New Hemisynthetic Oleanane Saponin with Antimicrobial Activities ()
1. Introduction
Saponins are natural compounds that widely occur in the plant kingdom and they are constituents of more than 100 family plants including endophytic fungi of terrestrial and marine origin. They are divided into two groups namely steroidal and triterpenoid saponins [1]. Their chemical structures are composed of a fat-soluble nucleus (aglycone) that is either a triterpenoid (C-30) or neutral or alkaloid steroid (C-27) attached to one or more side chains of water-soluble sugars (glycone) through ester or ether linkages to the aglycone nucleus at different carbon sites [2]. Based on the number of sugar chains attached to aglycone, saponins are categorized into mono, di and tridesmosidic. In monodesmosidic saponins, sugar chain is usually attached to C-3 and in bidesmosidic saponins, there are usually attached firstly to the position C-3 and either at position C-28 (Triterpenoid saponins) through an ester linkage or at C-26 (Frustanol saponins) through an ether linkage. D-glucose (Glc), D-glucuronic acid (GlcA), D-galactose (Gal), D-galacturonic acid (GalA), D-xylose (Xyl), D-fucose (Fuc), L-rhamnose (Rha) and L-arabinose (Ara), are the most common monosaccharides attached to aglycone. The nature and the functional groups on the aglycone moiety as well as nature and number of sugars can vary greatly, resulting in diverse group of saponins [2] [3]. Many biological activities of saponins have been reported such as antibacterial, antifungal, antiviral, insecticidal, molluscicidal, anti-inflammatory, anti-ulcer, haemolytic and hepatoprotective activities [4] - [10]. The structural complexity in the saponins reflected in their diversity of physicochemical, pharmacological and biological properties and led to the saponins as commercially important with wide variety of applications in food, cosmetics and pharmaceutical sectors [11]. Consider the variety of applications of saponins, chemical transformations could be carried out to enhance their biological activities [12]. The acetylation and methylation reactions carried out by Penders and Delaude, 1994 [13] on six oleanane saponins isolated from Melanthera scadens let to six hemisynthetic derivatives. However, the evaluation of their biological activities has not been realized. In the aims to determine the influence of these types of reactions on the antimicrobial activities of oleanane saponins, we decided to perform acetylation and methylation reactions on 3-O-β-D-glucopyranosyl-(1→2)-β-D-glucuronopyranosyl oleanolic acid previously isolated from Melanthera elliptica [10] and evaluate the antimicrobial activities of substrate and reaction product against pathogenic phenotypes selected on the basis of their relevance as human pathogens.
2. Material and Methods
2.1. Extraction, Isolation and Characterization of Substrate
Extraction, isolation and characterization of 3-O-β-D-glucopyranosyl-(1→2)-β-D-glucu-ronopyranosyl oleanolic acid (1) were performed by a method previously described by Tagousop et al. 2018 [10].
2.2. Hemisynthesis and Purification
The reaction was carried out using the method previously described by Penders and Delaude, 1994 [13] with some modifications (Scheme 1). In fact, in a 100 mL flask containing 50 mg (0.06 mmol) of substrate (compound 1), 25 mL of diazomethane and 25 mL of the pyridine-acetic anhydride mixture (1:1, v/v) was introduced. The flask was kept at ambient temperature (25˚C) with magnetic stirring and the reaction was monitored on a TLC plate. After 24 h, distilled water (25 mL) was added and the mixture was introduced into a separating funnel and 100 mL of EtOAc was added. After separation of the two phases, organic phase was dried on a rotary evaporator under reduced pressure to give 79 mg of residue. Purification on silica gel (0.063 - 0.200 mm) column chromatography (10 × 200 mm) of this residue using an isocratic elution with the mixture hexane-EtOAc (2:8, v/v) yielded 54.89 mg (85.6%) of compound 2.
2.3. Chromatographic Methods
Silica gel 60 Merck, 70 - 230 mesh was used to perform column chromatography while pre-coated silica gel 60 F254 (Merck) plates, were used to perform thin layer chromatography. The spots were visualized by spraying with 10% H2SO4 and heating at 100˚C for 2 min.
2.4. NMR Analysis
The 1D (1H and 13C-NMR) and 2D (COSY, NOESY, HSQC and HMBC) spectra were performed in deuterated solvents (DMSO-d6) on Bruker Avance III 600 spectrometer at 600 MHz/150 MHz. All chemical shifts (δ) were given in parts per million (ppm) with reference to tetramethylsilane (TMS) as internal standard and the coupling constants (J) are in Hz.
2.5. Spectrometric Analysis
The mass spectra (HR-TOFESIMS) were carried out on Micromass Q-TOF micro instrument (Manchester, UK). Samples were introduced by direct infusion in a solution of MeOH at a rate of 5 μL/min.
2.6. Microorganisms
The microorganisms used to determine the antimicrobial activities consisted of
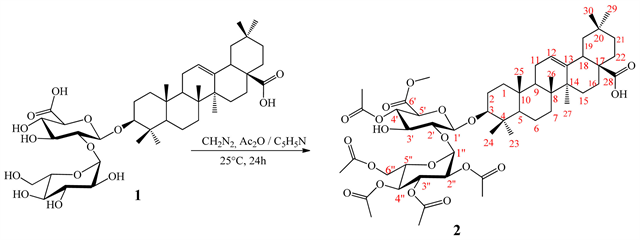
Scheme 1. General procedures used for semi-synthesis of compound 2.
three strains of bacteria (Escherichia coli S2, Staphylococcus aureus ATCC 25923 and Shigella flexneri SDINT) and three yeast strains (Candida tropicalis, Candida albicans ATCC 9002 and Cryptococcus neoformans IP 95,026) collected from the Research Unit of Microbiology and Antimicrobial Substances of the University of Dschang and provided from the University of Kolkata in India (for bacterial strains) and the “Institut Pasteur de Paris”, France (for fungal strains). These bacterial and fungal species were grown at 37˚C and maintained on nutrient agar (NA, Conda, Madrid, Spain) and Sabouraud Dextrose Agar (SDA, Conda) slants respectively.
2.7. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Microbicidal Concentration (MMC)
The minimum inhibitory concentration (MIC) values were determined using the broth microdilution method previously described by Clinical and Laboratory Sandards Institute (1997; 2009) [14] [15] with some modifications in terms of final concentration of samples and solutions of inocula. In fact, each sample was dissolved in dimethylsulfoxide diluted to 10% (v/v). The solution was then added to Mueller-Hinton Broth (MHB) for bacteria or Sabouraud Dextrose Broth (SDB) for yeasts to give a final concentration of 8192 μg/mL (instead of 4000 μg/mL). This was serially diluted to a concentration range of 0.125 to 4096 μg/mL. 100 μL of each concentration were subsequently added to each well (96-well microplate) containing 95 μL of MHB or SDB and 5 μL of inoculum for final concentrations ranging from 0.0625 to 2048 μg/mL. The inoculum was standardized to 2.5 × 105 cells/mL for yeast and 106 CFU/mL for bacteria (instead of 1.5 × 106 CFU/mL) using a JENWAY 6105 UV/Vis spectrophotometer. The final concentration of DMSO in each well was <1% (preliminary analyzes with 1% (v/v) DMSO did not inhibit the growth of the test organisms). The negative control well consisted of 195 μL of MHB or SDB and 5 μL of the standard inoculum. The plates were covered with sterile lids and then shaken to mix the contents of the wells with a plate shaker and incubated at 37˚C for 24 h (for bacteria) or 48 h (for yeast). The MIC values of the samples were determined by adding 50 μL of a solution of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) at 0.2 mg/mL followed by incubation at 37˚C for 30 minutes. This test is based on enzymatic reduction of the lightly colored tetrazolium salt to its formazan of intense purple-blue color. MIC values were defined as the lowest sample concentrations that prevented this color change indicating complete inhibition of microbial growth. For determination of the MMC values, a portion of liquid (5 μL) was taken from each well which showed no growth of microorganism on Mueller Hinton Agar or SDA and incubated at 37˚C for 24 h (for bacteria) or 37˚C for 48 h (for yeasts). The lowest concentrations that gave no growth after this subculture were taken as MMC values. Vancomycin (Sigma-Aldrich, Steinheim, Germany) and Fluconazole (Merck, Darmstadt, Germany) were used as positive controls for bacteria and yeasts respectively.
3. Results and Discussion
The acetylation and methylation reactions carried out on 3-O-β-D-glucopyranosyl-(1→2)-β-D-glucuronopyranosyl oleanolic acid (1) using pyridine-acetic anhydride mixture and diazomethane, led to a previously undescribe hemisynthetic derivative (2). The yield of synthesis (85.6%) suggests a successful conversion of compound 1 to compound 2. However, the loss of 14.4% was probably due to the purification process. The structure of compound 2 was established by interpretation of spectroscopic and spectrometric data followed by a comparison with those of the substrate (1). In fact, it was obtained as white powder which reacted positively with Liebermann-burchard and Molisch reagents. Its positive HR-TOFESIMS spectrum exhibited a sodium adduct peak at m/z 1041.5044 [M + Na]+ (calcd. for C53H78O19Na 1014.5035) corresponding to the molecular formula C53H78O19. Comparison of its 1H and 13C NMR data with those of compound 1 (Table 1) indicated the oleanane nature of aglycone moiety. However, the main difference was observed in the sugar moiety. Indeed, in addition to the signal of sugar we have on the 1H NMR spectrum (Table 2), the signals at δH: 1.98 (H-3''-OAc, s), 2.02 (H-4''-OAc, s), 2.06 (H-2''/6''-OAc, s), 2, 08 (H-4'-OAc, s) attributed to the methyl proton of acetyl groups and a signal at δH 3.70 (6'-OCH3, s) assigned to a proton of methoxy group. These informations were confirmed on its 13C NMR spectrum (Table 2) in which we observe the signals of five acetyl groups at δC: [170.5 (CO), 19.3 (CH3)], [170.0 (CO), 19.3 (CH3)], [170.2 (CO), 19.2 (CH3)], [169.9 (CO); 19.2 (CH3)], [170.9 (CO); 19.4 (CH3)], and a signal of methoxy group at δC 51, 6 (CH3).
Positions of the five acetyl groups were determined using HMBC spectrum through the correlations observed between the protons of sugars at δH: 4.82 (H-4'), 4.88 (H-2''), 5.24 (H-3''), 5.00 (H-4''), 4.30 (H-6'') and the carbonyls of acetyl groups at δC: 170.5, 170.0, 170.2, 169.9, 170.9 respectively (Scheme 2).
In addition to these correlations we observed another between proton at δH 3.70 (6'-OCH3) and carbonyl of glucuronic acid at δC 168.8 confirming the methylation of carboxylic function. Thus, the structure of compound 2 was established as: 4',2'',3'',4'',6''-penta-O-acetyl-6'-O-methyl-3-O-β-D-glucopyranosyl-(1→2)-β-D-glucuronopyranosyl oleanolic acid trivially named acetomethoxysaponin whose formation mechanism are proposed in Scheme 3 and Scheme 4.
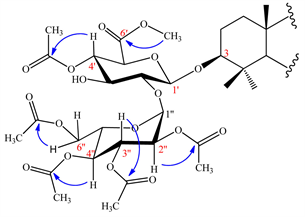
Scheme 2. Key HMBC of sugar moiety of compound 2.
![]()
Table 1. 1H RMN (DMSO-d6, 600 MHz) and 13C NMR (DMSO-d6, 150 MHz) data of aglycon moiety of compounds 1 and 2.
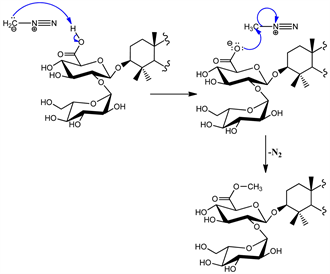
Scheme 3. Methylation mechanism of compound 2.
![]()
Table 2. 1H RMN (DMSO-d6, 600 MHz) and 13C NMR (DMSO-d6, 150 MHz) data of sugar moiety of compounds 1 and 2.
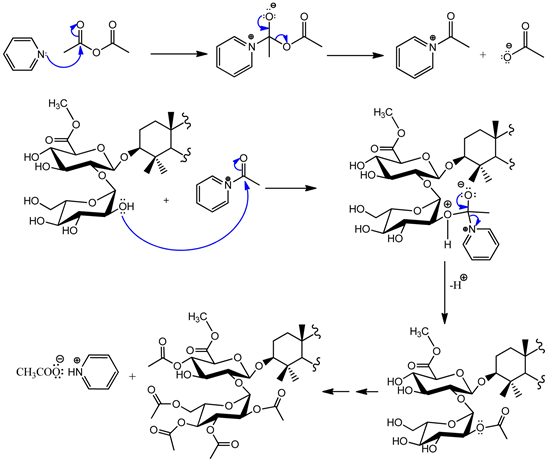
Scheme 4. Acetylation mechanism of compound 2.
After structural elucidation of hemisynthetic saponin, the antimicrobial activities of substrate and reaction product were carried out against three strains of bacteria (Escherichia coli S2, Staphylococcus aureus ATCC 25,923 and Shigella flexneri SDINT) and three yeast strains (Candida tropicalis, Candida albicans ATCC 9002 and Cryptococcus neoformans IP 95,026) selected on the basis of their relevance as human pathogens. The results are presented in Table 3.
In general, bacterial and fungal strains were more susceptible to the effects of the reaction product compared to the substrate. In fact, reaction product presented the lowest values of MIC againt E. coli (MIC = 16 μg/mL), S. aureus (MIC = 8 μg/mL), C. tropicalis (MIC = 16 μg/mL) C. albicans (MIC = 8 μg/mL) compare to the substrate against the same microbial strains: E. coli (MIC = 32 μg/mL), S. aureus (MIC = 16 μg/mL), C. tropicalis (MIC = 32 μg/mL), C. albicans (MIC = 16 μg/mL) whereas the two samples presented the same MIC values (MIC = 16 and 8 μg/mL) respectively against S. flexneri and C. neoformans. The antibacterial activity of compound 2 against E. coli and S. flexneri was less or equal to those of vancomycin (MIC = 16 - 32 μg/mL) used as reference antibiotic. The results of the present study showed that, the antimicrobial activities of compounds 1 and 2 varied with the bacterial and fungal strains. These variations may be due to genetic differences between the tested microorganisms. Furthermore, According to Li et al., 2009 [16], environmental conditions such as pH have a great influence on antimicrobial activities of saponins. Acetylation and methylation reactions of hydroxyl group carried out in this study contribute to modify the pH of the final solution containing compound 2. This difference of
![]()
Table 3. Antimicrobial activity (MIC and MMC in μg/mL) of substrate and reaction product as well as antimicrobial reference drugs.
MIC: Minimal Inhibitory Concentration; MMC Minimal Microbicide Concentration; *: Fluconazole for yeasts and Vancomycin for bacteria.
pH due to the chemical transformations would explain the difference observed at the level of the antimicrobial activities of the two compounds against the microbial strains tested. These results could also be explained in terms of antimicrobial action mechanism of saponin. According to Dourmashkin et al. (1962), [17] the mechanism of antimicrobial action of saponins is based on perturbation of membrane cell by the formation of pores. Based on this observation, Bangham and Horne (1962) [18] and Glauert et al. (1962) [19], concurrently reported that, the presence of cholesterol on the target membrane is essential for the saponins to induce pore formation. According to their reports, saponins and cholesterol associated spontaneously into a micelle-like complex and the hydrophilic sugar moieties are thought to be located in the central of the complex and leads to the development of aqueous pores. Such pores can increase the permeability of membrane and enabling the macromolecules and ions to pass through the membrane bilayer. The results of these studies suggest that, the acetyl and methyl groups have great influence on the spontaneous association between saponin and cholesterol and consequently increases the antimicrobial activities of reaction product.
4. Conclusion
The overall results of the present study indicate that, the new hemisynthetic saponin obtain after acetylation and methylation reactions are most active than the substrate against the tested microorganisms. Given the results, acetylation and methylation reactions followed by evaluation of antimicrobial activities have to be performed on a wide range of oleanane saponins.
Acknowledgements
The authors are grateful to the “Service Commun d’Analyses” and “Groupe Isolement et Structure”, of “Institut de Chimie Moléculaire de Reims” for the spectroscopic and spectrometric analysis on the ESIMS and NMR equipement of the PlAnet Platform.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.