Precise Synthesis of Acrylate Copolymer via RAFT Polymerization and Its Application ()
1. Introduction
Natural polymers form a great variety of self-assembled structures that are essential to their function. Extensive theoretical and experimental investigations have suggested that, beside the overall copolymer composition, the distribution of monomer units along polymer chains can be an important microstructural parameter for fine-tuning nano-morphologies, and thus the physical and functional properties of polymeric materials [1] [2]. The class of copolymers named random copolymer, block copolymer, and gradient copolymer. Among of them, block copolymer and gradient copolymer were difficult to synthesize using traditional radical polymerization. In recent years, with the development of controlled/living polymerization such as atom transfer radical polymerization (ATRP) [3] and reversible addition-fragmentation chain-transfer (RAFT), different sequence structure can be easily synthesized. In particular, RAFT polymerization enables the controlled polymerization of a wide range of functional monomers to form well-defined copolymers [4]. The acrylate copolymers with different sequence structure have different crystallization, thermal properties, mechanical properties and rheological properties. The different structural arrangement and the different movement modes of molecular chains, which also fully shows that the difference between properties is caused by the arrangement form of molecular chain segments and molecular movement [5] [6].
Acrylate copolymers containing a long-chain alkyl group such as stearyl acrylate (SA) and hydroxyethyl acrylate (HEA) are of great importance due to their potential applications as coating, water-proofing agent, and dispersant [7] [8] [9]. However, it is difficult to investigate the polymer morphology and distribution of polyester fabric, and structure-activity relationship between polymer structure and application properties.
Herein, we prepared random copolymer, block copolymer, and gradient copolymers with different sequence structure containing SA and HEA monomers using RAFT polymerization, as shown in Scheme 1 and Scheme 2. First, we prepared the targeted copolymers with controlling the same composition and ratio of monomers. Then, the chemical structure of the targeted copolymers were characterized by Fourier Transform Infra-Red (FT-IR) and Gel Permeation Chromatography (GPC). The crystallization property and aggregation structure of the targeted copolymers were investigated DSC and SAXS. Final, the water repellency of targeted copolymers were measured, which can be used to explain the internal relationship between structure and property and provide theoretical basis for practical production and application.
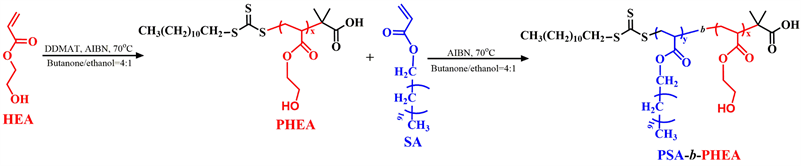
Scheme 1. Synthetic route of PHEA-b-PSA block copolymer.
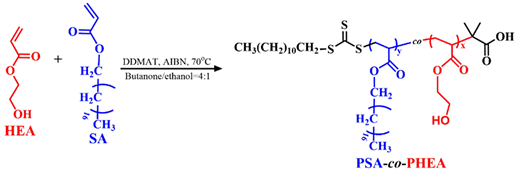
Scheme 2. Synthetic route of PHEA-co-PSA block copolymer.
2. Experimental
2.1. Materials
The following agents were purchased and used without further purification: Stearyl acrylate (SA, 99%, Sigma-Aldrich), hydroxyethyl acrylate (HEA, 98%, Alfa Aesar), 2-(dodecylthiocarbonothioylthio)-2-methylpropionic acid (DDMAT, 98%, Sigma-Aldrich). Azodiisobutyronitrile (AIBN, A. R., Enox) was purified by recrystallization from ethanol.
2.2. Characterizations
The number-average molecular weight (M̅n) and polydispersity index (Ð) of the targeted copolymers were analyzed with a gel permeation chromatography (GPC) instrument (Agilent 1100) using polystyrene as the standard and THF as the eluent. Differential scanning calorimetry was performed by a Perkin Elmer thermal analysis system equipped with a liquid-N2 low-temperature apparatus. The sample masses varied from 5.0 to 9.0 mg were used for the DSC measurements, and all DSC experiments were carried out under a N2 atmosphere. The heating rate was 10˚C/min [10] [11]. Small angle X-ray scattering (SAXS, Shanghai Synchrotron Radiation Facility, BL16B1) with a wavelength of 1.24 Å, a sample-detector distance of 1860 mm and an exposed time of 300 s [12].
2.3. Synthesis of PHEA-b-PSA Block Copolymer
The PHEA-b-PSA copolymer was synthesized via RAFT polymerization. All magnetic stirring bars and glass ware used in the experiments were dried at 120˚C for 24 h and cooled under vacuum to eliminate the moisture before use. Briefly, DDMAT (100 mg, 0.274 mmol), HEA (1.4 g, 12.1 mmol), and azobis(isobutyronitrile) (AIBN, 22.5 mg, 0.137 mmol) were added into the flask together with 10 mL of butanone/ethanol (v/v = 4/1). After being degassed through three exhausting-refilling nitrogen cycles, the mixture was stirred under a nitrogen atmosphere at 70˚C for 4 h. Then, 10 mL of butanone/ethanol (v/v = 4/1) containing SA (4.2 g, 12.9 mmol) was added into the flask, and keep 70˚C for 4 h under a nitrogen atmosphere. The flask was cooled to terminate the polymerization, the targeted copolymers was purified by removing the unreacted monomers. The final product PHEA-b-PSA copolymer was obtained by lyophilization method (Yield: 95.6%).
2.4. Synthesis of P(HEA-co-SA) Random Copolymer
RAFT polymerization and the random copolymerization of HEA and SA were carried out in a round-bottomed flask with a silicone septum under an argon atmosphere. Briefly, DDMAT (100 mg, 0.274 mmol), HEA (1.4 g, 12.1 mmol), SA (4.2 g, 12.9 mmol) and azobis(isobutyronitrile) (AIBN, 22.5 mg, 0.137 mmol) were added into the flask together with 20 mL of butanone/ethanol (v/v=4/1). After being degassed through three exhausting-refilling nitrogen cycles, the mixture was stirred under a nitrogen atmosphere at 70˚C for 4 h. The flask was cooled to terminate the polymerization, the targeted copolymers was purified by removing the unreacted monomers. The final product P (HEA-co-SA) random copolymer was obtained by lyophilization method. (Yield: 96.4%)
2.5. Synthesis of P (HEA-grad-SA) Gradient Copolymer
The P (HEA-grad-SA) gradient copolymer was synthesized via RAFT polymerization. All magnetic stirring bars and glass ware used in the experiments were dried at 120˚C for 24 h and cooled under vacuum to eliminate the moisture before use. Briefly, DDMAT (100 mg, 0.274 mmol), HEA (0.2 g, 1.7 mmol), SA (2.4 g, 7.4 mmol) and azobis(isobutyronitrile) (AIBN, 22.5 mg, 0.137 mmol) were added into the flask together with 10 mL of butanone/ethanol (v/v = 4/1). After being degassed through three exhausting-refilling nitrogen cycles, the mixture was stirred under a nitrogen atmosphere at 70˚C for 4 h. Then, 10 mL butanone/ethanol of HEA (0.4 g, 3.4 mmol) and SA (1.2 g, 3.7 mmol) were added into the flask under a nitrogen atmosphere at 70˚C for 4 h. Finally, 10 mL butanone/ethanol of HEA (0.8 g, 6.8 mmol) and SA (0.6 g, 1.85 mmol) were added into the flask and stirred 4 h at 70˚C. The flask was cooled to terminate the polymerization, the targeted copolymers was purified by removing the unreacted monomers. The final product P(HEA-grad-SA) gradient copolymer was obtained. (Yield: 97.4%)
3. Results and Discussion
We synthesized PHEA-b-PSA, PHEA-b-PSA-b-PHEA, PSA-b-P b HEA-b-PSA, P (HEA-co-SA), and P (HEA-grad-SA) via RAFT polymerization. The schematic diagram of targeted copolymers with different sequence structure are shown in Scheme 3.
As shown in Figure 1, the chemical structure of PHEA-b-PSA, PHEA-b-PSA-b-PHEA, PSA-b-PHEA-b-PSA, P (HEA-co-SA), and P(HEA-grad-SA)
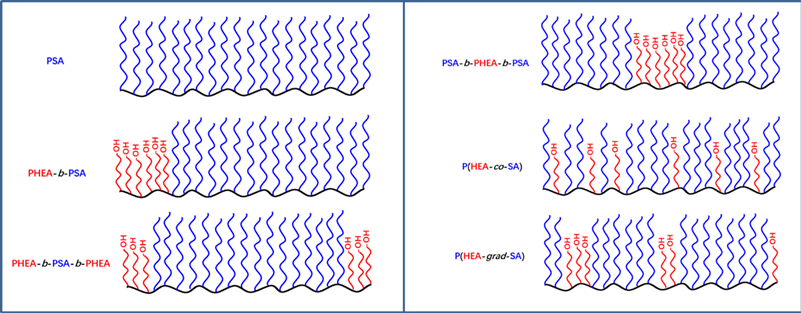
Scheme 3. The schematic diagram of targeted copolymers with different sequence structure. Abbreviated as PSA, PHEA-b-PSA, PHEA-b-PSA-b-PHEA, PSA-b-PHEA-b-PSA, P(HEA-co-SA), and P(HEA-grad-SA).
copolymers were characterized by FT-IR, which can demonstrate the characteristic peaks of the targeted copolymers, 3300 cm−1, 2800 cm−1, and 1750 cm−1 are the stretching vibration peaks of -OH, -CH, and -C=O in PHEA-b-PSA, PHEA-b-PSA-b-PHEA, PSA-b-PHEA-b-PSA, P(HEA-co-SA), and P (HEA-grad-SA) copolymers. We find that the targeted copolymers with different sequence structure have same characteristic absorption peak, this is to say, the differences of chemical structure could not be verified by FT-IR method.
Moreover, the molecular weight and molecular weight distribution of the different sequence structures were determined by GPC measurement in. The GPC trace of PSA (A), PHEA-b-PSA (B), PHEA-b-PSA-b-PHEA (C), PSA-b-PHEA-b-PSA (D), P(HEA-co-SA) (E), and P(HEA-grad-SA) (F) copolymer in Figure 2 exhibited a unimodal peak, this means that random copolymers, block copolymers and gradient copolymers have been successfully prepared.
Moreover, recorded DSC curves of PSA, PHEA-b-PSA, PHEA-b-PSA-b-PHEA, PSA-b-PHEA-b-PSA, P (HEA-co-SA), and P(HEA-grad-SA) copolymers are presented in Figure 3. The crystallization have difference due to different SA distribution of the random copolymers, block copolymers, and gradient copolymers, we find that the crystallization temperatures of different sequence structure are 49.6˚C, 39.5˚C, 47˚C, 37.3˚C, 48.3˚C, and 45.9˚C, respectively. The more concentrated the SA segment distribution in the copolymer, the crystallization temperature increases. Hence, we can control the crystallization of copolymers through changing the degree of aggregation of crystalline fragments.
Water repellency of polyester fabric was evaluated according to AATCC 22-2010 “Water Repellency: Spray Test”, as shown in Table 1. L0 represents the initial waterproof score and L5 represents the waterproof score after washing for five times. We find that the different sequence structure with same molecular weight possess the different waterproof score, the water repellency of PSA,
![]()
Figure 1. FT-IR spectra of PHEA-b-PSA, PHEA-b-PSA-b-PHEA, PSA-b-PHEA-b-PSA, P (HEA-co-SA), and P (HEA-grad-SA) copolymer.
![]()
Figure 2. GPC traces curves of PSA (A), PHEA-b-PSA (B), PHEA-b-PSA-b-PHEA (C), PSA-b-PHEA-b-PSA (D), P(HEA-co-SA) (E), and P(HEA-grad-SA) (F) copolymer.
![]()
Figure 3. DSC scans for PSA, PHEA-b-PSA, PHEA-b-PSA-b-PHEA, PSA-b-PHEA-b-PSA, P (HEA-co-SA), and P (HEA-grad-SA) copolymer.
![]()
Table 1. Water repellency of PHEA-b-PSA, PHEA-b-PSA-b-PHEA, PSA-b-PHEA-b-PSA, P(HEA-co-SA), and P(HEA-grad-SA), and PSA.
![]()
Figure 4. SEM images of PHEA-b-PSA, PHEA-b-PSA-b-PHEA, PSA-b-PHEA-b-PSA, P (HEA-co-SA), and P (HEA-grad-SA) copolymer coating polyester fabric. ((A), (B), (C), (D), and (E) represent the absence of a cross-linker. (A’), (B’), (C’), (D’), and (E’) represent the addition of a cross-linker).
PHEA-b-PSA, PHEA-b-PSA-b-PHEA, PSA-b-PHEA-b-PSA, P(HEA-co-SA), and P(HEA-grad-SA) copolymers could be improved with adding the cross-linked agents when the different copolymers finishing on polyester fabrics were performed. Among of them, the PHEA-b-PSA-b-PHEA have better water repellency. This is due to the HEA segments of PHEA-b-PSA-b-PHEA not only could act as a cross-linking role, but also fix the SA segment to come true the excellent washing resistance.
SEM images of the different copolymers coated polyester fabric were illustrated in Figure 4. Among of them, we find that the fabric with no cross-linked agents showed cylindrical structure and small amounts of copolymers aggregation in Figures 4(A)-(E). After the coated polyester fabric with adding the cross-linked agents, an evidently uneven film was markedly generated on the surface of the polyester fiber in Figures 4(A’)-(E’). The PHEA-b-PSA-b-PHEA block copolymers coated polyester fabric possess uniform circular distribution at high baking temperature, which confirmed that PHEA-b-PSA-b-PHEA had been successfully finished onto the surface of polyester fabric.
As we all know, the intensity of SAXS is dependent on the electron density difference between the phases of the system. Lorentz-corrected intensity Iq2 is a function of the scatter vector q under different shear temperatures along and perpendicular to the shear direction. From Figures 5(A)-(C), we can see that two scattering peaks appear in the block copolymers. As shown Figure 5(D) and Figure 5(E), the one peaks appear in the random and gradient copolymers, which indicate that the different copolymers have a great difference in the electron density of the system, this is due to the segmentation degree and crystallization of SA chain.
4. Conclusion
The random copolymer, block copolymer, and gradient copolymers with different
![]()
Figure 5. Iq2-q curves collected by SAXS and 2D SAXS patterns of PHEA-b-PSA ((A) and (A’)), PHEA-b-PSA-b-PHEA ((B) and (B’)), PSA-b-PHEA-b-PSA ((C) and (C’)), P(HEA-co-SA) ((D) and (D’)), and P(HEA-grad-SA) ((E) and (E’)).
sequence structure containing SA and HEA monomers have been synthesized via RAFT polymerization. The molecular weight and molecular weight distribution of the different copolymers have verified. The crystallization temperatures of different sequence structure are 49.6˚C, 39.5˚C, 47˚C, 37.3˚C, 48.3˚C, and 45.9˚C, respectively. What is more important, the PHEA-b-PSA-b-PHEA possess good water repellency due to the uniform circular distribution at high baking temperature with adding the cross-linked agents. Moreover, the different copolymers have difference in the electron density due to the segmentation degree and crystallization of SA chain.
Acknowledgements
Dr. Lei Li would like to thank the financial support from the Postdoctoral Research Program of Zhejiang Province (ZJ2020058). Dr. Lei Li thanks Ms. Shuhui Ding for her help in revising the manuscript.