Paper Menu >>
Journal Menu >>
 Keywords. Allometry; autotomy; chela display; cheliped; claw; handedness; regeneration Decapod crustacean chelipeds: an overview PITCHAIMUTHU MARIAPPAN, CHELLAM BALASUNDARAM and BARBARA SCHMITZ The structure, growth, differentiation and function of crustacean chelipeds are reviewed. In many decapod crusta- ceans growth of chelae is isometric with allometry level reaching unity till the puberty moult. Afterwards the same trend continues in females, while in males there is a marked spurt in the level of allometry accompanied by a sud- den increase in the relative size of chelae. Subsequently they are differentiated morphologically into crusher and cutter making them heterochelous and sexually dimorphic. Of the two, the major chela is used during agonistic encounters while the minor is used for prey capture and grooming. Various biotic and abiotic factors exert a negative effect on cheliped growth. The dimorphic growth pattern of chelae can be adversely affected by factors such as parasitic infection and substrate conditions. Display patterns of chelipeds have an important role in agonistic and aggressive interactions. Of the five pairs of pereiopods, the chelae are versatile organs of offence and defence which also make them the most vulnerable for autotomy. Regeneration of the autotomized chelipeds imposes an additional energy demand called “regeneration load” on the incumbent, altering energy allocation for somatic and/or reproductive processes. Partial withdrawal of chelae leading to incomplete exuvia- tion is reported for the first time in the laboratory and field in Macrobrachium species. 1. General morphology Chelipeds of decapod crustaceans have attracted human curiosity and fired human imagination since Aristotle (Hopkins 1993) probably because they figure so promi- nently both in structure and function in the life of these animals. Crustaceans are mostly aquatic arthropods which breathe through gills, have two pairs of antennae, and numerous paired appendages on thorax and abdomen (Stebbing 1893; Schmitt 1965) that are grouped into cepha- lic, thoracic and abdominal appendages in relation to the body tagmata. The cephalic and thoracic regions are usu- ally fused to form a cephalothorax and the appendages are known as cephalo-thoracic appendages. Decapod append- ages are the best example of serial homology with a serial modification in basic structure from the first to the last walking leg (Wood and Wood 1932). With the exception of the antennules, which are uniramous, other appendages are basically biramous and possess a basal segmented protopod with a coxa and basis and may have lateral (exites) or medial (endites) protrusions (Manton 1977; McLaughlin 1982). From the protopod arise the exopod and endopod. Of the two, the latter has undergone a variety of specialisations resulting in its transformation for vari- ous functions like sensory reception, feeding, walking, burrowing and swimming while the exopod is drastically reduced or may even be lost. This has further been facili- tated by mineralisation of the exoskeleton endowing rigi- dity and support to the appendages which are made flexible by the arthrodial membrane. All decapods usually have five pairs of well developed walking legs with exceptions in the sergistid family of the Dendrobranchiata, many of the Anomura, and a few Brachyura. In these animals the fifth or fourth and fifth pair of pereiopods are reduced in size for special func- tions. Occasionally both pairs may be vestigial or absent (McLaughlin 1982). Structural modifications of decapod appendages due to diversified functions and life style have been described in different groups (Tiegs and Manton 1958; Kaestner 1970; Schram 1978). The major modifications Journal of Biophysical Chemistry, 2009, 1, 1-13 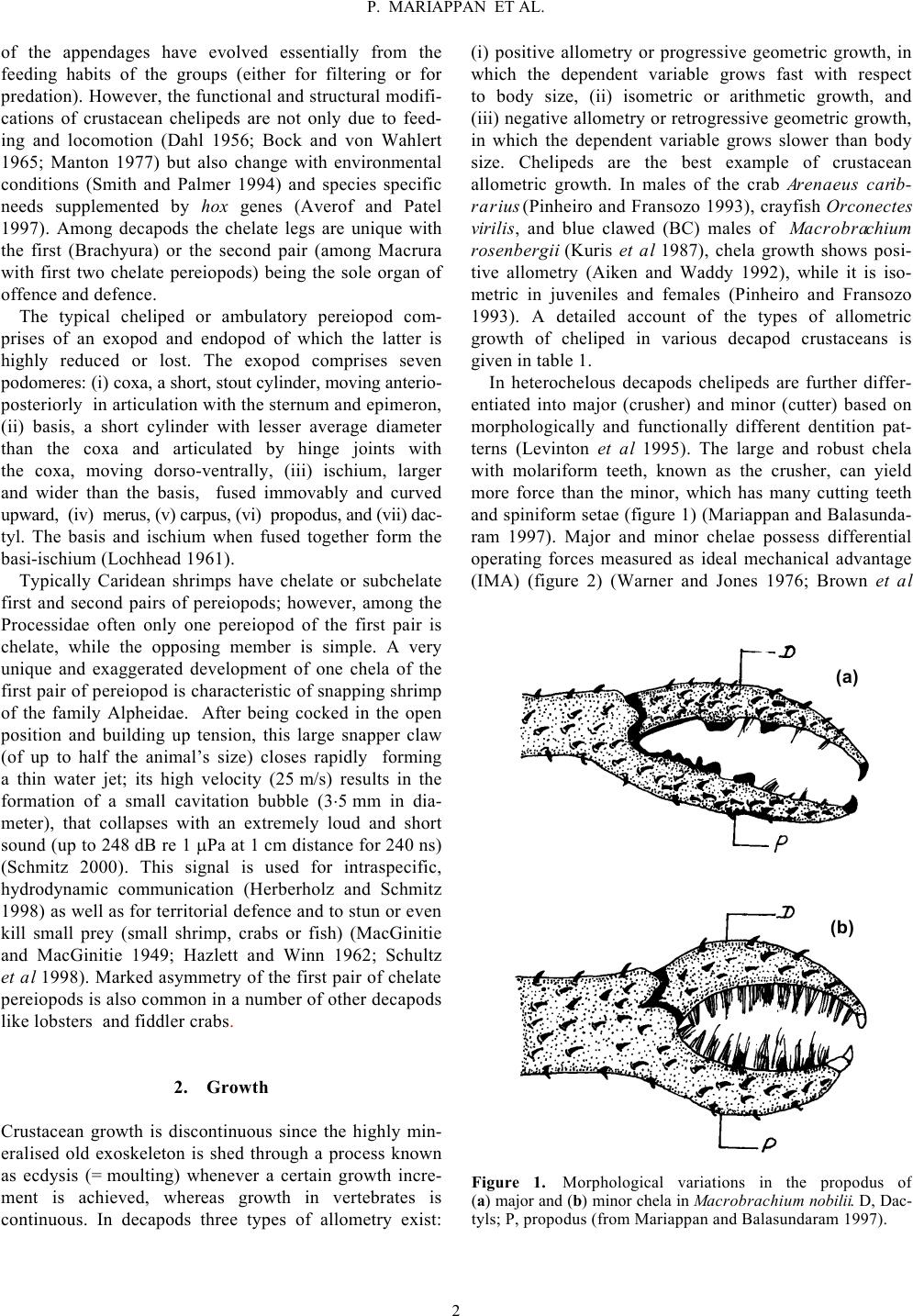 of the appendages have evolved essentially from the feeding habits of the groups (either for filtering or for predation). However, the functional and structural modifi- cations of crustacean chelipeds are not only due to feed- ing and locomotion (Dahl 1956; Bock and von Wahlert 1965; Manton 1977) but also change with environmental conditions (Smith and Palmer 1994) and species specific needs supplemented by hox genes (Averof and Patel 1997). Among decapods the chelate legs are unique with the first (Brachyura) or the second pair (among Macrura with first two chelate pereiopods) being the sole organ of offence and defence. The typical cheliped or ambulatory pereiopod com- prises of an exopod and endopod of which the latter is highly reduced or lost. The exopod comprises seven podomeres: (i) coxa, a short, stout cylinder, moving anterio- posteriorly in articulation with the sternum and epimeron, (ii) basis, a short cylinder with lesser average diameter than the coxa and articulated by hinge joints with the coxa, moving dorso-ventrally, (iii) ischium, larger and wider than the basis, fused immovably and curved upward, (iv) merus, (v) carpus, (vi) propodus, and (vii) dac- tyl. The basis and ischium when fused together form the basi-ischium (Lochhead 1961). Typically Caridean shrimps have chelate or subchelate first and second pairs of pereiopods; however, among the Processidae often only one pereiopod of the first pair is chelate, while the opposing member is simple. A very unique and exaggerated development of one chela of the first pair of pereiopod is characteristic of snapping shrimp of the family Alpheidae. After being cocked in the open position and building up tension, this large snapper claw (of up to half the animal’s size) closes rapidly forming a thin water jet; its high velocity (25 m/s) results in the formation of a small cavitation bubble (3⋅5 mm in dia- meter), that collapses with an extremely loud and short sound (up to 248 dB re 1 µPa at 1 cm distance for 240 ns) (Schmitz 2000). This signal is used for intraspecific, hydrodynamic communication (Herberholz and Schmitz 1998) as well as for territorial defence and to stun or even kill small prey (small shrimp, crabs or fish) (MacGinitie and MacGinitie 1949; Hazlett and Winn 1962; Schultz et al 1998). Marked asymmetry of the first pair of chelate pereiopods is also common in a number of other decapods like lobsters and fiddler crabs. 2. Growth Crustacean growth is discontinuous since the highly min- eralised old exoskeleton is shed through a process known as ecdysis (= moulting) whenever a certain growth incre- ment is achieved, whereas growth in vertebrates is continuous. In decapods three types of allometry exist: (i) positive allometry or progressive geometric growth, in which the dependent variable grows fast with respect to body size, (ii) isometric or arithmetic growth, and (iii) negative allometry or retrogressive geometric growth, in which the dependent variable grows slower than body size. Chelipeds are the best example of crustacean allometric growth. In males of the crab Arenaeus carib- rarius (Pinheiro and Fransozo 1993), crayfish Orconectes virilis, and blue clawed (BC) males of Macrobrachium rosenbergii (Kuris et al 1987), chela growth shows posi- tive allometry (Aiken and Waddy 1992), while it is iso- metric in juveniles and females (Pinheiro and Fransozo 1993). A detailed account of the types of allometric growth of cheliped in various decapod crustaceans is given in table 1. In heterochelous decapods chelipeds are further differ- entiated into major (crusher) and minor (cutter) based on morphologically and functionally different dentition pat- terns (Levinton et al 1995). The large and robust chela with molariform teeth, known as the crusher, can yield more force than the minor, which has many cutting teeth and spiniform setae (figure 1) (Mariappan and Balasunda- ram 1997). Major and minor chelae possess differential operating forces measured as ideal mechanical advantage (IMA) (figure 2) (Warner and Jones 1976; Brown et al Figure 1. Morphological variations in the propodus of (a) major and (b) minor chela in Macrobrachium nobilii. D, Dac- tyls; P, propodus (from Mariappan and Balasundaram 1997). (a) (b) 2 P. MARIAPPAN ET AL. 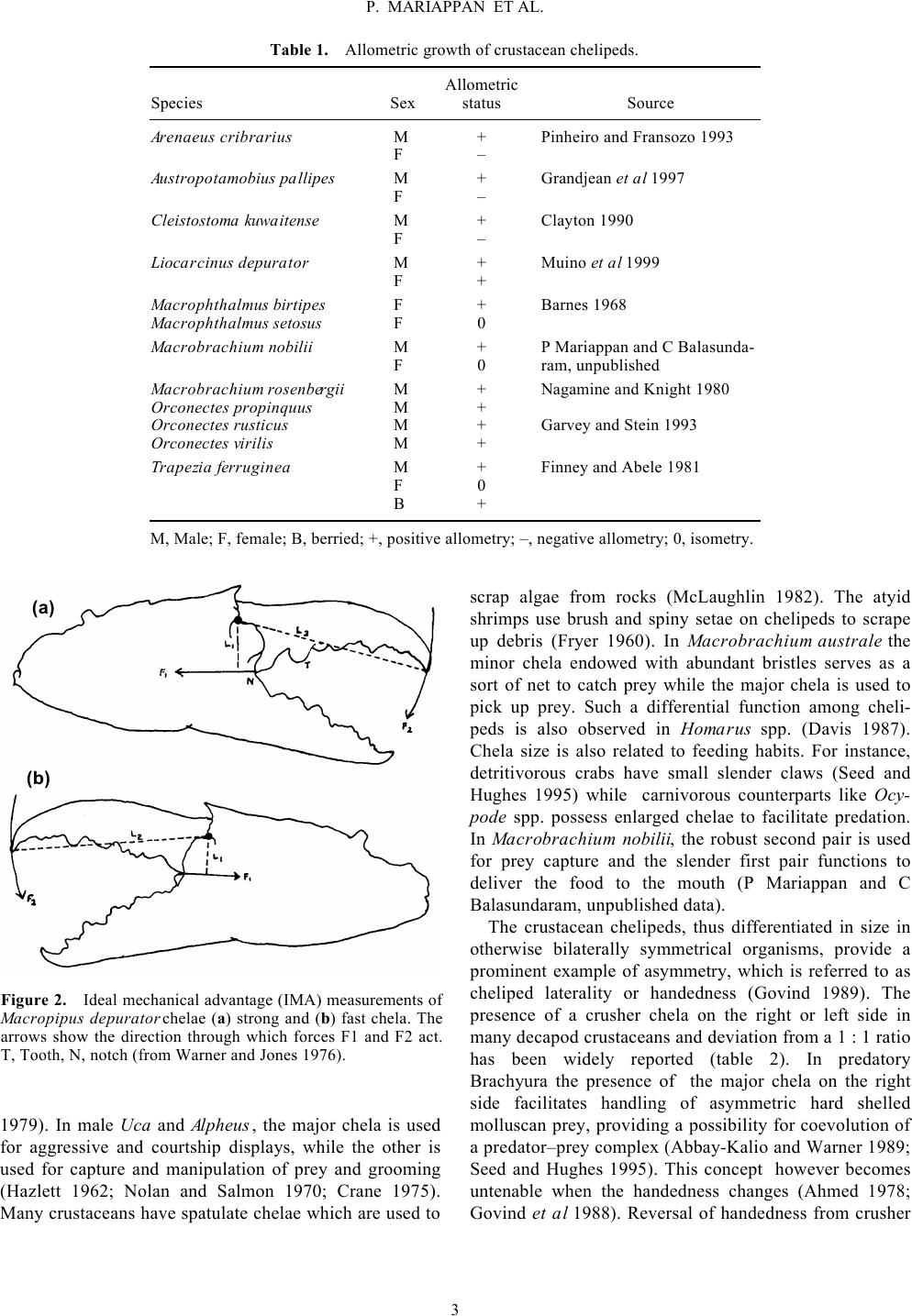 1979). In male Uca and Alpheus, the major chela is used for aggressive and courtship displays, while the other is used for capture and manipulation of prey and grooming (Hazlett 1962; Nolan and Salmon 1970; Crane 1975). Many crustaceans have spatulate chelae which are used to scrap algae from rocks (McLaughlin 1982). The atyid shrimps use brush and spiny setae on chelipeds to scrape up debris (Fryer 1960). In Macrobrachium australe the minor chela endowed with abundant bristles serves as a sort of net to catch prey while the major chela is used to pick up prey. Such a differential function among cheli- peds is also observed in Homarus spp. (Davis 1987). Chela size is also related to feeding habits. For instance, detritivorous crabs have small slender claws (Seed and Hughes 1995) while carnivorous counterparts like Ocy- pode spp. possess enlarged chelae to facilitate predation. In Macrobrachium nobilii, the robust second pair is used for prey capture and the slender first pair functions to deliver the food to the mouth (P Mariappan and C Balasundaram, unpublished data). The crustacean chelipeds, thus differentiated in size in otherwise bilaterally symmetrical organisms, provide a prominent example of asymmetry, which is referred to as cheliped laterality or handedness (Govind 1989). The presence of a crusher chela on the right or left side in many decapod crustaceans and deviation from a 1 : 1 ratio has been widely reported (table 2). In predatory Brachyura the presence of the major chela on the right side facilitates handling of asymmetric hard shelled molluscan prey, providing a possibility for coevolution of a predator–prey complex (Abbay-Kalio and Warner 1989; Seed and Hughes 1995). This concept however becomes untenable when the handedness changes (Ahmed 1978; Govind et al 1988). Reversal of handedness from crusher Table 1. Allometric growth of crustacean chelipeds. Species Sex Allometric status Source Arenaeus cribrarius M + Pinheiro and Fransozo 1993 F – Austropotamobius pallipes M + Grandjean et al 1997 F – Cleistostoma kuwaitense M + Clayton 1990 F – Liocarcinus depurator M + Muino et al 1999 F + Macrophthalmus birtipes F + Barnes 1968 Macrophthalmus setosus F 0 Macrobrachium nobilii M + F 0 P Mariappan and C Balasunda- ram, unpublished Macrobrachium rosenbergii M + Nagamine and Knight 1980 Orconectes propinquus M + Orconectes rusticus M + Garvey and Stein 1993 Orconectes virilis M + Trapezia ferruginea M + Finney and Abele 1981 F 0 B + M, Male; F, female; B, berried; +, positive allometry; –, negative allometry; 0, isometry. Figure 2. Ideal mechanical advantage (IMA) measurements of Macropipus depurator chelae (a) strong and (b) fast chela. The arrows show the direction through which forces F1 and F2 act. T, Tooth, N, notch (from Warner and Jones 1976). (a) (b) 3 P. MARIAPPAN ET AL.  to cutter and vice-versa or from pincer claw to snapper claw, when a chela is lost, has been well documented in some heterochelous crabs, lobsters, and snapping shrimp (Wilson 1903; Yamaguchi 1977; Mellon 1981; Govind 1989; Young et al 1994). In other species with plasticity in chela development into major or minor forms, the esta- blishment of laterality (handedness) is determined by eco- logical factors (Davis 1987; Smith and Palmer 1994; Goldstein and Noetzli 1997), and the reversal of handed- ness depends on the age of the animal (Cheung 1976). However, in species where there is no reversal, genetic factors determine laterality (Bush 1930; Yamaguchi 1977). Apart from functional differences, structural variations between crusher and cutter also have been elucidated (Ogonowski and Lang 1979; Ogonowski et al 1980). After autotomy, the resultant changes in the composition of chela muscles at the time of chela development, reversal, and regeneration are well documented in lob- sters (Homarus americanus), and snapping shrimp Alpheus heterochaelis (Stephens and Mellon 1979; Mellon and Stephens 1980; Govind and Lang 1981; Quigley and Mellon 1984; Govind et al 1987, 1988; Govind and Pearce 1988a, b, 1994; Govind 1989). In Gecarcinus lateralis, there is an attendant break- down in claw muscle protein that occurs at moulting which allows the reduced claw to be drawn through the comparatively small foramen at the proximal end of the propus (Skinner 1966; Mykels and Skinner 1981). Sexual dimorphism in cheliped size has also been esta- blished in crabs (Crothers 1967), lobsters and crayfish (Snedden 1990), mantis shrimp (Schuster and Caldwell 1989), snapping shrimp (Read and Govind 1997), and freshwater prawns (Mariappan and Balasundaram 1997). Generally such a dimorphism between a cheliped pair (Darby 1934) is mainly based on size rather than form (Lee 1995) and when adjusted for size variations their functions are similar as in Ozius verreauzii (Hughes 1989). However in Alpheus heterochaelis the male pincer claw really differs in form from that of the female struc- turally (Read and Govind 1997). The development of a dimorphic pattern begins at the time of puberty moult (Hartnoll 1974; Pinheiro and Fransozo 1993, 1998), which is a prerequisite for functional sexual maturity (see e.g. Hyas lyratus, Stevens et al 1993). In some decapods the attainment of puberty moult is identified by the level of change in propodus length (e.g. Nephrops norvegicus, Farmer 1974). Differences in chela allometry are used to Table 2. Handedness in decapod crustaceans. Species Handedness Source Calappa philargius R Ng and Tan 1985 Callinectes sapidus R Hamilton et al 1976 Carcinus maenas R Abby-Kalio and Warner 1989 Glabropilumnus laevimanus R Tweedie 1950 Globopilumnus globosus R Tweedie 1950 Heteropanope glabra R Tweedie 1950 Heterozius rotundifrons R Jones 1978 Macrobrachium nobilii R Mariappan and Balasundaram 1997 Menippe mercenaria R Cheung 1976 Necora puber R Norman and Jones 1991 Neopanope texana R Swartz 1972 Pilumnus hirtellus R Tweedie 1950 Uca lactea R Yamaguchi 1973, 1977 Uca vocans R Barnwell 1982 Uca tetragonon R Barnwell 1982 Uca formosensis R Barnwell 1982 Ocypode gaudichaudii L Trott 1987 Synalpheus brevicarpus L Herrick 1911 Alpheus dentipes – Dawes 1934 Alpheus heterochaelis – Young et al 1994 Chlorodopsis melanochira – Tweedie 1950 Homarus americanus – Herrick 1911 Macrobrachium australe – Davis 1987 Nephrops norvegicus – Farmer 1974 Ocypode quadrata – Haley 1969 Thalassina anomala – Pillai 1990 Uca formosensis – Shih et al 1999 Xantho exartus – Tweedie 1950 R, Right handed; L, left handed; –, equal distribution of right and left handed animals. 4 P. MARIAPPAN ET AL.  differentiate immature from mature phases in Pagurus prideauxi (Paulian 1936). Factors like feeding, mate- guarding, and fighting influence the development of such dimorphic patterns of chelipeds (Vermeij 1977; Hughes 1989). Parasites exert a remarkable negative effect on the growth of chelipeds in various crustaceans. Bopyrids, entoniscids and sacculinids are the common parasites known to affect the normal growth of chelipeds. Infection of a bopyrid Gyge branchialis on Upogebia littoralis and Probopyrus pandalicola on Palaemonetes, Ione thoracica on Callianassa laticauda, an entoniscid Entonella mono- ensis and a sacculinid Sacculina polygenea on Hemigrap- sus sanguinesus showed a significant reduction of chela size when compared to uninfected forms (Tucker 1930; Reverberi 1943; Morris 1948; Hartnoll 1960; Yamaguchi and Aratake 1997). In Macrobrachium rosenbergii the development of polymorphic males is common in natural as well as com- munally cultured populations. These males are differenti- ated into (i) small males (SM), with delicate, clear or light pink claws and with a low ratio of claw to body length and much smaller than the other two morphotypes, (ii) orange-clawed males (OC) with non-spineous, often orange claws, having a higher claw to body length ratio, and (iii) blue-clawed males (BC) with blue, spineous claws and a high ratio of claw to body length. Small males can transform into blue-clawed males through orange- clawed forms in the absence of dominant BC males or when raised in isolation (Ra’anan and Cohen 1985; Kuris et al 1987). Among mature males of Pisa spp., Jassa fal- cata and Inachus leptochirus, even within the same age group there is a remarkable difference in the size and Table 3. Variations in the percentage of limb loss in field populations of various decapod crustaceans. Species Category Per cent Source Atergatis flloridus M 41⋅30 Norman 1995 F 18⋅40 Callinectes sapidus – 24⋅80 Smith 1990a, b Cancer magister – 25⋅00 Shirley and Shirley 1988 Cancer magister – 45⋅00 Durkin et al 1984 Cancer pagurus M 13⋅20 Bennett 1973 F 9⋅90 Carcinus maenas M 12⋅50 Abello et al 1994 F 7⋅90 Carcinus maenas M* 1⋅70 Sekkelsten 1988 M** 17⋅90 Carcinus maenas M 53⋅30 McVean 1976 F 55⋅00 Chionoecetes bairdi J 34⋅60 Edwards 1972 M 43⋅00 F 23⋅00 Cyrtograpsus angulatus – 80⋅00 Spivak and Politis 1989 Homarus americanus M 44⋅40 Moriyasu et al 1999 F 61⋅30 Homarus americanus – 21⋅00 Estrella and Armstrong 1994 Homarus americanus M 40⋅00 Briggs and Mushacke 1979 F 30⋅20 Macrobrachium nobilii J 10⋅90 M 15⋅22 Mariappan and Balasundaram 1999b F 22⋅30 Necora puber J 23⋅00 Norman and Jones 1991 M 32⋅80 F 28⋅80 Nephrops norvegicus M 62⋅00 Chapman and Rice 1971 F 41⋅00 Panulirus argus – 40⋅30 Davis 1981 Paralithodes camtschatica J 29⋅40 Edwards 1972 – 14⋅80 Paralithodes camtschatica M 15⋅30 Niwa and Kurata 1964 F 19⋅50 J, Juveniles; M, males; F, females; –, not categorised. Carapace width: *20–34⋅9, **65–79⋅9 mm. 5 P. MARIAPPAN ET AL.  shape of the chela (Sexton and Reid 1951; Hartnoll 1963). Season-induced cyclic changes in chela polymorphism has been reported in males of Orconectes propinquus (Stein 1976). 3. Autotomy Autotomy refers to a reflex severance of one or more limbs in response to injury or its threat, which occurs al- ways in a predetermined breakage plane (Wood and Wood 1932; Robinson et al 1970; McVean 1982). A number of factors contribute to the prevalence of auto- tomy, which has been extensively studied and reviewed from time to time (Wood and Wood 1932; Bliss 1960; McVean 1982; Juanes and Smith 1995). Crustaceans widely practice self amputation of one or more limbs dur- ing inter- and intraspecific competition for limited re- sources like food, shelter, mate and also as a strategy to avoid predation and wound limitation (Wood and Wood 1932; Bliss 1960; McVean 1982). Apart from such biological reasons, commercial factors like intentional harvesting of chelipeds in species like Menippe merce- naria (Savage and Sullivan 1978), incidental damage by fishing gear (Kirkwood and Brown 1998), and culling of undesirable individuals (Kennelly et al 1990) are also responsible for the loss of chelipeds. In the polymorphic male population of M. rosenbergii, cheliped loss is a periodic event among the dominant blue-clawed males (bulls) on attaining a critical value of 1 : 2⋅8 ± 0⋅18 body length/chela length as a growth strategy (Schmalbach et al 1984). Males of M. nobilii (28%) (carapace length: 1⋅6–2⋅5 cm) resort to chela autotomy during exuviation even when reared individually under ideal laboratory conditions (Mariappan and Balasundaram 1999a); even multiple limb autotomy occurs in M. malcolmsonii in the field (P Mariappan and C Balasundaram, unpublished data). The limb loss varies from species to species (1⋅7% in Carcinus maenas, Sekkelsten 1988; 80% in Cyrtograpsus angulatus, Spivak and Politis 1989), within a species (C. maenas, 1⋅7%, Sekkelsten 1988; 55%, McVean 1976) and as a function of size within a species (Necora puber, 12% in juveniles and 38% in adults, Norman and Jones 1991) (table 3). To a certain extent temporal and geo- graphic variations also contribute to autotomy in a given population (Shirley and Shirley 1988; Smith 1990a). Though the autotomised animals get immediate advantage in terms of survival, in the long term the need to divert body resources for regeneration has an adverse effect on the regular energy budget. Further the injured animal becomes less dominant and remains more vulnerable to further attacks in a community; autotomy also limits its access to shelter, food gathering potential, and its abi- lity to find a mate (Kuris and Mager 1975; Sekkelsten 1988; Davenport et al 1992; Abello et al 1994; Smith 1995). 4. Regeneration Crustaceans have the ability to replace lost limbs by means of regeneration, which is linked with moulting (Prizbram 1901; Bliss 1960; Skinner 1985). However, at any given time, in a wild population of Cancer magis- ter the proportion of animals with regenerating limbs (5%) is comparatively lower than that of animals with lost limbs (18%) (Shirley and Shirley 1988), suggesting an increased vulnerability of autotomised animals to preda- tion (McVean and Findlay 1979). In some species the process of limb regeneration affects the moult increment and moult interval but in others no such effect has been reported (Smith 1990b; Spivak 1990; Cheng and Chang 1993). Regeneration of a lost limb to its original size depends upon age and time of loss in a given moult cycle. Normally the lost limb regenerates within 2–3 moults, faster in juveniles than in adults (Skinner 1985; Smith 1990b). 5. Abnormalities in chelipeds Abnormalities or malformation of chelipeds have been reported widely in various decapod crustaceans like lob- ster (Homarus americanus, Faxon 1881; H. gammarus and Nephrops norvegicus, cf. Shelton et al 1981), crayfish (Procambarus clarkii, Chokki and Ishihara 1994; Naka- tani et al 1997), crab (Geryon affinis granulatus, Oka- moto 1991; Macrophthalmus japonicus, Suzuki 1963), and the Japanese edible crab (Chionectes japonicus, Mo- toh 1971). Most of these claw abnormalities are mainly due to a lateral outgrowth in the propodus, which results es- pecially from abnormal wound healing following the damage of the propodus (Okamoto 1991; Nakatani et al 1992); this phenomenon could also be induced in the laboratory (Murayama et al 1994; Nakatani and Kitahara 1999). 6. Cheliped display Communication in crustaceans often involves the display of antennae and chelipeds. The roles of the chelipeds in agonistic and aggressive interactions during inter- and intraspecific competition for a limited resource is well documented in the literature (Hazlett 1972; Salmon and Hyatt 1983). The possession of chelipeds plays a major role in acquisition and retention of shelters in Homarus americanus (O’Neill and Cobb 1979) and Macrobrachium nobilii (Balasundaram and Mariappan 1998). Different 6 P. MARIAPPAN ET AL.  kinds of acts or movements for cheliped presentation have been reported in various crustaceans (Liocarcinus depura- tor and Necora puber, Huntingford et al 1995; H. ameri- canus, Atema and Cobb 1980; Macrobrachium rosenbergii, Barki et al 1991; M. australiense, Lee and Fielder 1983). A detailed account of the use of chelipeds in communication is provided by Salmon and Hyatt (1983). Cheliped extension, meral spread, strike, lifting of claw, scissoring, thrust, cheliped striking, embrace, nip and push are the major events mediated by chelipeds (table 4 and figure 3). 7. Courtship and mating The role of chelipeds in courtship display and the pres- ence of chelipeds as an aid in mate access have been extensively studied. In Uca pugilator there is a marked diffe- rence in display patterns between mature and immature males (Salmon et al 1978). Since chelae have a major role in displays during agonistic interactions, the degree of dominance is expressed by the type of chela morphometry. An animal with robust chelae has easy access to mates during inter-male competition and also through sexual selection by females. Autotomy also plays a crucial role in mating since such handicaps lead to a negative effect on mate access as observed in small and medium sized males of Carcinus maenas (Sekkelsten 1988). Variations in mating and reproductive patterns are observed among polymorphic males of Macrobrachium rosenbergii. The dominant blue-clawed males (bulls, BC males) effectively court and protect mates (Ra’anan and Sagi 1985), while intermediate males (OC males) show reduced reproductive activity in the presence of BC males (Ra’anan and Cohen 1985). Submissive small males are also sexually less competent, but mate successfully in the absence of BC and OC males (Sagi 1984). 8. Implications of chelae for decapod culture In communal culture of crustaceans, the possession of large crusher chelae triggers aggression between individu- als leading to physical damage of body parts (especially chelipeds) aggravating the rate of limb loss and mortality. Indeed chelipeds constitute 10–26% of the body weight in Table 4. Cheliped mediated displays in decapod crustaceans. Act Description Cheliped extension2,3,5,6 Extension of chelae towards the opponent without contact Cheliped presentation2 Ambulatory legs in walking position and both chelipeds in presentation position Cheliped shaking3,5 Rapid oscillations of the dactyls while (without touching propodus) the claw is partly extended in the direction of another prawn Complete lifting3,5 Lifting of the claws and anterior part of the body towards another individual Incomplete lifting3 Similar to complete lifting, but the claws remain in the hori- zontal plane Crouching6 Chelipeds are folded tightly against the body Fending6 Outward swinging of one or both chelipeds Grasping1,6 Seizing of another individual with thoracic appendages 3–5 Meral spread1,3 Outward spreading of the enlarged meri of the appendages Nip3,4 One animal closes down the tips of its chelae on a body part of another animal Push3,4,5 One animal pushes one of its chelae against a body part of another animal Shielding6 Holding the chelipeds like a shield Strike1,3 A blow delivered by one individual to another with the dac- tyls of one or both raptorial appendages Scissoring3,5,6 Bringing the two claws together from the complete lifting position in a scissoring motion Thrust3 Rapid simultaneous opening of the two claws in the direc- tion of another prawn Sources: 1) Dingle 1969, 2) Hazlett and Bossert 1965, 3) Barki et al 1991, 4) Peebles 1979, 5) Lee and Fielder 1983, 6) Jachowski 1974. 7 P. MARIAPPAN ET AL.  Figure 3. Agonistic acts in decapod crustaceans (Jachowski 1974; Barki et al 1991). 8 P. MARIAPPAN ET AL.  Macrobrachium nobilii (Mariappan and Balasundaram 1999a), 20% in Carcinus maenas and Liocarcinus hol- satus (Lee and Seed 1992), and 50% in Menippe merce- naria (Simonson and Steele 1981). In H. americanus, the possession of the crusher claw is essential for acquisition of limited resources, as well as establishment and mainte- nance of dominance hierarchies (O’Neill and Cobb 1979). In such cases the autotomised animal becomes subjugated and more subordinated during further attacks. In C. sapidus, the loss of chelipeds was shown to have not only a negative effect on foraging ability and prey handling time (Juanes and Hartwick 1990; Smith and Hines 1991), but also the incumbent has to channelise more metabolic energy for the regeneration of chelipeds. Thus in species like Callinectes sapidus (Ary et al 1987; Smith 1990b), the loss of chelipeds leads to a reduction in moult incre- ment due to energy diversion; such energy demand is called regeneration load (Skinner 1985), which may reduce reproductive output (Norman and Jones 1993; Luppi et al 1997). Chelotomy, dactylotomy and immobi- lisation of the dactyls have been shown to reduce the degree of cannibalism in H. americanus (Kendall et al 1982) and in M. rosenbergii (Karplus et al 1989; Diaz et al 1990). However the decreased survival rate due to forced severance of limbs and subsequent regeneration are major constraints that reduce the harvest size (Powell et al 1998). 9. Conclusion Though autotomy, moulting and regeneration of chelipeds have been reviewed periodically, a collective perusal of literature attempted in this review reveals that the diverse functional and structural modifications of chelipeds are not only influenced by feeding and locomotion patterns, but also by environmental conditions and species-specific needs. A number of biotic and abiotic factors influence the development of chelae. The chelae are most vulnera- ble to autotomy and their regeneration imposes a regene- ration load in the regular energy budget of the animal resulting in a telling effect on the other regular somatic and reproductive processes. In aquaculture experimental removal of chelae minimizes aggressive interactions but the problem is recurrent due to regeneration potential and hence is of limited applicability. Since it takes more than one moult for total regeneration of the chelae, their use as a taxonomic character is doubtful. Acknowledgements Financial assistance from the Council of Scientific and Industrial Research, New Delhi, to PM in the form of a Senior Research Fellowship and University Grants Com- mission, New Delhi, to CB in the form of a major research project is gratefully acknowledged. Thanks are also due to Dr S Prem Mathi Maran, Chennai, for line drawings. References Abby-Kalio N J and Warner G F 1989 Heterochely and handed- ness in the shore crab Carcinus maenas (L.) (Crustacea: Brachyura); Zool. J. Linn. Soc. 96 19–26 Abello P, Warman C G, Reid D G and Naylor E 1994 Chela loss in the shore crab, Carcninus maenas (Crustacea: Brach- yura) and its effect on mating success; Mar. Biol . 121 247– 252 Ahmed M 1978 Development of asymmetry in the fiddler crab Uca cumulanta Crane, 1943 (Decapoda, Brachyura); Crusta- ceana 34 294–300 Aiken D E and Waddy S L 1992 The growth process in cray- fish; Rev. Aquat. Sci. 6 335–381 Ary R D, Bartell C K and Poirrier M A 1987 The effects of chelotomy on molting in the blue crab, Callinectes sapidus; J. Shellfish Res. 6 103–108 Atema J and Cobb J S 1980 Social behavior; in The biology and management of lobsters (eds) J S Cobb and B F Phillips (New York: Academic Press) vol 1, pp 409–450 Averof M and Patel N H 1997 Crustacean appendage evolution associated with changes in Hox genes expression; Nature (London) 388 682–686 Balasundaram C and Mariappan P 1998 Observations on the sheltering behaviour of Macrobrachium nobilii (Henderson and Matthai 1910); in Natl. Symp. Sustainable Aquaculture, Feb. 20–21, 1998, University of Delhi, New Delhi. Abstract No. 2 Barki A, Karplus I and Goren M 1991 Morphotype related dominance hierarchies in males of Macrobrachium rosenber- gii (Crustacea, Palaemonidae); Behaviour 117 145–160 Barnes R S K 1968 Relative carapace and chela proportions in some Ocypodid crabs (Brachyura, Ocypodidae); Crustaceana 14 131–136 Barnwell F H 1982 The prevalence of male right-handedness in the Indo-West Pacificfiddler crabs Uca vocans (Linnaeus) and U. tetragonon (Herbst) (Decapoda: Ocypodidae); J. Crust. Biol . 2 70–83 Bennett D B 1973 The effect of limb loss and regeneration on the growth of the edible crab, Cancer pagurus L.; J. Exp. Mar. Biol. Ecol. 13 45–53 Bliss D E 1960 Autotomy and regeneration; in The physiology of crustacea (ed.) T H Waterman (New York: Academic Press) vol 1, pp 561–589 Bock W J and von Wahlert G 1965 Adaptation and the form- function complex; Evolution 19 269–299 Briggs P T and Mushacke F M 1979 The American lobster and the pot fishery in the inshore waters of the south shore of Long Island, New York; N.Y. Fish Game J. 27 156–178 Brown S C, Cassuto S R and Loos R W 1979 Biomechanics of chelipeds in some decapod crustaceans; J. Zool. 188 143– 159 Bush S F 1930 Asymmetry and relative growth of parts in the two sexes of the hermit crab, Eupagurus prideauxi; Wilhelm Roux’ Arch. Entwicklungsmech. Org . 123 39–79 Chapman C J and Rice A L 1971 Some direct observations on the ecology and behaviour of the Norway lobster Nephrops norvegicus; Mar. Biol. 10 321–329 Cheng J-H and Chang E S 1993 Determinants of postmolt size 9 P. MARIAPPAN ET AL.  in the American lobster (Homarus americanus). 1. D sub(1) super(3) is the critical stage; Can. J. Fish. Aquat. Sci. 50 2106–2111 Cheung T S 1976 A biostatistical study of the functional consis- tency in the reversed claws of the adult male stone crabs, Menippe mercenaria (Say); Crustaceana 31 137–144 Chokki H and Ishihara T 1994 The second specimen of Pro- cambarus (Scapulicambarus) clarkii (Girard) bearing mal- formed chela; Bull. Owakidani Nat. Hist. Mus. Hakone 12 1–3 (in Japanese) Clayton D A 1990 Crustacean allometric growth: a case for caution; Crustaceana 58 270–290 Crane J 1975 The fiddler crabs of the world (Ocypodidae: Genus Uca) (New Jersey: Princeton University Press) Crothers J H 1967 The biology of the shore crab Carcinus mae- nas (L.); Field Stud. 2 407– 434 Dahl E 1956 Some crustacean relationships; in Bertil hanstrom: Zoological papers in honor of his sixty-fifth birthday (ed.) K G Wingstrand (Sweden: Lund Zool Inst) pp 138–147 Darby H H 1934 The mechanism of asymmetry in the Alphei- dae; Carnegie Inst. Washington Publ. 28 349–361 Davenport J, Spikes M, Thornton S M and Kelly B O 1992 Crab-eating in the diamond black terrapin Malaclemys terra- pin: dealing with dangerous prey; J. Mar. Biol. Assoc. U.K.; 72 835–848 Davis T A 1987 Laterality in Crustacea; Proc. Indian Natl. Sci. Acad. B53 47–60 Dawes B 1934 A study of normal and regenerative growth in pistol shrimp, Alpheus dentipes (Guèrin); Wilhelm Roux’ Arch. Entwicklungsmech. Org. 131 543–574 Diaz G G, Nakagawa H and Kasahara S 1990 Effect of propo- dus excision on growth and survival in giant freshwater prawn Macrobrachium rosenbergii; J. Fac. Appl. Biol. Sci. (Hiroshima Univ.) 29 19–24 Dingle H 1969 Statistical and information analysis of aggressive communication in the mantis shrimp Gonodactylus bredini Manning; Anim. Behav. 17 561–575 Durkin J T, Buchanan K D and Blahm T H 1984 Dungeness crab leg loss in the Columbia river estuary; Mar. Fish. Rev. 46 22–24 Edwards J S 1972 Limb loss and regeneration in two crabs: the king crab, Paralithodes camtschatica and the tanner crab Chionoecetes bairdi; Acta. Zool. 53 105–112 Estrella B T and Armstrong M P 1994 Massachusetts coastal commercial lobster trap sampling program May–November 1993; Mass. Div. Mar. Fish. 30 Farmer A S 1974 The development of external sexual characters of Nephrops norvegicus (L.) (Decapoda: Nephropidae); J. Nat. Hist. 8 241–255 Faxon W 1881 On some crustacean deformities; Bull. Mus. Comp. Zool. 8 257–274 Fryer G 1960 The feeding mechanism of some atyid prawns of the genus Caridina; Trans R. Soc. Edinburgh 54 335– 381 Finney W C and Abele L G 1981 Allometric variation and sexual maturity in the obligate coral commensal Trapezia fer- ruginea Latreille (Decapoda, Xanthidae); Crustaceana 41 113–130 Garvey J E and Stein R A 1993 Evaluating how chela size influences the invasion potential of an introduced crayfish (Orconectes rusticus); Am. Midl. Nat. 129 172–181 Goldstein J S and Noetzli C H 1997 Substrate variability as critical developmental factor in the claw asymmetry of the North American lobster, Homarus americanus; Today’s Aquaculture 6 4–5 and 11 Govind C K 1989 Asymmetry in lobster claws; Am. Sci. 77 468–474 Govind C K, Mellon, DeF and Quigley M M 1987 Muscle and muscle fiber type transformation in clawed crustaceans; Am. Zool . 27 1079–1098 Govind C K and Lang F 1981 Physiological identification and asymmetry of lobster claw closer motorneurons; J. Exp. Biol . 94 329–339 Govind C K and Pearce J 1988a Independent development of bilaterally homologous closer muscles in lobster claws; Biol. Bull . 175 430–433 Govind C K and Pearce J 1988b Remodeling of nerves during claw reversal in adult snapping shrimps; J. Comp. Neurol. 268 121–130 Govind C K and Pearce J 1994 Muscle remodelling in adult snapping shrimps via fat-fiber degeneration and slow-fiber genesis and transformation; Cell Tissue Res. 276 445–454 Govind C K, Pearce J and Potter D J 1988 Neural attrition following limb loss and regeneration in juvenile lobsters; J. Neurobiol. 15 4209–4222 Grandjean F, Romain D, Avila-Zarza C, Bramard M, Souty- Grosset C and Mocquard J P 1997 Morphometry, sexual di- morphism and size at maturity of the white-clawed crayfish, Austropotamobius pallipes pallipes (Lereboullet) from a wild French population at Deux-Sevres (Decapoda, Astacidea); Crustaceana 70 31–44 Haley S R 1969 Relative growth and sexual maturity of the Texas ghost crab, Ocypode quadrata (Fabr.) (Brachyura, Ocypodidae); Crustaceana 17 285–297 Hamilton P V, Nishimoto R T and Halusky J G 1976 Cheliped laterality in Callinectes sapidus (Crustacea: Portunidae); Biol. Bull. 150 393–401 Hartnoll R G 1960 Entionella monensis sp. nov., an entoniscis parasite of the crab Eurynome aspera (Pennant); J. Mar. Biol. Assoc. U.K. 39 101–107 Hartnoll R G 1963 The biology of Manx spider crabs; Proc. Zool. Soc. London 141 423– 496 Hartnoll R G 1974 Variations in growth pattern between some secondary sexual characters in crabs (Decapoda, Brachyura); Crustaceana 27 131–136 Hartnoll R G 1982 Growth; in The biology of crustacea (ed.) L G Abele (New York: Academic Press) vol. 2, pp 111– 196 Hazlett B A 1962 Aspects of the biology of snapping shrimp (Alpheus and Synapheus); Crustaceana 4 82–83 Hazlett B A 1972 Responses to agonistic postures by the spider crab Microphrys bicornutus; Mar. Behav. Physiol . 1 85–92 Hazlett B A and Bossert W H 1965 A statistical analysis of the aggressive communication systems of some hermit crabs; Anim. Behav. 13 357–373 Hazlett B A and Winn H E 1962 Sound production and associ- ated behavior of Bermuda crustaceans (Panulirus, Gonodac- tylus, Alpheus and Synalpheus); Crustaceana 4 25– 38 Herberholz J and Schmitz B 1998 Role of mechanosensory stimuli in intraspecific agonistic encounters of the snapping shrimp (Alpheus heterochaelis); Biol. Bull. 195 156–167 Herrick F H 1911 Natural history of American lobster; Bull.U.S. Bur. Fish. 29 149–408 Hopkins P M 1993 Regeneration of walking legs in the fiddler crab Uca pugilator; Am. Zool. 33 348–356 Hughes R N 1989 Foraging behaviour of a tropical crab, Ozius verreauxii; Proc. R.. Soc. London B237 201–212 Huntingford F A, Taylor A C, Smith, I P and Thorpe K E 1995 Behavioural and physiological studies of aggression in swimming crabs; J. Exp. Mar. Biol. Ecol. 193 21–39 10 P. MARIAPPAN ET AL.  Jachowski R L 1974 Agonistic behavior of the blue crab, Callinectes sapidus Rathbun; Behaviour 50 232–251 Jones M B 1978 Aspects of the biology of the big-handed crab, Heterozius rotundifrons (Decapoda: Brachyura), from Kai- koura, New Zealand; N.Z. J. Zool. 5 783– 794 Juanes F and Hartwick E B 1990 Prey size selection in dunge- ness crabs: the effect of claw damage; Ecology 71 744– 758 Juanes F and Smith L D 1995 The ecological consequences of limb damage and loss in decapod crustaceans: a review and prospectus; J. Exp. Mar. Biol. Ecol. 193 197–223 Kaestner A 1970 Invertebrate zoology (translated by H W Levi and L R Levi), vol 3, (New York: Wiley Interscience) Karplus I, Samsonov E, Hulata G and Milstein A 1989 Social control of growth in Macrobrachium rosenbergii. I. The effect of claw ablation on survival and growth of communally raised prawns; Aquaculture 80 325–335 Kendall R A, Van Olst J C and Carlberg J M 1982 Effects of chelae immobilization on growth and survivorship for indi- vidually and communally raised lobsters, Homarus ameri- canus; Aquaculture 29 359–372 Kennelly S J, Watkins D and Craig J R 1990 Mortality of dis- carded spanner crabs, Ranina ranina (Linnaeus) in a tangle- net fishery-laboratory and field experiments; J. Exp. Mar. Biol. Ecol. 140 39–48 Kirkwood J M and Brown I W 1998 Effect of limb damage on the survival and burial time of discarded spanner crabs Ranina ranina (Linnaeus); Mar. Freshwater Res. 49 41–45 Kuris A M and Mager M 1975 Effect of limb regeneration on size increase a molt of the shore crabs Hemigrapsus oregonen- sis and Pachygrapsus crassipes; J. Exp. Zool. 193 353– 360 Kuris A M, Ra’anan Z, Sagi A and Cohen D 1987 Morphotypic differentiation of male Malaysian giant prawns, Macro- brachium rosenbergii; J. Crust. Biol. 7 219–237 Lee C L and Fielder D R 1983 Agonistic behaviour and the development of dominance hierarchies in the freshwater prawn, Macrobrachium australiense Holthuis, 1950 (Crusta- cea: Palaemonidae); Behaviour 83 1–17 Lee S Y 1995 Cheliped size and structure: the evolution of multi-functional decapod organ; J. Exp. Mar. Biol. Ecol. 193 161–176 Lee S Y and Seed R 1992 Ecological implications of the cheli- ped size in crabs: some data from Carcinus maenas and Lio- carcinus holsatus; Mar. Ecol. Prog. Ser. 84 151–160 Levinton J S, Judge M L and Kurdziel J P 1995 Functional dif- ferences between the major and minor claws of fiddler crabs (Uca, family Ocypodidae, Order Decapoda, Subphylum Crusta- cea): A result of selection or developmental constraint?; J. Exp. Mar. Biol. Ecol . 193 147–160 Lochhead J H 1961 Locomotion; in The physiology of crustacea (ed.) T H Waterman (New York: Academic Press) vol 2, pp 313–364 Luppi A T, Bas C C, Spivak E D and Anger K 1997 Fecundity of two grapsid crab species in the Laguna Mar Chiquita, Argentina; Arch. Fish. Mar. Res. 45 149–166 MacGinitie G E and MacGinitie N 1949 Natural history of marine animals (New York: McGraw Hill) Manton S M 1977 The arthropods: habits, functional morphology and evolution (London, New York: Oxford University Press) Mariappan P and Balasundaram C 1999a Molt related limb loss in Macrobrachium nobilii; Curr. Sci. 75 637–639 Mariappan P and Balasundaram C 1999b Prevalence of auto- tomy in field populations of Macrobrachium nobilii; Indian J. Fish. 46 61–66 Mariappan P and Balasundaram C 1997 Cheliped laterality in the freshwater prawn Macrobrachium nobilii (Henderson and Matthai 1910); Curr. Sci. 73 875–877 McLaughlin P A 1982 Comparative morphology of crustacean appendages; in The biology of crustacea (ed.) D E Bliss (New York: Academic Press) vol 2, pp 197–256 McVean A 1976 The incidence of autotomy in Carcinus mae- nas (L.); J. Exp. Mar. Biol. Ecol. 24 177–187 McVean A 1982 Autotomy; in The biology of crustacea (ed.) D E Bliss (New York: Academic Press) vol 4, pp 107–132 McVean A and Findlay I 1979 The incidence of autotomy in an estuarine population of the crab Carcinus maenas (L.); J. Mar. Biol. Assoc. U.K. 59 341–354 Mellon DeF Jr 1981 Nerves and the transformation of claw type in snapping shrimps; Trends Neurosci. 4 245–248 Mellon DeF Jr and Stephens P J 1980 Modifications in the arrangement of thick and thin filaments in transformed shrimp muscle; J. Exp. Zool . 213 173–179 Moriyasu M, Landsburg W, Wade E and Maynard D R 1999 The role of an estuary environment for regeneration of claws in the American lobster, Homarus americanus H. Milne Edwards, 1837 (Decapoda); Crustaceana 72 417–433 Morris J A 1948 Studies on the host-parasite relationship of Probopyrus pandalicola (Packard); Cathol. Univ. Am. Biol. Stud. 8 1–20 Motoh H 1971 Abnormalities found in the left cheliped of Japa- nese edible crab, Chionectes japonicus Rathbun; Res. Crust. 4–5 184–190 Muino R, Fernandez L, Gonzalez-Gurraiaran E, Freire J and Vilar J A 1999 Size at maturity of Liocarcinus depurator (Brachyura: Portunidae): a reproductive and morphometric study; J. Mar. Biol. Assoc. U.K. 79 295–303 Murayama O, Nakatani I and Nishita M 1994 Induction of lat- eral outgrowths on the chelae of the crayfish, Procambarus clarkii (Girard); Crust. Res. 23 69–73 Mykles D L and Skinner D M 1981 Preferential loss of thin filaments during molt-induced atrophy in crab claw muscle; J. Ultrastruct. Res. 75 314–325 Nagamine C M and Knight A W 1980 Development, matura- tion, and function of some sexually dimorphic structures of the Malaysian prawn, Macrobrachium rosenbergii (De Man) (Decapoda, Palaemonidae); Crustaceana 39 141–152 Nakatani I and Kitahara 1999 Induction of outgrowths at wounds on the cheliped of Procambarus clarkii (Decapoda, Cambari- dae); J. Crust. Biol. 19 1–7 Nakatani I, Okada Y and Yamaguchi T 1997 An extra claw on the first and on the third cheliped of the crayfish, Procamba- rus clarkii (Decapoda, Cambaridae); Crustaceana 70 788– 798 Nakatani I, Yamaguchi T and Murayama O 1992 Abnormalities found in the chela of the crayfish, Procambarus clarkii (Gi- rard); Res. Crust. 21 207–209 Ng P K L and Tan L W H 1985 ‘Right handedness’ in the heterochelous calappoid and xanthoid crabs – suggestion for functional advantage; Crustaceana 49 98–100 Niwa K and Kurata H 1964 Limb loss and regeneration in the adult king crab Paralithodes camtschatica; Bull. Hokkaido Reg. Fish. Res. Lab. 28 51–55 (Transl. from Japanese by Fish. Res. Board Can. Transl. Ser. No. 1190, 1969) Nolan B A and Salmon M 1970 The behavior and ecology of snapping shrimp (Crustacea: Alpheus heterochaelis and Alpheus normanni); Forma Functio 2 289– 335 Norman C P 1995 Limb loss in the poisonous crab Atergatis floridus (Linnaeus) advantages of possessing toxins?; Crust. Res. 24 137–145 11 P. MARIAPPAN ET AL.  Norman C P and Jones M B 1991 Limb loss and its effect on handedness and growth in the velvet swimming crab Necora puber (Brachyura: Portunidae); J. Natl. Hist. 25 639– 645 Norman C P and Jones M B 1993 Reproduction ecology of the velvet swimming crab, Necora puber (Brachyura: Portuni- dae), at Plymouth; J. Mar. Biol. Assoc. U.K. 73 379–389 Ogonowski M M and Lang F 1979 Histochemical evidence for enzyme differences in crustacean fast and slow muscle; J. Exp. Zool. 207 143–151 Ogonowski M M, Lang F and C K Govind 1980 Histochemistry of lobster claw-closer muscles during development; J. Exp. Zool . 213 359–367 O’Neill D J and Cobb J S 1979 Some factors influencing the outcome of shelter competition in lobsters (Homarus ameri- canus); Mar. Behav. Physiol. 6 33–45 Okamoto K 1991 Abnormality found in the cheliped of Geryon affinis granulatus Sakai; Res. Crust. 20 63–65 Paulian R 1936 L’existence d’un stade critique dans la crois- sance relative de l’ Eupagurus prideauxi (Crustacée ano- moure); C.R. Seances Soc. Biol. Ses Fil. 121 435–437 Peebles J B 1979 The role of prior residence and relative size in competition for shelter by the Malaysian prawn Macro- brachium rosenbergii; Fish. Bull. 76 905–911 Pillai G 1990 Notes on the chelae of the mangrove lobster Tha- lassina anomala (Decapoda, Thalassinidae); Crustaceana 59 89–95 Pinheiro M A A and Fransozo A 1993 Relative growth of the speckled swimming crab Arenaeus cribrarius (Lamarck, 1818) (Brachyura, Portunidae), near Ubatuba, State of Sao Paulo, Brazil; Crustaceana 65 377–389 Pinheiro M A A and Fransozo A 1998 Sexual maturity of the speckled swimming crab Arenaeus cribrarius (Lamarck, 1818) (Decapoda, Brachyura, Portunidae), in the Ubatuba lit- toral, Sao Paulo State, Brazil; Crustaceana 71 434–452 Powell M L, Hammer H S and Watts S A 1998 Observations on the frequency of claw loss in the crayfish Procambarus clarkii; J. World Maricult. Soc. 29 485–490 Przibram H 1901 Experimentelle studien uber regeneration; Arch. Ent. Mech. Org. 11 321–345 Quigley M M and Mellon DeF Jr 1984 Changes in myofibrillar gene expression during fibre-type transformation in the claw closer muscles of the snapping shrimp Alpheus heterochelis; Dev. Biol. 106 262–265 Ra’anan Z and Cohen D 1985 The ontogeny of social structure and population dynamics in the freshwater prawn, Macro- brachium rosenbergii (de Man); in Crustacean issues II. Crustacean growth (eds) F M Schram and A Wenner (Rotter- dam: Balkema) pp 271–311 Ra’anan Z and Sagi A 1985 Alternative mating strategies in male morphotypes of the freshwater prawn Macrobrachium rosenbergii (de Man); Biol. Bull. 169 592–601 Read A T and Govind C K 1997 Regeneration and sex-biased transformation of the sexually dimorphic pincer claw in adult snapping shrimps; J. Exp. Zool . 279 356–366 Reverberi G 1943 Sul significato della “castrazione parassi- taria”. La trasformazione del sesso nei Crostacei parassiti da Bopiridi e da Rizocefali; Pubbl. Stn. Zool. Napoli 19 225– 316 Robinson M H, Abele L G and Robinson B 1970 Attack auto- tomy: A defense against predators; Science 169 300–301 Sagi A 1984 Alternative reproduction strategies in male popu- lation of the freshwater prawn Macrobrachium rosenbergii, M.Sc. Thesis, Hebrew University, Jerusalem Salmon M and Hyatt G W 1983 Communication; in The biology of crustacea (ed.) D E Bliss (New York: Academic Press) vol. 7, pp 1–40 Salmon M, Hyatt G, McCarthy K and Costlow J D Jr 1978 Dis- play specificity and reproductive isolation in the fiddler crabs Uca panacea and U. pugilator. Z. Tierpsychol. 48 251–276 Savage T and Sullivan J R 1978 Growth and claw regeneration of the stone crab, Menippe mercenaria; Florida Mar. Res. Publ. 32 1–23 Schmalbach E A, Harpaz S, Kahan D, Galun R and Frankenberg E 1984 Periodic cheliped autotomy of the males of the Malaysian prawn Macrobrachium rosenbergii; Naturwissen- schaften 71 325–326 Schmitt W L 1965 Crustaceans (Ann Arbor: University of Michigan Press) Schmitz B 2000 Sound production in Crustacea with special reference to the Alpheidae; in Physiology of the Crustacean nervous system (ed.) K Wiese (Springer-Verlag) (in press) Schram F R 1978 Arthropods: A convergent phenomenon; Fieldiana 39 61–108 Schultz S, Wuppermann K and Schmitz B 1998 Behavioural interactions of the snapping shrimp (Alpheus heterochaelis) with conspecifics and sympatric crabs (Eurypanopeus depres- sus); Zool. Anal. Complex Syst. (Suppl I) 101 85 Schuster S M and Caldwell R L 1989 Male defense of the breeding cavity and factors affecting the persistence of breed- ing pairs in the stomatopod, Gonodactylus bredini (Manning) (Crustacea: Hoplocarida); Ethology 82 192–207 Seed R and Hughes R N 1995 Criteria for prey size-selection in molluscivorous crabs with contrasting claw morphologies; J. Exp. Mar. Biol. Ecol. 193 177–195 Sekkelsten G I 1988 Effect of handicap on mating success in male shore crabs Carcinus maenas; Oikos 51 131–134 Sexton E W and Reid D M 1951 The life history of the multi- form species Jassa falcata (Montagu) (Crustacea, Amphi- poda) with a review of the bibliography of the species; J. Linn. Soc. London Zool. 57 29–88 Shelton P M J, Truby P R and Shelton R G J 1981 Naturally occurring abnormalities (Bruchdreifachbildungen) in the che- lae of three species of Crustacea (Decapoda) and a possible explanation; J. Embryol. Exp. Morphol. 63 285–304 Shih H-T, Mok H-K, Chang H-W and Lee S-C 1999 Morpho- logy of Uca formosensis, 1921 (Crustacea: Decapoda: Ocy- podidae), an endemic fiddler crab from Taiwan, with notes on its ecology; Zool. Stud. 38 164–177 Shirley S M and Shirley T C 1988 Appendage injury in dunge- ness crabs, Cancer magister, in Southeastern Alaska; Fish. Bull . 86 156–160 Simonson J L and Steele P 1981 Cheliped asymmetry in the stone crab, Menippe mercenaria, with notes on claw reversal and regeneration; Northeast Gulf Sci. 5 21–30 Skinner D M 1966 Breakdown and reformation of somatic muscle during the molt cycle of land crab, Gecarcinus later- alis; J. Exp. Zool. 163 115–124 Skinner D M 1985 Molting and regeneration; in The biology of crustacea (eds) D E Bliss and T H Mantel (New York: Aca- demic Press) vol 9, pp 43–143 Smith L D 1990a The frequency and ecological consequences of limb autotomy in the blue crab, Callinectes sapidus Rath- bun, Ph D thesis, University of Maryland, Maryland, USA Smith, L D 1990b Patterns of limb loss in the blue crab, Callinectes sapidus Rathbun, and the effects of autotomy on growth; Bull. Mar. Sci. 46 23–36 Smith L D 1995 Effects of limb autotomy and tethering on juve- nile blue crab survival from cannibalism; Mar. Ecol. Prog. Ser. 116 65–74 12 P. MARIAPPAN ET AL.  Smith L D and Hines A H 1991 The effect of cheliped loss on blue crab Callinectes sapidus Rathbun foraging rate on soft- shell clams Mya arenaria L.; J. Exp. Mar. Biol. Ecol. 151 245–256 Smith L D and Palmer A R 1994 Effects of manipulated diet on size and performance of brachyuran crab claws; Science 264 710–712 Snedden W A 1990 Determinants of male mating success in the temperate crayfish Orconectes rusticus: chela size and sperm competition; Behaviour 115 100–113 Spivak E D 1990 Limb regeneration a common South American littoral crab Cyrtograpsus angulatus; J. Natl. Hist. 24 393– 402 Spivak E D and Politis M A 1989 High incidence of limb auto- tomy in crab population from a coastal lagoon in the province of Buenos Aires, Argentina; Can. J. Zool. 67 1976– 1985 Stebbing T R R 1893 A history of Crustacea recent mala- costraca (London: Kegan Paul, Trench, Treubner and Co Ltd.) Stein R A 1976 Sexual dimorphism in crayfish chelae: func- tional significance linked to reproductive activities; Can. J. Zool. 54 220–227 Stephens P J and Mellon DeF Jr 1979 Modification of structure and synaptic physiology in transformed shrimp muscle; J. Comp. Physiol . 132 97–108 Stevens B G, Donaldson W E, Haaga J A and Munk J E 1993 Morphometry and maturity of paired Tanner crabs, Chionoe- cetes bairdi, from shallow and deepwater environments; Can. J. Fish. Aquat. Sci. 50 1504–1516 Suzuki H 1963 An abnormality found in the cheliped of Mac- ropthalmus japonicus De Haan; Res. Crust. 1 51–53 Swartz R C 1972 Postlarval growth and reproduction in the painted ghost crab Neopanope texana sayi, Ph D thesis, Col- lege of William and Mary, Tiegs O W and Manton S M 1958 The evolution of the Arthro- poda; Biol. Rev . 33 255–337 Trott T J 1987 The prevalence of left-handedness in the painted ghost crab Ocypode gaudichaudii H. Milne Edwards and Lucas (Decapoda, Brachyura, Ocypodidae); Crustaceana 52 213–215 Tucker B W 1930 On the effects of an epicaridan parasite, Gyge branchialis, on Upogebia littoralis; Q. J. Microsc. Sci. (N.S.) 74 1–118 Tweedie M W F 1950 The fauna of the Cocos-Keeling Islands, Brachyura and Stomatopoda; Bull. Raffles Mus . 22 105–148 Vermeij G J 1977 Patterns in claw size: the geography of crush- ing; Syst. Zool. 26 138–151 Warner G F and Jones A R 1976 Leverage and muscle type in crab chelae (Crustacea: Brachyura); J. Zool. 180 57–68 Wilson E B 1903 Notes on the reversal of asymmetry in the regeneration of chelae in Alpheus heterochelis; Biol. Bull . 4 197–210 Wood F D and Wood W H 1932 Autotomy in decapod Crusta- cea; J. Exp.. Zool . 62 1–55 Yamaguchi T 1973 Asymmetry and dimorphism of chelipeds in the fiddler crab, Uca lactea De Haan; Zool. Mag. 82 154–158 Yamaguchi T 1977 Studies on the handedness of the fiddler crab, Uca lactea; Biol. Bull. 152 424–436 Yamaguchi T and Aratake H 1997 Morphological modifications caused by Sacculina polygenea in Hemigrapsus sanguineus (De Haan) (Brachyura: Grapsidae); Crust. Res. 26 125–145 Young R E, Pearce J and Govind C K 1994 Establishment and maintenance of claw bilateral asymmetry in snapping shrimps; J. Exp. Zool . 269 319–326 13 P. MARIAPPAN ET AL. |

