 Pharmacology & Pharmacy, 2011, 2, 361-369 doi:10.4236/pp.2011.24047 Published Online October 2011 (http://www.SciRP.org/journal/pp) Copyright © 2011 SciRes. PP 361 Bucolome N-Glucuronide Formation: Species Differences and Identification of Human UDP-Glucuronosyltransferase Isoforms Humihisa Kanoh, Makiko Tada, Yoshihiro Uesawa, Kiminori Mohri* Clinical Pharmaceutics Laboratory, Department of Pharmacy and Health Sciences, Faculty of Pharmacy and Pharmaceutical Sciences, Meiji Pharmaceutical University, Tokyo, Japan. Email: *k-mohri@my-pharm.ac.jp Received August 4th, 2011; revised September 13th, 2011; accepted September 20th, 2011. ABSTRACT The barbituric acid derivative bucolome (BCP) is a nonsteroidal anti-inflammatory drug. The present study investi- gated whether BCP N-glucuronide (BCP-NG, the primary metabolite of BCP) was produced in mammalian species other than rats, and attempted to identify the UDP-glucuronosyltransferase (UGT) isoform (s) responsible for forma- tion of BCP-NG in humans. BCP-NG was detected in all species tested. The results were as follows (pmol equivalent/ min/mg protein): rat, 479 ± 83; Mongolian gerbil, 378 ± 9; rabbit, 275 ± 26; guinea pig, 257 ± 10; human, 242 ± 18; hamster, 177 ± 22; and mouse, 167 ± 15. Since human liver microsomes formed BCP-NG, we investigated the metabo- lites of BCP excreted in the urine of a patient after oral administration of BCP (600 mg). BCP and BCP-NG were ex- creted in the urine at amounts of 2.9 mg (about 0.5% of the do se) and 14.4 mg (about 2.5% of the dose) over 12 hours. In order to identify the UGT isofo rms involved in formation of BCP-NG in humans, we investigated BCP-NG formation by the microsomes of insect cells expressing each of twelve UGT isoforms (hUGT1A1, 1A3, 1A4, 1A6, 1A7, 1A8, 1A9, 1A10, 2B4, 2B7, 2B15, and 2B17). As a result, BCP-NG formation (pmol equivalents/min/mg protein) was observed with microsomes expressing hUGT1A1 (142), 1A3 (196), 1A4 (8), 1A7 (8), 1A8 (66), 1A9 (38), 1A10 (9), 2B4 (7) and 2B7 (16). In particular, the activity of hUGT1A1 and 1A3 was high. These results suggest that the UGT isoforms re- sponsible for formation of BCP-NG exist in various mammalian species, including humans, and that the UGT 1A family is primarily responsible for BCP N-glucuronide formation in humans. Keywords: Bucolome, Bucolome N-Glucuronide, UGT Isoforms, Barbiturate, Barb iturate N-Glucuronide 1. Introduction Glucuronic acid conjugation is an enzymatic reaction catalyzed by UDP-glucuronosyltransferase (UGT; EC 2.4.1.17), and it is one of the most important reactions in phase II drug metabolism. UGT is widely present in many species from bacteria to humans, and glucuronic acid conjugation is estimated to account for about 35% of phase II drug metabolism [1]. Bucolome (BCP, Figure 1) is a barbituric acid derivative nonsteroidal anti-inflam- matory drug that exhibits analgesic and anti-inflamma- tory actions without having sedative or hypnotic effects, unlike many barbiturates. It has been used for the treat- ment of rheumato id arthritis [2]. It has been reported that BCP promotes the secretion of bile in dogs and rats [3,4] and induces uric acid excretion in humans [5], so BCP is also used as an antipodagric. In recent years, it has been shown that BCP inhibits CYP2C9 [6]. In patients on warfarin therapy in Japanese hospitals, BCP is often ad- ministered concomitantly to maintain the plasma war- farin concentration through inhibition of the metabolism of S-warfarin by CYP2C9 [7]. Although BCP is used for various purposes, as noted above, its metabolic pathway and the enzymes involved in humans have not been evaluated in detail, with only old references being avail- able [5,8-11]. In order to clarify whether the formation of BCP-NG occurs in mammals other than rats, we investigated BCP- NG formation in vitro using liver microsomes from rats, guinea pigs, mice, hamsters, Mongolian gerbils, rabbits, and humans. Although the N-glucosides of amobarbital [12] and phenobarbital [13] have been reported to be the primary metabolites of these barbiturate derivatives in  Bucolome N-Glucuronide Formation: Species Differences and Identification of Human 362 UDP-Glucuronosyltransferase Isoforms Figure 1. Chemical structures of bucolome and bucolome N-glucuronide. humans, their N-glucuronides have not been identified. Based on the results of BCP-NG formation by human liver microsomes in the present study, we also assessed BCP metabolites in the urine of a patient with hyperu- ricemia who was administered BCP at 600 mg/day. We found BCP-NG excretion in the urine at 2.5% of th e dose over 12 hours. In order to identify the UGT isoforms involved in N-glucuronidation of BCP, we also studied BCP-NG formation using insect cell microsomes ex- pressing 12 different human UGT isoforms (hUGT1A1, 1A3, 1A4, 1A6, 1A7, 1A8, 1A9, 1A10, 2B4, 2B7, 2B15, and 2B17). This report describes the UGT isoforms catalyzing BCP-NG formation in humans. 2. Materials and Methods 2.1. Chemicals, Experimental Animals, and Enzymes All animal procedures were approved by the Meiji Pharmaceutical University Committee for Ethics of Ex- perimentation and Animal Care (approved No. 2304). BCP was synthesized from cyclohexylurea (Tokyo Che- mical Industry Co., Ltd., Tokyo) and n-butylmalonate (Tokyo Chemical Industr y) by the method of Send a et al. [2]. Paramidine (300 mg) was purchased from Aska Pharmaceutical Co., Ltd., (Tokyo). BCP-NG was ob- tained by the previously reported method [14]. Lubrol WX was purchased from Sigma-Aldrich Co. (St. Louis, MO). Phenylbutazone (PBZ, internal standard, I.S.) and saccharo-1,4-lactone were purchased from Nacalai Tes- que, Inc. (Kyoto), while magnesium chloride (MgCl2), 2-amino-2-hydroxymethyl-1,3-propanediol (Tris), am- monium sulfate, UDP-glucuronic acid (UDP-Ga), metha- nol (MeOH), and ethanol (EtOH) were all from Wako Pure Chemical Industries Ltd. (Osaka). Alamethicin was purchased from Enzo Life Sciences Inc. (New York). Solutions of BCP and PBZ adjusted to 1 mg/ml with DMSO were used as standard solutions, which were di- luted with the solvent immediately before use. Reagents were stored at 30˚C and were used within 3 months. Male Wistar-ST rats weighing 300 - 500 g (clean), male Hartley guinea pigs weighing 550 - 700 g (clean), male ddY mice weighing 60 - 80 g (SPF), male Syrian ham- sters weighing 185 - 200 g (SPF), male Mongolian ger- bils weighing 60 - 63 g (SPF), and male Japanese white rabbits weighing 2.0 - 2.2 kg (clean) were all purchased from Japan SLC Inc. (Shizuoka). The animals were housed under conventional conditions until use. Recom- binant hUGT isoform microsomes for various human UGT isoforms (UGT1A1, 1A3, 1A4, 1A6, 1A7, 1A8, 1A9, 1A10, 2B4, 2B7, 2B15, and 2B17) were expressed in insect cells using a baculovirus and 50-donor pooled human liver microsomes were obtained from BD Gentest (San Jose, CA). All chemicals used were analytical or special grade products, and the water was double-dis- tilled. Liver microsomes of rats, mice, guinea pigs, ham- sters, Mongolian gerbils, and rabb its were prepared from 100 g of liver tissue harvested from four animals by the previously reported method [15]. Protein concentrations were determined by the method of Lowry et al. [16]. 2.2. HPLC and Chromatography Conditions BCP-NG formation in vitro and the levels of BCP and BCP-NG in the urine of a patient were measured by the method of Mohri et al. with slight modifications (2001). The HPLC system consisted of a JASCO Intelligent HPLC pump (Model PU-1580, JASCO Co. Ltd., Tokyo) equipped with a JASCO Intelligent UV detector (Model UV-1570), a JASCO Intelligent Sampler (Model 851-AS) and a JASCO Chromatography Data Station (Chrom NAV). The detector was set at 268 nm, with a sensitivity of 0.005 absorbance units full scale (a.u.f.s.). BCP, PBZ (I.S.), and BCP-NG were separated at room temperature (23˚C) on a reversed-phase Capcell Pak ODS column (UG120) [6.0 mm in internal diameter (I.D.) 15 cm in length and particle size of 5 m] (Shiseido Co. Ltd., To- kyo) equipped with a guard column packed with the same stationary phase [4.6 mm I.D. 1 cm]. The mobile phase (0.05 M phosphate buffer (pH 5.7):MeOH:THF; 50:40:4 v/v) was pumped through the column at a rate of 1.5 ml/min, after being passed through a 0.45 mm filter (Millipore, Bedfo rd, MA) prior to use and degassed with an ERC-3322 degasser (Erma Co. Ltd., Saitama) under reduced pressure. All HPLC analyses were performed in triplicate. 2.3. LC/MS Analysis of BCP-NG A Shimadzu HPLC mass spectrometer (LCMS-2010EV, Shimadzu Corp., Kyoto) was used, with an LC/MS pump (LC-20AB) attached to an autosampler (SIL-20A), as well as a degasser (DGU-20A5), column oven (CTO- 20SA), and system controller (CBM-20A). A Shim-pack Copyright © 2011 SciRes. PP  Bucolome N-Glucuronide Formation: Species Differences and Identification of Human 363 UDP-Glucuronosyltransferase Isoforms VP-ODS column (2.0 mm i.d. × 50 mm) attached to a GVP-ODS guard column was used at an oven tempera- ture of 40˚C, and analysis was performed with the ESI method in negative ion mode after setting the mobile phase flow rate at 0.2 ml/min, N2 gas flow rate at 1.5 l/min, probe voltage at 1.5 kV, probe temperature at 250˚C, CDL voltage at 20.0 V, and block temperature at 200˚C. Reversed-phase HPLC was done by flow injec- tion and the elution conditions were as follows: the ratio of mobile phase A (0.1 % formic acid) to mobile phase B (acetonitrile) was altered from 7:3 at 0 minutes to 5:5 at 5 minutes according to a linear gradient and further to 2:8 at 8 minutes according to a linear gradient by increasing acetonitrile. It was then maintained at this level for 5 minutes, reduced stepwise to 7:3 (v/v), and maintained at that level for 6 minutes. Ion detection was performed by the electrospray ionization procedure at an m/z value of 265 for BCP, 307 for PBZ (I.S.), and 441.20 for BCP- NG. 2.4. BCP-NG Formation by Liver Microsomes of Humans and Experimental Animals The concentration of each reagent in the reaction mixture yielding maximum BCP-NG formation was determined in advance using rat liver microsomes (final concentra- tion range tested: lubrol 0 - 0.4 mg/ml, rat liver micro- somes 0 - 5 mg/ml, MgCl2 0 - 10 mM, saccharolactone 0 - 8 mM, BCP 0 - 5 mM, 0.5 M Tris-HCl pH6 - pH8, UDP-Ga 0 - 10 mM, and incubation time 0 - 60 min). Enzymatic reactions were performed in a total volume of 250 l containing UDP-Ga (final concentration, 8 mM), liver microsomes solubilized with lubrol (final concen- tration, 0.1 mg/ml) of each species (rat, mouse, guinea pig, hamster, Mongolian gerbil, rabbit, and human; 2 mg/ml microsomal protein), MgCl2 (final concentration, 8 mM), saccharo-1,4-lactone (final concentration, 4 mM), BCP (final concentration, 2 mM), and 0.5 M Tris-HCl (pH 7.4) (final concentration, 0.2 M). After the reaction mixture had been preincubated at 37˚C for 5 minutes, the reaction was started by addition of UDP-Ga or BCP at 37˚C for 20 minutes, and then the reaction was stopped by adding 0.5 g ammonium sulfate. After adding 200 ml of an EtOH solution containing 24 M PBZ, the resulting mixture was mixed vigorously for 30 seconds. It was then centrifuged at 18,000 × g for 10 minutes at 4˚C, and 10 l of 0.1% NaHCO3 was added to 150 l of the supernatant. Next, 10 l of the supernatant was di- rectly injected into the HPLC apparatus and the concen- tration of BCP-NG formed by the enzymatic reaction was determined using the BCP calibration curve, as de- scribed previously [15]. All measurements were per- formed in triplicate. 2.5. Stabilization of BCP-NG Since BCP-NG was stable under weakly basic conditions, 10 l of 0.1% NaHCO3 was added to 150 l of super- natant. 2.6. Ethanol Extraction Method An excess of solid ammonium sulfate (0.5 g) was added to the reaction solution, wh ich was mixed vigorously an d then centrifuged at 18,000 g for 10 minutes at 4˚C to separate water and EtOH. This procedure using the salt- ing out method is able to transfer highly water-soluble compounds such as glucuronides into the EtOH layer at high concentrations. 2.7. Km, Vmax, and Intrinsic Metabolic Clearance (CLmet) of BCP and UDP-Ga in Experimental Animals In order to compare BCP-NG formation among the ex- perimental animals tested (rat, guinea pig, mouse, ham- ster, Mongolian gerbil, and rabbit), the Km, Vmax, and intrinsic metabolic clearance (CLmet = Vmax/Km) were obtained. To obtain Km and Vmax values fo r BCP, its con- centration was varied over the range from 0.01 mM to 3 mM. The amount of UDP-Ga used in this reaction was 22 mM for rats, 8 mM for mice, 8 mM for guinea pigs, 6 mM for hamsters, 10 mM for Mongolian gerbils, and 6 mM for rabbits. To obtain Km and Vmax values for UDP-Ga, its concentration was varied over the range from 0.01 mM to 24 mM. BCP was used at 2 mM in this enzymatic reaction. Human microsomes were pooled from 50 persons. Since both racial differences and inter- individual variations are very large, we did not obtain Km and Vmax values for BCP N-glucuronidation using human liver microsomes, because Homo sapiens is not a homo- geneous species. The initial velocity of BCP-NG forma- tion in relation to the BCP dose and UDP-Ga dose was analyzed using Lineweaver-Burk plots. All measure- ments were performed in triplicate. 2.8. Analysis of Urinary BCP and BCP-NG in a Patient with Hyperuricemia We received written informed consent from a patient to measure the levels of BCP and BCP-NG in the urine. Total urine was collected from a 23-year-old patient with hyperuricemia at 0, 1, 2, 3, 4, 7, 8, 9, and 12 hours after administration of 600 mg of BCP (2 tablets of Para- midin™ 300 mg), and the urine volume and the urinary levels of BCP and BCP-NG concentrations were deter- mined at each sampling point. The pH of the urine sam- ples was adjusted to about 8 by adding solid NaHCO3. To 250 l of urine, 200 l of an EtOH solution contain- Copyright © 2011 SciRes. PP 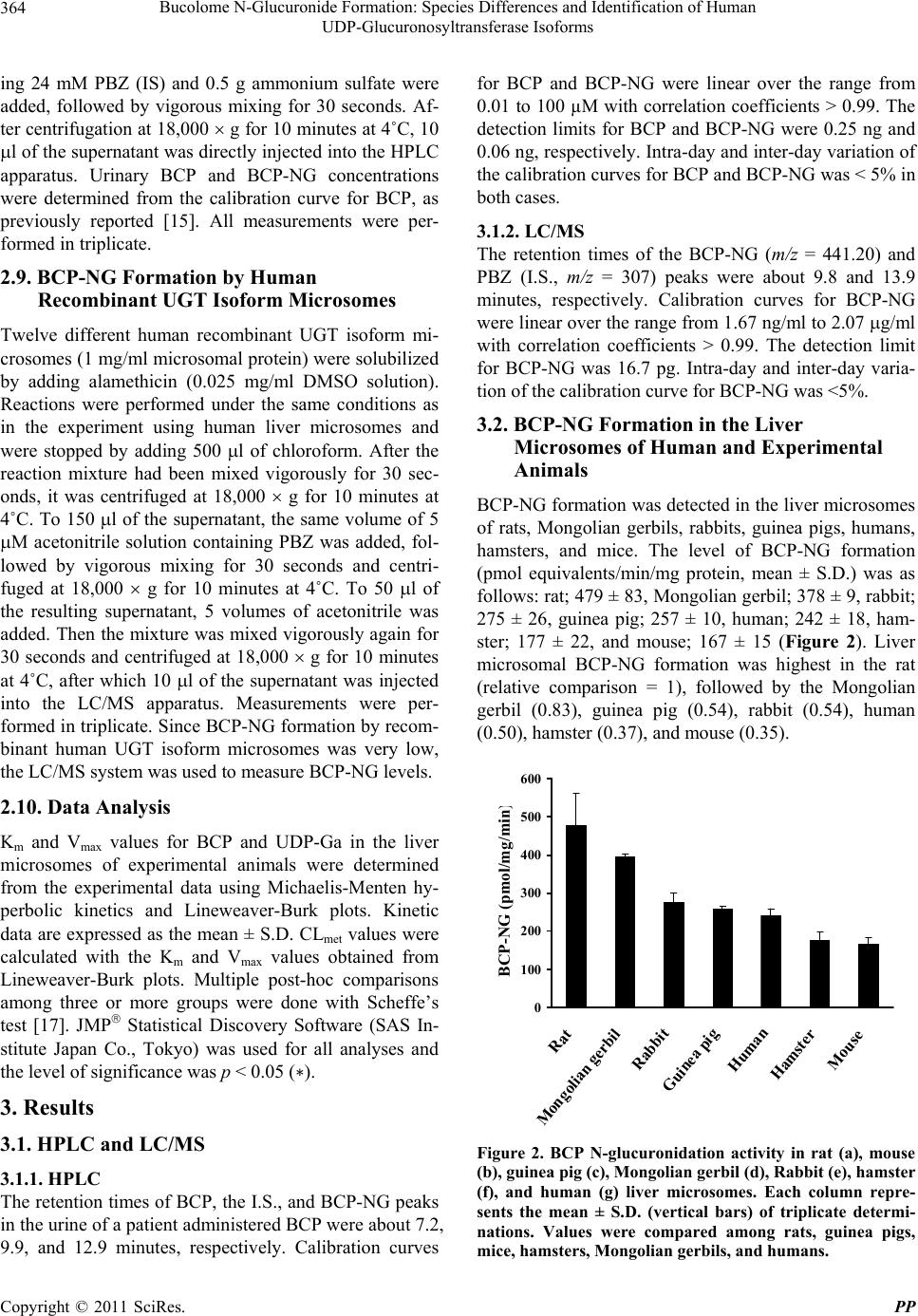 Bucolome N-Glucuronide Formation: Species Differences and Identification of Human 364 UDP-Glucuronosyltransferase Isoforms ing 24 mM PBZ (IS) and 0.5 g ammonium sulfate were added, followed by vigorous mixing for 30 seconds. Af- ter centrifugation at 18,000 g for 10 minutes at 4˚C, 10 l of the supernatant was directly injected into the HPLC apparatus. Urinary BCP and BCP-NG concentrations were determined from the calibration curve for BCP, as previously reported [15]. All measurements were per- formed in triplicate. 2.9. BCP-NG Formation by Human Recombinant UGT Isoform Microsomes Twelve different human recombinant UGT isoform mi- crosomes (1 mg/ml microsomal protein) were solubilized by adding alamethicin (0.025 mg/ml DMSO solution). Reactions were performed under the same conditions as in the experiment using human liver microsomes and were stopped by adding 500 l of chloroform. After the reaction mixture had been mixed vigorously for 30 sec- onds, it was centrifuged at 18,000 g for 10 minutes at 4˚C. To 150 l of the supernatant, the same volume of 5 M acetonitrile solution containing PBZ was added, fol- lowed by vigorous mixing for 30 seconds and centri- fuged at 18,000 g for 10 minutes at 4˚C. To 50 l of the resulting supernatant, 5 volumes of acetonitrile was added. Then the mixture was mixed vigorously again for 30 seconds and centrifuged at 18,000 g for 10 minutes at 4˚C, after which 10 l of the supernatant was injected into the LC/MS apparatus. Measurements were per- formed in triplicate. Since BCP-NG formation by recom- binant human UGT isoform microsomes was very low, the LC/MS system was used to measure BCP-NG levels. 2.10. Data Analysis Km and Vmax values for BCP and UDP-Ga in the liver microsomes of experimental animals were determined from the experimental data using Michaelis-Menten hy- perbolic kinetics and Lineweaver-Burk plots. Kinetic data are expressed as the mean ± S.D. CLmet values were calculated with the Km and Vma x values obtained from Lineweaver-Burk plots. Multiple post-hoc comparisons among three or more groups were done with Scheffe’s test [17]. JMP Statistical Discovery Software (SAS In- stitute Japan Co., Tokyo) was used for all analyses and the level of significance was p < 0.05 (כ). 3. Results 3.1. HPLC and LC/MS 3.1.1. HPLC The retention times of BCP, the I.S., and BCP-NG peaks in the urine of a patient administered BCP were about 7.2, 9.9, and 12.9 minutes, respectively. Calibration curves for BCP and BCP-NG were linear over the range from 0.01 to 100 µM with correlation coef ficients > 0.99. The detection limits for BCP and BCP-NG were 0.25 ng and 0.06 ng, respectively. Intra-day and inter-day variation of the calibration curves for BCP and BCP-NG was < 5% in both cases. 3.1.2. LC/MS The retention times of the BCP-NG (m/z = 441.20) and PBZ (I.S., m/z = 307) peaks were about 9.8 and 13.9 minutes, respectively. Calibration curves for BCP-NG were linear over th e range from 1.67 ng/ml to 2.07 g/ml with correlation coefficients > 0.99. The detection limit for BCP-NG was 16.7 pg. Intra-day and inter-day varia- tion of the calibration curve for BCP-NG was <5%. 3.2. BCP-NG Formation in the Liver Microsomes of Human and Experimental Animals BCP-NG formation was detected in the liver microsomes of rats, Mongolian gerbils, rabbits, guinea pigs, humans, hamsters, and mice. The level of BCP-NG formation (pmol equivalents/min/mg protein, mean ± S.D.) was as follows: rat; 479 ± 83, Mon golian gerbil; 378 ± 9, rabbit; 275 ± 26, guinea pig; 257 ± 10, human; 242 ± 18, ham- ster; 177 ± 22, and mouse; 167 ± 15 (Figure 2). Liver microsomal BCP-NG formation was highest in the rat (relative comparison = 1), followed by the Mongolian gerbil (0.83), guinea pig (0.54), rabbit (0.54), human (0.50), hamster (0.37), and mouse (0.35). 0 100 200 300 400 500 600 Rat Mongolian gerbil Rabbit Guinea pig Human Hamster Mouse BCP-NG (pm ol/mg/min Figure 2. BCP N-glucuronidation activity in rat (a), mouse (b), guinea pig (c), Mongolian gerbil (d), Rabbit (e), hamster (f), and human (g) liver microsomes. Each column repre- sents the mean ± S.D. (vertical bars) of triplicate determi- nations. Values were compared among rats, guinea pigs, mice, hamsters, Mongolian gerbils, and humans. Copyright © 2011 SciRes. PP  Bucolome N-Glucuronide Formation: Species Differences and Identification of Human UDP-Glucuronosyltransferase Isoforms Copyright © 2011 SciRes. PP 365 3.3. Kinetic Analysis of BCP-NG Formation by Liver Microsomes in Experimental Animals The Km, Vmax, and CLmet v alues for BCP and UDP-Ga are shown in Tables 1(a) and 1(b). The kinetic profile of BCP N-glucuronidation for BCP and UDPGa in the liver microsomes of the 6 mammalian species tested showed a single-enzyme Michaelis-Menten pattern, and no sig- moidal kinetics were noted. The rate of BCP-NG forma- tion in relation to the BCP dose and UDP-Ga dose was analyzed using Lineweaver-Burk plots. Statistically sig- nificant species differences were observed for the Km, Vmax, and CLmet values of BCP and UDP-Ga. 3.4. Analysis of BCP and BCP-NG in the Urine of a Patient with Hyperuricemia The HPLC chromatogram of urine obtained 2 hours after administration of BCP is shown in Figure 4. Cumulative BCP excretion in the urine up to 12 hours after admini- stration was about 3 mg, which was about 0.5% of the dose administered. Cumulative BCP-NG excretion was 14.4 mg, which was about 2.5% of the BCP dose. 3.5. BCP-NG Formation by Recombinant Human UGT Isoforms BCP-NG activity was noted in 9 (hUGT 1A1, 1A3, 1A4, 1A7, 1A8, 1A9, 1A10, 2B4, and 2B7) of the 12 hUGT isoforms (UGT1A1, 1A3, 1A4, 1A6, 1A7, 1A8, 1A9, 1A10, 2B4, 2B7, 2B15, and 2B17), and was particularly high for hUGT1A1 and 1A3 (Figure 3). The initial ve- locities of the BCP-NG activity of hUGT1A1, 1A3, 1A4, 1A7, 1A8, 1A9, 1A10, 2B4, and 2B7 were 142, 196, 8, 8, 66, 38, 9, 7, and 16 pmol equivalents/min/mg protein, respectively, while those of the other recombinant UGT isoforms were 0.2 pmol equivalents/min/mg protein. Statistically significant differences were observed for apparent BCP-NG formation by the hUGT isoforms. Table 1. Kinetic parameters for BCP and UDP-Ga during BCP N-glucuronidation by liver microsomes pre- pared from each animal species. (a) Kinetic parameters for BCP Species Km (mM) Vmax (nmol /min/m g) CLmet (L/min/mg) Rat (a) 1.02 ± 0.04 1.46 ± 0.07b,c,d,e,f 1.43 ± 0.09 Mouse (b) 0.16 ± 0.07 0.17 ± 0.02 1.20 ± 0.49 Guinea pig (c) 0.35 ± 0.06 0.33 ± 0.02 0.96 ± 0.13 Mongolian gerbil (d) 1.25 ± 0.14b 0.91 ± 0.03b,c,e,f 0.74 ± 0.06 Rabbit (e) 0.49 ± 0.14 0.34 ± 0.01 0.72 ± 0.20 Hamster ( f) 1.93 ± 0.78b,c,e 0.56 ± 0.16b,c 0.30 ± 0.06a,b Correlation coefficient with BCP-NG formation 0.144 0.893 0.461 (b) Kinetic parameters for UDP-Ga Species Km (mM) Vmax (nmol/min/m g ) CLmet (L/min/mg) Rat (a) 8.350 ± 0.490b,c,d,e,f 1.220 ± 0.130b,c,d,e,f 0.146 ± 0.017 Mouse (b) 0.505 ± 0.070 0.177 ± 0.018 0.351 ± 0.014a,c,d,e,f Guinea pig (c) 1.210 ± 0.230 0.315 ± 0.036 0.262 ± 0.018a,f Mongolian gerbil (d) 2.200 ± 0.270b,c 0.558 ± 0.035b,c,e,f 0.255 ± 0.016a,f Rabbit (e) 1.370 ± 0.110 0.341 ± 0.027 0.250 ± 0.032a,f Hamster ( f) 1.560 ± 0.140b 0.214 ± 0.021 0.138 ± 0.017 Correlation coefficient with BCP-NG formation 0.827 0.925 –0.384 Values are the mean ± S.D. of triplicate experiments. CLmet was calculat ed as Vmax/Km. Small su perscript l etters (a, b, c, d, e, f) indicate th e results of multiple comparison by Scheffé’s test in each species (p < 0.05).  Bucolome N-Glucuronide Formation: Species Differences and Identification of Human 366 UDP-Glucuronosyltransferase Isoforms 0 50 100 150 200 250 1A1 1A3 1A4 1A6 1A7 1A8 1A9 1A10 2B4 2B7 2B15 2B17 BCP-NG (pmol/min/mg ) Figure 3. BCP N-glucuronidation activity of recombinant human UGT isoforms. Each column represents the mean ± S.D. (vertical bars) of triplicate determinations. The lower limit of quantitation for the assay under these conditions was 1.67 ng of BCP-NG. 0 5000 10000 15000 0510 15 20 Time (min) Signal intensity (v) IS (a) 0 5000 10000 15000 0510 15 20 Time (min) Signal intensity (v) BCP ISBCP-NG (b) Figure 4. HPLC chromatogram of a urine sample from a patient with hyperuricemia administered BCP. (a) Before dosing; (b) 2 h after dosing. 4. Discussion Although many N-glucuronides have been reported, most of them are compounds that undergo N-glucuronidation, including primary aromatic amines (UGT1A1, 1A4, 1A9), hydroxylamines (UGT1A1, 1A10), amides (2B7), tertiary aliphatic amines (1A4, 2B10), and aromatic N-heterocycles (1A4, 2B7, 2B10). A number of aromatic N-heterocycles with five- and six-member rings, such as imidazoles, pyrazoles, triazines, tetrazoles, and pyridines are subject to N-glucuronidation. However, N-glucuro- nidation of the pyrimidine skelton of barbiturates has not been mentioned in recent review articles [18-20]. There have been a few reports of N-glucosides and N-glucuronides in which glucose or glucuronic acid is directly attached to a nitrogen (N) atom in the pyrimidine skelton of barbiturates. It has b een reported that amobar- bital and phenobarbital N-glucosid es, in which glucose is directly bound to an N atom of the pyrimidine skeleton, are the primary metabolites of barbiturate derivatives in humans, but N-glucuronides of barbiturate derivatives have not been reported to date in humans [12,13,21,22]. Neighbors et al. [23] reported that an N-glucoside and an N-glucuronide were found in the urine of mice adminis- tered phenobarbital. BCP-NG, the first N-glucuronide of a barbiturate derivative, has only been reported in rats, but there had been no investigation of whether or not BCP-NG is produced in other species. Therefore, we established an in vitro method to assay BCP-NG forma- tion using rat liver microsomes, and studied BCP-NG formation in the liver microsomes of Mongolian gerbils, guinea pigs, rabbits, hamsters, mice, and humans. BCP-NG formation was observed in all species, but the activity varied greatly among the different species (Fig- ure 2). These results suggest that UGT isoforms forming BCP-NG exist in a wide range of mammalian species. We calculated Km, Vmax, and CLmet valu es for BCP and UDP-Ga under the conditions showing maximum BCP- NG formation by the liver microsomes of rats, Mongo- lian gerbils, guinea pigs, rabbits, hamsters, and mice (Tables 1(a) and 1(b)). A significant relation was ob- served between BCP-NG formation in these 6 animals and the Vmax and Km values for UDP-Ga (R = 0.925 and R = 0.827, respectively) (Table 1(b). However, although a significant relation was observed between BCP-NG formation in the 6 animals and Vmax values for BCP (R = 0.894), no relation was observed between BCP-NG for- mation and Km values for BCP (R = 0.144). Glucuronic acid conjugation is a two-substrate reaction (BCP and UDP-Ga). It is thus considered that BCP-NG formation occurs as follows: first, UDP-Ga binds to UGT in the endoplasmic reticulum, and then BCP binds to UGT- UDP-Ga complex, resulting in the formation of BCP-NG. Thus, if UGT binds more readily with UDP- Ga, an ani- mal will produce BCP-NG easily, resulting in a high Vmax value for UDP-Ga. Luukkanen et al. [24] reported that the glucuronidation of entacapone by UGT1A9 was inhibited by 1-naphthol in an entacapone-competitive fashion and was noncom- petitive with respect to UDP-Ga. Inhibition by UDP, on the other hand, was noncompetitive with respect to enta- Copyright © 2011 SciRes. PP  Bucolome N-Glucuronide Formation: Species Differences and Identification of Human 367 UDP-Glucuronosyltransferase Isoforms capone and competitive with respect to UDPGA. They stated that the reaction involved a compulsory ordered bi bi mechanism based on analysis of inhibition profiles, in which UDP-Ga was the first binding substrate and enta- capone was the second binding substrate. The mecha- nism of BCP-NG formation, in which the first binding substrate is UDP-Ga and the second binding substrate is BCP, agrees well with the results of Luukkanen et al. [24]. The differences of kinetic parameters for BCP and UDP-Ga among animal species are believed to be due to complex reactions caused by species differences of UGT isoforms, so when drug metabolism data obtained from laboratory animals are extrapolated to human, differences in the metabolism of animal species must be taken into consideration. Since BCP-NG was formed by human liver micro- somes, we investigated metabolites of BCP in the urine of a patient with hyperuricemia who was administered 600 mg of BCP (2 tablets of Paramidin 300 mg). Our results demonstrated that BCP was metabolized to BCP-NG and excreted in the urine (about 2.5% of the BCP dose over 12 hours) (Figure 4). Thus, it was clari- fied that N-glucuronidation is also the primar y metabolic pathway of BCP in humans. It is known that N-glucoside is the primary human metabolite of barbiturates, so we investigated BCP N-glucoside formation in human liver microsomes by using UDP-glucose (8 mM) instead of UDP-Ga. After 2 hours of incubation under the same conditions as for BCP-NG formation in vitro, HPLC was performed, but an unknown new peak could not be de- tected on the chromatogram (data not shown). Further investigation will b e needed to determine the factors that select N-glucoside and/or N-glucuronide as the metabo- lite of barbituric acid derivatives. In order to identify UGT isoforms that catalyze the N-glucuronidation of BCP in humans, we studied BCP- NG formation using microsomes of recombinant insect cells expressing each human UGT isoform (hUGT1A1, 1A3, 1A4, 1A6, 1A7, 1A8, 1A9, 1A10, 2B4, 2B7, 2B15, and 2B17) (Figure 3). As a result, BCP-NG formation was noted in hUGT1A1, 1A3, 1A4, 1A7, 1A8, 1A9, 1A10, 2B4, and 2B7, with the specific activity (pmol equivalents/min/mg protein) being particularly high for 1A1 (142.0) and 1A3 (196.2), followed by 1A8 (66.2), 1A9 (38.3), and 2B7 (15.9). The activity of hUGT1A4, 1A7, 1A10, and 2B4 was 10 pmol equivalents/min/mg protein or lo wer. Ohno et al. [25] established a method for the quantifi- cation of mRNAs for hUGT isoforms using real-time RT-PCR, and identified and quantified the levels of hUGT mRNA isoforms in various human organs. Ac- cording to their report, the mRNAs for hUGT1A1, 1A3, 1A5, 1A6, 1A7, 1A8, 1A9, 1A10, 2B4, 2B7, 2B10, 2B11, 2B15, and 2B17 were found in human liver tissue. The expression of hUGT1A1 mRNA was about 30% of that for hUGT2B7 mRNA, while that of hUGT2B4 mRNA was about 9 times higher than that of 2B7 mRNA. BCP-NG formation by 2B4 and 2B7 was lower than by the UGT1A family, but when their relative abundance in the liver (approximately 27 times that of 1A1 for 2B4 and approximately 3 times that of 1A1 for 2B7) is taken into consideration, 2B4 and 2B7 also seem likely to con- tribute to the formation of BCP-NG in humans. In recent years, it has been reported that hepatic UGT1A4 and 2B10 catalyze the N-glucuronidation of aromatic N-heterocycles in humans [18,19]. However, BCP-NG formation by hUGT1A4 was <10 pmol equiva- lents/min/mg protein (Figure 3). The level of hUGT1A4 mRNA in tissues other than the liver is either very low [18,19] or below the detection limit [25]. Therefore, it seems that the contribution of UGT1A4 to BCP-NG formation is negligible. hUGT2B10 has only been de- tected in the liver and small intestine [18,19], and the level of hUGT2B10 mRNA in the small intestine is on ly 0.05% of that in the liver. The ortholog of human 2B10 has not been detected in animals [20], while the UGT1A1 family has been detected in various species [20,26-27] and a number of different tissues [28-30]. We found that BCP-NG was formed in the liver mi- crosomes of rats, Mongolian gerbils, rabbits, guinea pigs, hamsters and mice, as well as in hu mans (Figure 2). Pre- viously, we reported that BCP-NG formation was ob- served with the microsomal fractions of the liver, small and large intestines, and kidney in rats [30]. It is sup- posed that the hUGT isoforms catalyzing BCP-NG for- mation in rats differ from UGT2B10 that is localized to the liver and small intestine in humans. However, mi- crosomes expressing hUGT2B10 are not commercially available, so we could not examine BCP-NG formation directly. Further investigations will be needed to clarify the role of BCP-NG f ormation by hUGT2B10. We previously reported that the liver microsomes of UGT1A family-deficient Gunn rats [29] show dramati- cally low BCP-NG formation (only 8.5% of that in nor- mal rats), while BCP-NG formation by phenobarbital- and clofibric acid-pretreated microsomes was 1.5- and 1.6-fold higher than by untreated microsomes, respec- tively [31]. 5. Conclusions The results obtained in this study suggest that the UGT1A family plays the primary role in the formation of BCP-NG in mammals, including humans, and that UGT2B isoforms may have a complementary role. This Copyright © 2011 SciRes. PP  Bucolome N-Glucuronide Formation: Species Differences and Identification of Human 368 UDP-Glucuronosyltransferase Isoforms is the first report about detection of the N-glucuronid e of a barbiturate derivative in humans. REFERENCES [1] C. Guillemette, “Pharmacogenomics of Human UDP- Glucuronosyltransferase Enzymes,” The Pharmacogeno- mics Journal, Vol. 3, No. 3, 2003, pp. 136-158. doi:10.1038/sj.tpj.6500171 [2] S. Senda, H. Izumi and H. Fujimura, “On Uracil Deriva- tives and Related Compounds. 6,5-Alkyl-2,4,6-trioxoper- hydrophyrimidine Derivatives as Antiphlogistics,” Arz- neimittel-Forschung, Vol. 17, No. 12, 1967, pp. 1519-1523. [3] K. Kitani, S. Tsuruoka, R. Miura and Y. Morita, “The Effect of Bucolome on the Canalicular Bile Formation and Sulfobromophthalein Transport Maximum in the Dog,” Biochemical Pharmacology, Vol. 25, No. 12, 1976, pp. 1377-1381. doi:10.1016/0006-2952(76)90107-6 [4] K. Kitani, M. Nokubo, S. Kanai, R. Miura and T. Uesugi, “The Biliary Excretion of Bucolome in the Rat: A Possi- ble Cause for Choleresis,” Biochemical Pharmacology, Vol. 26, No. 24, 1977, pp. 2457-2461. doi:10.1016/0006-2952(77)90461-0 [5] T. Yashiki, T. Matsuzawa, T. Kondo, Y. Uda and T. Shima, “Studies on the Metabolic Fate and the Pharma- cokinetics of 5-N-Butyl-1-cyclohexyl-2,4,6-trioxoperhy- dropyrimidine (BCP) in Man. I. Identification of the Me- tabolites of BCP in Human Urine,” Chemical & Pharma- ceutical Bulletin, Vol. 19, No. 3, 1971, pp. 468-477. [6] M. Kobayashi, M. Takagi, K. Fukumoto, R. Kato, K. Tanaka and K. Ueno, “The Effect of Bucolome, a CYP2C9 Inhibitor, on the Pharmacokinetics of Losartan,” Drug Metabolism and Pharmacokinetics, Vol. 23, No. 2, 2008, pp. 115-119. doi:10.2133/dmpk.23.115 [7] H. Takahashi, T. Kashima, S. Kimura, N. Murata, T. Takaba, K. Iwade, T. Abe, H. Tainaka, T. Yasumori and H. Echizen, “Pharmacokinetic Interaction between Warfarin and a Uricosuric Agent, Bucolome: Application of in Vi- tro Approaches to Predicting in Vivo Reduction of (S) Warfarin Clearance,” Drug Metabolism and Disposition, Vol. 27, No. 10, 1999, pp. 1179-1186. [8] T. Yashiki, T. Kondo, Y. Uda and H. Mima, “Studies on the Metabolic Fate and the Pharmacokinetics of 5-N-Bu- tyl-1-cyclohexyl-2,4,6-trioxoperhydropyrimidine (BCP) in Man. II. Determination of BCP and Its Metabolite, 1- Cyclohexyl-5-(3-hydroxybutyl)-2,4,6-trioxoperhydropyri- midine by Ultraviolet Absorption Method,” Chemical & Pharmaceutical Bulletin, Vol. 19, No. 3, 1971, pp. 478- 486. [9] T. Yashiki, Y. Uda, T. Kondo and H. Mima, “Studies on the Metabolic Fate and the Pharmacokinetics of 5-N-Bu- tyl-1-cyclohexyl-2,4,6-trioxoperhydropyrimidine (BCP) in Man. III. Gas-Liquid Chromatographic Determination of BCP and Its Metabolites,” Chemical & Pharmaceutical Bulletin, Vol. 19, No. 3, 1971, pp. 487-492. [10] T. Yashiki, T. Matsuzawa, M. Yamada, T. Kondo and Y. Uda, “Studies on the Metabolic Fate and the Pharma- cokinetics of 5-N-Butyl-1-cyclohexyl-2,4,6-trioxoperhy- dro-pyrimidine (BCP) in Man. IV. Pharmacokinetics of BCP in Man Following Oral Administration,” Chemical & Pharmaceutical Bulletin, Vol. 19, No. 5, 1971, pp. 869- 880. [11] T. Yashiki, T. Matsuzawa, M. Yamada, T. Kondo, H. Mima, M. Yamamoto, T. Yamada, M. Nakajima and K. Doi, “Studies on the Metabolic Fate and the Pharmacoki- netics of 5-N-Butyl-1-cyclohexyl-2,4,6-trioxoperhydro- pyrimidine (BCP) in Man. V. Pharmacokinetics of BCP in Man and in Rabbit Following Intravenous Administra- tion of BCPNa,” Chemical & Pharmaceutical Bulletin, Vol. 19, No. 5, 1971, pp. 881-891. [12] B. K. Tang, W. Kalow and A. A. Grey, “Amobarbital Metabolism in Man: N-Glucoside Formation,” Research Communications in Chemical Pathology and Pharma- cology, Vol. 21, No. 1, 1978, pp. 45-53. [13] B. K. Tang, W. Kalow and A. A. Grey, “Metabolic Fate of Phenobarbital in Man N-Glucoside Formation,” Drug Metabolism and Disposition, Vol. 7, No. 5, 1979, pp. 315- 318. [14] K. Mohri, T. Uesugi and K. Kamisaka, “Bucolome N- Glucuronide: Purification and Identification of a Major Metabolite of Bucolome in Rat Bile,” Xenobiotica, Vol. 15, No. 7, 1985, pp. 615-621. doi:10.3109/00498258509045891 [15] K. Mohri, Y. Uesawa and T. Uesugi, “Metabolisme of Bucolome in Rats Stability and Biliary Excretion of Bu- colome N-Glucuronide,” Journal of Chromatography B: Biomedical Sciences and Applications, Vol. 759, No. 1, 2001, pp. 153-159. doi:10.1016/S0378-4347(01)00218-3 [16] O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Ran- dall, “Protein Measurement with the Folin Phenol Re- agent,” The Journal of Biological Chemistry, Vol. 193, No. 1, 1951, pp. 265-275. [17] H. Scheffe, “The Analysis of Variance,” John Wiley & Sons Inc., New York, 1959. [18] S. Kaivosaari, P. Toivonen, L. M. Hesse , M. Koskinen, M. H. Court and M. Finel, “Nicotine Glucuronidation and the Human UDP Glucuronosyltransferase UGT2B10,” Mo- lecular Pharmacology, Vol. 72, No. 3, 2007, pp. 761-768. doi:10.1124/mol.107.037093 [19] S. Kaivosaari, P. Toivonen, O. Aitio, J. Sipilä, M. Koski- nen, J. S. Salonen and M. Finel, “Regio- and Stereospeci- fic N-Glucuronidation of Medetomidine: The Differences between UDP Glucuronosyltransferase (UGT) 1A4 and UGT2B10 Account for the Complex Kinetics of Human Liver Microsomes,” Drug Metabolism and Disposition, Vol. 36, No. 3, 2008, pp. 1529-1537. doi:10.1124/dmd.108.021709 [20] S. Kaivosaari, M. Finel and M. Koskinen, “N-Glucuroni- dation of Drugs and Other Xenobiotics by Human and Animal UDP-Glucuronosyltransferases,” Xenobiotica, Vol. 41, No. 8, 2011, pp. 652-659. doi:10.3109/00498254.2011.563327 [21] B. K. Tang, “Drug Glucosidation,” Pharmacology & The- Copyright © 2011 SciRes. PP  Bucolome N-Glucuronide Formation: Species Differences and Identification of Human UDP-Glucuronosyltransferase Isoforms Copyright © 2011 SciRes. PP 369 rapeutics, Vol. 46, No. 1, 1990, pp. 53-56. doi:10.1016/0163-7258(90)90034-Y [22] S. G. Paibir and W. H. Soine, “High-Performance Liquid Chromatographic Analysis of Phenobarbital and pheno- barbital Metabolites in Human Urine,” Journal of Chro- matography B: Biomedical Sciences and Applications, Vol. 691, No. 1, 1997, pp. 111-117. [23] S. M. Neighbors and W. H. Soine, “Identification of Phe- nobarbital N-Glucuronides as Urinary Metabolites of Phe- nobarbital in Mice,” Drug Metabolism and Disposition, Vol. 23, No. 5, 1995, pp. 548-552. [24] L. Luukkanen, J. Taskinen, M. Kurkela, R. Kostiainen, J. Hirvonen and M. Finel, “Kinetic Characterization of the 1A Subfamily of Recombinant Human UDP-Glucurono- syltransferases,” Drug Metabolism and Disposition, Vol. 33, No. 7, 2005, pp. 1017-1026. doi:10.1124/dmd.105.004093 [25] S. Ohno and S. Nakajin, “Determination of mRNA Ex- pression of Human UDP-Glucuronosyltransferases and Application for Localization in Various Human Tissues by Real-Time Reverse Transcriptase-Polymerase Chain Reaction,” Drug Metabolism and Disposition, Vol. 37, No. 1, 2009, pp. 32-40. doi:10.1124/dmd.108.023598 [26] T. Zhang, P. Haws and Q. Wu, “Multiple Variable First Exons: A Mechanism for Cell- and Tissue-Specific Gene Regulation,” Genome Research, Vol. 14, No. 1, 2004, pp. 79-89. doi:10.1101/gr.1225204 [27] P. I. Mackenzie, K. W. Bock, B. Burchell, C. Guillemette, S. Ikushiro, T. Iyanagi, J. O. Miners, I. S. Owens, D. W. Nebert, “Nomenclature Update for the Mammalian UDP Glycosyltransferase (UGT) Gene Superfamily,” Phar- macogenetics and Genomics, Vol. 15, 2005, pp. 677-685. doi:10.1097/01.fpc.0000173483.13689.56 [28] C. D. King, M. D. Green, G. R. Rios, B. L. Coffman, I. S. Owens, W. P. Bi sho p and T. R. Tephly , “The Gluc uronida- tion of Exogenous and Endogenous Compounds by Stably Expressed Rat and Human UDP-Glucuronosyltransferase 1.1,” Archives of Biochemistry and Biophysics, Vol. 332, No. 1, 1996, pp. 92-100. doi:10.1006/abbi.1996.0320 [29] J. R. Chowdhury, R. Kondapalli and N. R. Chowdhury, “Gunn Rat: A Model for Inherited Deficiency of Bilirubin Glucuronidation,” Advances in Veterinary Science & Com- parative Medicine, Vol. 37, 1993, pp. 149-173. [30] H. Kanoh, M. Tada, S. Ikushiro and K. Mohri, “Charac- terization of Bucolome N-Glucuronide Formation: Tissue specificity and Identification of Rat UDP-Glucuronosyl- transferase Isoform (s),” Pharmacology & Pharmacy, Vol. 2, No. 3, 2011, pp. 151-158. doi:10.4236/pp.2011.23021 [31] H. Kanoh, K. Okada and K. Mohri, “Identification of the UDP-Glucuronosyltransferase Responsible for Bucolome N-Glucuronide Formation in Rats,” Pharmazie, Vol. 65, No. 11, 2010, pp. 840-844.
|