [30] , sodium pyruvate [17] (“s-pyr”, for H2O2, Fuji-film Wako Pure Chemical Co., Ltd., Osaka, Japan), nitro blue tetrazolium [31] (“nbt”, for
, Nacalai Tesque Chemicals) and sodium azide [17] (for short “NaN3”, for 1O2, Fuji-film Wako Pure Chemical) were mainly used to determine which ROS is generated. At first these scavengers were solved in the buffer solution into the concentration 5.0 × 10−2 - 5.0 × 10−6 mol·L−1 and then obtained 0.25 mL or 0.50 mL scavenger solution were used. Each solution and a 4.0 mL pure buffer solution were mixed. CL measurement was performed as the same as CL intensity measurement, after a 6.0 × 101 s’ warming up of the detector, the intensity of CL was integrated between 6.0 × 101 - 6.0 × 102 s. The amount of difference (D) for each ROS was evaluated using the equation of
D = SCL (9 min, without scavenger) − SCL (9 min, with scavenger) (1)
2.2.2. Physicochemical Property
Microstructural observation with a field emission-type scanning electron microscope (FE-SEM; SU8020, Hitachi High-Technologies Co., Ltd., Tokyo, Japan) and a transmission electron microscope (TEM, JEM-2100F, JEOL, Tokyo, Japan) equipped with the energy-dispersive X-ray spectroscopy (EDS, JED-2300T, JEOL) were performed. Then, the Cu samples were cut into a small specimen suitable for TEM observation using a focused ion beam (FIB, FB2200, Hitachi High-Technologies Co., Ltd.).
Thin-film X-ray diffraction (XRD; Smartlab, Rigaku, Tokyo, Japan) analysis using CuKα radiation (wavelength of 0.15418 nm) was utilized for identification of the crystalline phases and evaluation of lattice parameter of thin-film copper oxides, Cu2O and CuO. Measuring conditions were as follows: accelerating electric voltage and current were 45 kV and 200 mA, respectively, scanning speed 1˚/min, scanning angle 2q: 20˚ - 80˚, angle of incidence 0.5˚ using a parallel X-ray beam. Under these conditions, the penetration depth of X-ray was estimated to be around 200 nm from the outermost surface [31] . Reference intensity ratio (RIR) analysis [32] was utilized to determine the mass % of Cu2O and CuO.
3. Results and Discussion
Figure 1 shows a brief diagram explaining the flowchart of experimental results.
3.1. CL Intensity of Heated Cu Plates and Their Surface Morphology
As described at 2.2.2 in 2. Experimental procedure, chemiluminescence (CL) of Cu plates was measured using the luminol solution. Figure 2 shows dependence of CL intensity of as-received Cu plate on the concentration of luminol solution. Based on the previous experimental data [11] , their concentrations were varied from 5.0 × 10−4 to 5.0 × 10−9 mol/L (M). Except for extraordinary high CL intensity curve obtained using luminol’s concentration of 5.0 × 10−4 M, the CL intensity was decreased gradually with decreasing luminol’s concentration from 5.0 × 10−5 to 5.0 × 10−9 M. By considering the suitable intensity and linearity, the aqueous luminol solutions with concentration of 5.0 × 10−5 or 5.0 × 10−6 M were utilized in the present study. Then the Cu plates were rapidly heated for 4.2 × 102 s and quenched in air to form copper thin-film oxides.
![]()
Figure 1. A brief diagram explaining the flowchart of experimental results.
![]()
Figure 2. Dependence of CL intensity of as-obtained Cu plate on the concentration of luminol solution.
Figure 3 exhibits the CL curves of these heated Cu plates and the summation of CL intensity SCL (9 min) is displayed; here the luminol’s concentration of 5.0 × 10−5 M was adopted. SCL (9 min) increased from 111.6 × 103 count, i.e., 111.6 k·count, to 736.6 k·count with increasing temperature up to 573 K and decreased rapidly to 48.5 k·count at 673 K, suggesting that SCL (9 min) depends much on the amount of copper oxides generated during the heat treatment in air and there might be the most suitable heating temperature. Luminol can emit light in pH value around 11.0 [16] , then in order to eliminate pH effect we searched the light emission reagent which can perform in pH around 7. MPEC [25] is a reagent which can perform light emission in neutral pH, however, its intensity is very weak, so we used MPEC with the higher concentration than Luminol. Figure 4 shows SCL (9 min) of Cu plates prepared under various temperatures, which were determined using luminol (the concentration and the amount of instillation, 5.0 × 10−5 M and 2.50 × 10−7 m3, 0.25 mL) and MPEC (5.0 × 10−1 M, 0.25 mL) under pH = 10.8 and 7.5 buffer solutions (4.0 mL), respectively. Both SCL (9 min) exhibited the highest value around 573 K; the best heating temperature is around 573 K. However, it was found that the top temperature sometimes shifted to the lower temperature, for example, 373 K or even room temperature, depending on the surface conditions of Cu plates as-received. Then, the surfaces of Cu plates heated at various temperatures were observed using an SEM under the magnification around 20,000 and compared. Figure 5 shows their images of various Cu plates; as-received (No. 1), and heated at 373 K (No. 2), 473 K (No. 3), 573 K (No. 4), and 673 K (No. 5) for 4.2 × 102 s in air. Among the Cu plates from as-received to 473 K, little change has been recognized, however, 573 K-heated Cu plate showed the granular surface. Furthermore, at 673 K a small amount of needle-like granular were observed. As the highest SCL (9 min) value was attained on the Cu plate heated at 573 K, the outermost surfaces of Cu plates heated at 50 K lower and higher than the top temperature (573 K) were observed using the high-resolution TEM. Figure 6 displays the cross-section images of their outermost surfaces, in the top of each photograph upper left gray portion is bulk Cu, along to the right lower direction white thin film and black mass were observed on the surface of bulk Cu. Here, the magnifications of 1) 523 K and 2) 573 K are the same (see the scale bar of 100 nm), however, the right 3) 623 K is a little lower magnification (see the scale bar of 200 nm) because of the thick oxide film of Cu2O, which will be described latter. Here we notice that the heating temperature increased from 523 to 573 K, “island-shaped, nub” film was first formed and grew along the base; “a hetero growth thin film (Cu oxide film on metal Cu)” was observed. This growth might be caused by “Volmer-Weber mode” [33] mechanism, that is, in the case that the surface tension of grown substance (Cu2O) is larger than adhesion force and poor wettability between Cu2O and Cu.
In the lower images with the higher magnification, the green line indicates the intensity of oxygen content along with a horizontal thin red line. As shown in Table 1, TEM observation gave the thickness of films about 40 to 100 nm for 1) 523 K and 2) 573 K samples, respectively, at 623 K-heated samples “non-uniform” layer. The former thickness values (40 and 100 nm) correspond well to those (40 and 100 nm) of Cu2O formed on oxygen-free (<5 ppm) Cu heated at 473 K for 25 and 100 min in air, respectively, which is reported by M. Honkanen et al. [12] . Of course in the present study the heating conditions were much different: the temperature (523 K and 573 K) was much higher, and the soaking time (7 min) was very short. M. Honkanen et al. [12] also, described that below 473 K in air Cu2O was first formed, and then above 473 K, Cu2O changed into CuO by reacting with O.
![]()
Figure 3. CL curves of Cu plates heated at various temperatures for 4.2 × 102 s in air.
![]()
Figure 4. Summation of CL of Cu plates prepared under various heating temperatures, using Luminol (5.0 × 10−5 M, 0.25 mL) and MPEC (5.0 × 10−1 M, 0.25 mL) at pH = 10.8 and 7.5 buffer solutions (4.0 mL), respectively.
![]()
Figure 5. SEM photo images of the surfaces of Cu plates heated at various temperatures.
![]()
Figure 6. TEM images of the cross-section of surfaces of Cu plates heated at various temperatures for 4.2 × 102 s in air.
![]()
Table 1. Characteristics of oxide films formed on the surface of Cu plate heated at various temperatures.
(b) Lattice parameters of Cu2O and CuO
*Thin film XRD was measured on the surface layers from the top to around 200 nm under.
By using the EDX analysis on thin films, the compositional ratios of Cu and O were determined. From the results of thin-film XRD analysis on Cu plates heated, which will be described in next Figure 7, the mass% of Cu2O and CuO and their crystallite sizes were estimated (Table 1(a)). The content of Cu2O was increased from 6.0 to 22.6 mass% gradually with increasing heating temperature up to 623 K, however, that of CuO increased suddenly from 0.40 to 6.05 mass% between 573 and 623 K. On the other hand, the crystallite size of Cu2O reduced in size from 19.9 to 9.2 nm with increasing temperature, which may be reflected by the abrupt growth of CuO between 573 and 623 K. Figure 7 shows the thin-film XRD patterns of Cu plates as-received (300 K) and heated at 473, 573, and 673 K for 4.2 × 102 s in air. XRD peaks of Cu2O and CuO are not recognized on as-received Cu plate, however, heated at 473 K only a small Cu2O peak appeared around 2q = 36.6˚, and at 573 K both Cu2O and CuO peaks are observed. Furthermore, at 673 K many Cu oxides peaks were recognized. Based on these patterns, mass% of Cu2O and CuO and crystallite sizes were estimated [32] . Table 1(b) shows the lattice parameters of cubic Cu2O and monoclinic CuO formed on the Cu plate surfaces, with reported PDF data. At-a-glance, the lattice parameter a (042874 to 0.42654 nm) and unit volume V (0.078810 to 0.077605 nm3) of Cu2O phase are larger than those of the reported PDF data (a = 0.42520 nm and V = 0.07884 nm3) and decreased gradually with increasing temperature, however, their changing degrees are very small. On the other hand, those of CuO phase except b values are almost the same with margin for error. Figure 8 displays the Cu2O and CuO contents (mass%) near the outmost surface of the Cu plates heated at various temperatures. Cu2O gradually increased from 373 K and rapidly rose at 573 K, on the other hand, CuO showed little change up to around 573 K, which corresponding to the first Cu2O formation at lower temperature than CuO on the Cu surface during heating in air. From this, temperature of 573 K might be the stage of sudden alternation, suggesting that copper oxides are active for generation of ROS.
3.2. CL Intensity of Heated Cu Plates with Scavenger
From the preliminary experiments, as we noticed that there must be the most suitable amount of each scavenger to suppress the generation of ROS, so investigation
![]()
Figure 7. Thin-film XRD patterns of Cu plates heated at various temperatures for 4.2 × 102 s in air.
![]()
Figure 8. Contents of Cu2O and CuO formed on the surface of Cu plates heated from room temperature to 673 K for 4.2 × 102 s in air.
of the relationship between SCL values and the amount of scavengers was performed. Figure 9 displays the variations of SCL as a function of the amount of various scavengers. In this figure, the amount of luminol is constant (5.0 × 10−5 M, 0.25 mL) and, for example, the amount of instillation of nbt solution with the same concentration (5.0 × 10−5 M) as luminol was changed; the bottom point for SCL is achieved around 0.50 mL, suggesting that the most suitable amount of nbt is 0.50 mL. And as the same manner, the most suitable amount of other scavengers were determined; for 2-propanol 0.25 mL, for s-pyr 0.50 mL and for NaN3 0.50 mL tentatively. In the case of the last scavenger NaN3, the 0.50 mL could reduce SCL value than that without scavenger and 1.0 mL (4 times higher mol ratio) addition might be too much.
Figure 10 summarized CL curves of as-received Cu plates added with the most suitable amounts of scavengers, using a constant amount of luminol (5.0 × 10−5 M, 0.25 mL) and the buffer solution (pH = 10.9, 5.0 mL); the values of SCL are also shown in this figure. Scavengers such as 2-propanol, nbt, s-pyr revealed the CL reduction, i.e., among ROS which is formed on the Cu plate surface, hydroxyl radial ·OH, superoxide anion ·O2 and hydrogen peroxide H2O2 were contained, however, singlet oxygen 1O2 was not recognized. From the difference (D) between SCL values measured without and with scavengers, for example, D·SCL (2-propanol) = SCL (without 111.6) – SCL (with 53.1) = 58.5 k·count might be corresponding to the amount of ·OH. These amounts of each ROS formed on the heated Cu plates will be summarized later. Figures 11-14 are the CL curves measured without and with each scavenger, using Cu plates heated at 373, 473, 573 and 673 K, respectively, for 4.2 × 102 s in air. In Figure 13 and Figure 14, the scavenger’s effect for singlet oxygen 1O2 disappeared. Here, we have to mention that each scavenger cannot reduce only one ROS, i.e., one scavenger might eliminate more than one ROS at the same time. Then, we calculate main ROS by normalizing. Figure 15 is the summation of results showing the components of ROS formed on the heated Cu plates. At 573 K the highest amount of ROS was obtained and their main ROS were H2O2,
and ·OH, however, we should consider the amount of H2O2 and ·OH are combined, because the formation of H2O2 is strongly related with the amount of ·OH due to the following equation of ·OH + ·OH = H2O2. Furthermore, even though some amounts of singlet oxygen 1O2 are recognized at both 373 and 473 K, we might ignore its formation.
![]()
Figure 9. Summation of CL, SCL, of as-received Cu plate as a function of the mol ratio of scavengers vs. luminol.
![]()
Figure 10. CL curves of as-obtained Cu plates measured using luminol and various scavengers.
![]()
Figure 11. CL curves of 373 K-heated Cu plates measured using luminol and various scavengers.
![]()
Figure 12. CL curves of 473 K-heated Cu plates measured using luminol and various scavengers.
![]()
Figure 13. CL curves of Cu plate heated at 573 K for 4.2 × 102 s in air; luminol 5.0 × 10−5 M, 0.25 mL, buffer pH = 10.95, 5.0 mL, scavengers, such as, 0.25 mL 2-pro, 0.50 mL nbt and 0.50 mL s-pyr with the same concentration of 5.0 × 10−5 M were added.
![]()
Figure 14. CL curves of Cu plate heated at 673 K for 4.2 × 102 s in air; luminol 5.0 × 10−5 M, 0.25 mL, buffer pH = 10.95, 5.0 mL, scavengers, such as, 0.25 mL 2-pro, 0.50 mL nbt and 0.50 mL s-pyr with the same concentration of 5.0 × 10−5 M were added.
Finally, the mechanism for generation of hydroxyl radical ·OH, hydrogen per oxide H2O2, and superoxide anion 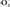 on the copper surface are proposed in Figure 16. As already described, a very fine and small amount of thin copper oxide films, Cu2O and CuO are formed on the outermost surface in air. Cu bulk plate is drawn as a rectangular shape and placed in water or air. In the case of former, water contacts with air and the latter, air contains water vapor. Based on some chemical knowledge, i.e., standard electrode potential Eo(Cu) = 0.34 V, SHE [standard hydrogen electrode]) [34] , copper’ stability in oxygen dissolved acidity or neutral aqueous solution, and a potential-pH diagram of Cu-H2O at room temperature in air [35] , Cu can be oxidized in the alkaline aqueous solution
on the copper surface are proposed in Figure 16. As already described, a very fine and small amount of thin copper oxide films, Cu2O and CuO are formed on the outermost surface in air. Cu bulk plate is drawn as a rectangular shape and placed in water or air. In the case of former, water contacts with air and the latter, air contains water vapor. Based on some chemical knowledge, i.e., standard electrode potential Eo(Cu) = 0.34 V, SHE [standard hydrogen electrode]) [34] , copper’ stability in oxygen dissolved acidity or neutral aqueous solution, and a potential-pH diagram of Cu-H2O at room temperature in air [35] , Cu can be oxidized in the alkaline aqueous solution
![]()
Figure 15. Summation of CL, SCL and components of ROS for Cu plates heated at various temperatures for 4.2 × 102 s in air.
![]()
Figure 16. Schematic diagram for “Fenton reaction” to produce “ROS, ·OH,![]() ” on the surface of Cu plate with thin copper oxides.
” on the surface of Cu plate with thin copper oxides.
with pH = 7 - 14 by dissolved oxygen O2; that is Cu --> Cu2O or CuO. However, these products are supposed to be minute quantity due to a short reaction time for CL measurement of 6.0 × 102 s at 293 K. Therefore, in Figure 16 (i) Cu in the bulk copper coelute into water at the surface; Cu --> Cu+ + e− (or Cu2+ + 2e−), (ii) O2 in air dissolves into H2O, (iii) and through the reaction (1/4·O2 + 1/2·H2O), it (OH) reacts with e− (or 2e−) at metal surface, (iv) then ·OH is formed. Furthermore, (v) two ·OH changes into H2O2 + 2e−. On the other hand, [* Cu+ and Cu2+ in (i)] reacts with [H2O2 in (v)] into (vi) hydroxyl radical ·OH or (vii) superoxide anion![]() . These (vi) and (vii) equations are called “Fenton Reaction” [13] . Here, at the first thin-film layer Cu2O and the second layer CuO formed on the outermost surface of bulk Cu, as presented in upper side of Figure 16, (2Cu+ + 1/2·O2 + 2e−) and (Cu2+ +1/2·O2 + 2e−) are produced, respectively. These Cu+ and Cu2+ react with H2O2, as presented by the equation (vi) and (vii); “Fenton Reaction” [13] takes place. It should be noted that these reactions tend to take place at “active step” of the edge place near islands of Cu2O/CuO, not continuous thin-film.
. These (vi) and (vii) equations are called “Fenton Reaction” [13] . Here, at the first thin-film layer Cu2O and the second layer CuO formed on the outermost surface of bulk Cu, as presented in upper side of Figure 16, (2Cu+ + 1/2·O2 + 2e−) and (Cu2+ +1/2·O2 + 2e−) are produced, respectively. These Cu+ and Cu2+ react with H2O2, as presented by the equation (vi) and (vii); “Fenton Reaction” [13] takes place. It should be noted that these reactions tend to take place at “active step” of the edge place near islands of Cu2O/CuO, not continuous thin-film.
4. Conclusion
By focusing on generation of reactive oxygen species (ROS) on the copper surface, the antibacterial activity of copper has been tried to explain. Especially, the change in ROS formed on the heated Cu plate was investigated: hydroxyl radical ·OH, hydrogen per oxide H2O2, and superoxide anion ![]() are the most of ROS. These data concerning about the components of ROS formed on the surface of heated Cu is reported for the first time using Chemiluminescence with the combination of luminol and scavengers. In addition, the generation mechanism of these three ROS on the outermost surface consisting of thin film Cu2O, CuO layer and bulk Cu also is proposed.
are the most of ROS. These data concerning about the components of ROS formed on the surface of heated Cu is reported for the first time using Chemiluminescence with the combination of luminol and scavengers. In addition, the generation mechanism of these three ROS on the outermost surface consisting of thin film Cu2O, CuO layer and bulk Cu also is proposed.
Acknowledgements
The authors thank Ms. M. Toda of the Doshisha University Research Centre for Interfacial Phenomena, for FE-SEM and TEM observations of the samples.