American Journal of Plant Sciences
Vol.4 No.5A(2013), Article ID:32253,7 pages DOI:10.4236/ajps.2013.45A008
Effects of Growth Regulators on Biomass and the Production of Secondary Metabolites in Peppermint (Mentha piperita) Micropropagated in Vitro
![]()
1Dpto. Biología Molecular, FCEFQyN, Universidad Nacional de Río Cuarto, Campus Universitario, Río Cuarto, Argentina; 2Instituto Multidisciplinario de Biologia Vegetal, Universidad Nacional de Córdoba, Córdoba, Argentina.
Email: *ebanchio@exa.unrc.edu.ar
Copyright © 2013 Maricel Valeria Santoro et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 16th, 2013; revised April 21st, 2013; accepted May 6th, 2013
Keywords: Micropropagated Plants; Mentha piperita; Growth Regulators; IBA; BAP; Secondary Metabolites; Essential Oils
ABSTRACT
The effects of plant growth regulators on peppermint (Mentha piperita) cultured in vitro were studied for the purpose of maximizing growth and essential oil production in micropropagated plants. The basal medium was experimentally supplemented with the auxin 4-indol-3-ylbutyric acid (IBA) and the cytokinin 6-benzylaminopurine (BAP) individually and in combination. Supplementation with BAP alone resulted in the highest values for root length, root dry weight, shoot length, and numbers of nodes, leaves, and ramifications. Treatment with IBA alone or with IBA + BAP resulted in a ~50% increase in shoot fresh weight. The production of secondary metabolites was affected only by the addition of cytokinin, which resulted in a ~40% increase in the total yield of essential oils (EOs). Similar trends were observed for yields of the major EO components (menthone, menthol, pulegone, and menthofuran). Our findings demonstrate that the application of growth regulators increases EO production and biomass concomitantly in an herbaceous species rich in commercially valuable terpenes.
1. Introduction
Peppermint (Mentha piperita L.; family Labiatae) is an important and commonly used flavoring agent worldwide. Fresh or dried leaves of Mentha species are used as condiments, and the essential oils (EOs) of these plants are used as flavorings for foods and beverages, as fragrances, and as fungicides or insecticides in many pharmaceutical and industrial products [1,2]. Menthol, a crystalline compound obtained from peppermint oil, has a pleasant flavor and aroma and a cooling anesthetic effect, and is used in confectionery, pharmaceutical, oral health care, cosmetic, tea, and tobacco products.
Every year, the aroma and fragrance industries account for sales of ~$18 billion and the international trade of EOs increases by ~10%. Many of the compounds used as fragrances and as flavoring agents are chemically synthesized from petroleum derivatives. Because hard metals are often used as catalysts in the synthetic processesit is desirable to find more natural sources for these compounds [3]. EOs are the most important raw material in the fragrance and aroma industry. They are also used in the food and pharmaceutical industries because of their therapeutic antimicrobial and antioxidant activities. Some EOs have biological activities that make them useful as herbicides, pesticides, and anticancer compounds [4]. A common problem in the cultivation of aromatic plants is the quantitative and qualitative variability in plant responses to the environment [4,5]. The productions of EOs does not depend solely on plant genetics or on the developmental stage. The environment and its changes significantly affect biochemical pathways and physiological processes in the plants and may consequently alter metabolism and EO biosynthesis [1]. To meet the demands of world markets and industrial requirements, it is important to maintain a constant production and quality of EOs, particularly in terms of their chemical composition [5].
Plant tissue culture techniques in controlled laboratory environments provide an alternative to field agriculture for the production of economically important secondary metabolites [6-8]. The production of secondary metabolites from undifferentiated plant cell cultures has been intensively studied [7]. However, very few compounds have been commercially manufactured in vitro. Several strategies have been employed to enhance the production of desired phytochemicals from cultured cells, e.g., genetic engineering (which maximizes certain biochemical pathways at the expense of others), the selection of fastgrowing cell clones that display high metabolic activity, modification of culture media, and the use of elicitors to stimulate desired pathways [7,8]. However, certain secondary products with commercial value, such as monoterpenes and other EOs, are not produced by callus cultures or cell suspension cultures [9]. Such cultures lack the complex tissue and organ differentiation found in intact plants [10]. On the other hand, micropropagated plants, the normal sites of secondary metabolism in nature, easily produce commercially desirable secondary metabolites in vitro [8,10,11]. In the mint family (Labiatae), EOs are synthesized primarily in leaf epidermal cells and stored primarily in glandular leaf trichomes [12]. These glandular trichomes are present on the leaf surfaces of tissue culture plantlets and readily produce volatile Eos [11,13].
The effects of growth regulators on EO production are highly variable. Variations in EO yield or content are often observed. Chemical changes are caused by some growth regulators but not by others. In view of the ability of growth regulators to influence plant growth and development, physiological and biochemical processes, and even gene regulation, there are very many ways in which applications of these compounds can potentially alter EO production [14].
Methods for enhancing the growth of economically important plant species undergo constant evolution and improvement. In the case of mint, a number of growth media have been screened for suitability for shoot and root induction and for rapid development and propagation in vitro [4,5,7]. In contrast, no information is available regarding the composition of EOs in regenerated in vitro clones of M. piperita. We report here the effects of the growth regulators 4-indol-3-ylbutyric acid (IBA; an auxin), 6-benzylaminopurine (BAP; a cytokinin), and their combination on the growth, morphogenesis, and secondary metabolism of M. piperita plants micropropagated in vitro.
2. Experimental
2.1. Plant Material and in Vitro Regeneration
Young shoots from M. piperita plants grown in the Traslasierra Valley (Córdoba province, Argentina) were surface disinfected by soaking for 1 min in 17% sodium hypochlorite solution and rinsed 3X in sterile distilled water. The disinfected shoots were cultured in 100 mL of BM consisting of Murashige and Skoog [15] salts plus 0.7% (w/v) agar and 1.5% (w/v) sucrose [16]. All culture media contained 7.5 g/L agar and 30 g/L sucrose.
Stage I. Initial shoot tip culture. After 30 days, apical meristems with foliar primordia that did not show contamination were removed aseptically from the terminal buds of shoots obtained as described above. Explants were cultured in 40 mL BM in test tubes.
Stage II. Growth and in vitro multiplication. Plantlets obtained from shoot tips were multiplied by single node culture, and the pH of the BM was adjusted to 5.6 - 5.8 prior to autoclaving (20 min, 121˚C). Explants were placed in a growth chamber with controlled conditions of light (16/8 h light/dark cycle), temperature (22˚C ± 2˚C), and relative humidity (~70%) [16].
Stage III. Treatment with growth regulators. Single nodes from aseptically cultured plantlets were placed in glass vials (100 mL) in 40 mL BM with five growth regulator supplementation treatments: 1) BAP 0.6 mg/L; 2) IBA 0.6 mg/L; 3) BAP 0.3 mg/L; 4) BAP 0.6 mg/L + IBA 0.6 mg/L; 5) control (BM only). The vials were sealed with translucent polypropylene caps, arranged in a completely randomized design, and placed in a growth chamber with controlled conditions as described for Stage II. At least 7 plants were used for each treatment. Plants were harvested after 30 days.
2.2. Plant Growth Measurement
Plants were removed from the vial after 4 weeks of culture, and the roots were rinsed with water to remove BM. For each treatment, the plants were evaluated in terms of growth (shoot length and fresh weight, root length and dry weight), morphogenesis (numbers of leaves, nodes, and ramifications), and production of secondary metabolites. The shoot fresh material was weighed and kept in the freezer until the hydrodistillation.
2.3. Extraction of EOs
Each shoot sample was weighed, subjected to hydroidstillation in a micro Clevenger-like apparatus for 30 min, and the volatile fraction was collected in dichloromethane [16]. The internal standard used was 0.1 μL dodecalactone in 50 μL ethanol.
M. piperita plants contain ~3% volatile oils, consisting of >40 different compounds. The four major EO components, which account for ~60% of the total oil volume, are (+)pulegone, (−)menthone, (−)menthol, and (+)menthofuran. These compounds were quantified in relation to dodecalactone, which was added during the distillation procedure. The FID response factors for each compound generated essentially equivalent areas (difference < 5%).
The identification of the components of the sample was performed by GC-MS. A Perkin-Elmer Clarus 600 GC-MS coupled with a quadrupole analyzer and photodiode detector was employed for the identification. Turbo Mass software was used to control and acquire data from the GC-MS. A capillary column DB-5 (60 m 0.25 mm i.d. and 0.25 m coating thickness) was used for the separation of the components. Helium was used as carrier gas at 49.6 psi. The temperature program was 60˚C for 5 min, from 60˚ to 250˚C at 4˚C/min, with a final hold time of 10min. The injector and detector were maintained at 250˚C and 280˚C, respectively. The sample, 0.2 μL, was injected with a 1:100 split ratio.
Ionization was carried out in the mass spectrometer under vacuum by electron impact with a 70-eV ionization energy. The GC transfer line was maintained at 200˚C. Chromatograms were acquired in “scan” mode scanning the quadrupole from m = z 50 to m = z 300 (scan time: 0.2 s, inter-scan time: 0.1 s).
Retention indices of the sample components were determined on the basis of homologous n-alkane hydrocarbons under the same conditions. The compounds were identified by comparing their retention indices and mass spectra with published data [16] and libraries NIST and Adams. The main components were further identified by coinjection of authentic standards (SIGMA, USA).
GC analysis was performed using a Perkin Elmer Clarus 500. Total Chrom v.6.3.1 software was used to control and acquire data from the GC. All the separations were conducted through a Alltech fused silica DB 5 capillary column (30 m, 0.25 mm ID, 0.25 µm film thickness) using nitrogen as a carrier gas (49.6 psi). The split injection mode was selected. Samples were analyzed using the following Chromatographic conditions: oven temperature program: initial temperature 60˚C (held for 5 min), 5˚C/ min to 240˚C (held for 10 min). An injector and detector temperature of 250˚C was used.
2.4. Statistical Analysis
7 plants were used for each treatment, and experiments were replicated 3 times. For the statistical analysis were used the means values from the 7 plants (each replica; we do not pooled the samples of different replicates into one treatment).Values presented were means of repeated experiments. The statistical analysis of the data was based on analysis of variance (ANOVA). Differences among means were evaluated by Fisher’s least significant difference (LSD) test (P < 0.05 considered significant) using Infostat software program version 2011 (Group Infostat, Universidad Nacional de Córdoba, Argentina). The data presented as percentages were subjected to arcsine transformation before analysis and then converted back to percentage form for presentation.
3. Results
The growth of micropropagated M. piperita was greatly affected by the application of growth regulators. Root dry weight was 3-fold higher in plants grown with IBA, BAP, or IBA + BAP than in controls (plants grown in basal medium [BM]) or plants grown with 50% IBA (P < 0.005). Root length was decreased by all treatments expect BAP, for which the values were similar to those of controls (P < 0.005). Shoot weight was increased 30% by IBA and IBA + BAP treatments and decreased 50% by BAP and 50% IBA treatments (P = 0.01), compared with control values. Shoot length was increased ~40% by BAP, IBA, and IBA + BAP treatment compared with control and 50% IBA treatment (P = 0.04) (Table 1(a)). Plant morphogenesis was also altered by the application of the regulators. The numbers of leaves, nodes, and ramifications for plants treated with 50% IBA were not significantly different from control values. In contrast, the numbers of leaves and nodes were higher than control values in plants treated with IBA, BAP, or IBA+BAP (n˚ leaves, P < 0.005; n˚ nodes P < 0.001), the highest leaves and node numbers was obtained on medium containing BAP increasing 50% and 250% respectively (Table 1(b)).
The EO yield in plants treated with IBA or with IBA + BAP was not significantly different from that in controls


Table 1. Effects of growth regulators on growth and morphogenesis of micropropagated M. piperita plants (mean ± SE). Vertical bars indicate mean values ± standard error (SE) of 3 replicates. Letters above bars indicate significant differences according to Fisher’s LSD test (P < 0.05).
but was increased ~45% in plants treated with BAP (P < 0.001). (Figure 1). Similar trends were observed for the concentrations of the major EO components: (+)pulegone, (−)menthone, (−)menthol, and (+)menthofuran. BAPtreated plants showed an approximately two-fold increase in menthone concentration and a three-fold increase in menthol and pulegone concentrations compared with control values (P < 0.001) (Figure 1). Treatment of plants with IBA or with 50% IBA did not cause significant changes in concentrations of the major EO components (P < 0.05).
Treatment with cytokinin BAP altered the relative percentages (R%) of EOs (Table 2). The R% for pulegone, the primary EO component, for menthone and for menthol was approximately two-fold higher in BAPtreated plants than in controls (P < 0.001). The R% for menthofuran were also two-fold higher in BAP and IBA treated plants than in controls (P < 0.001).
4. Discussion
Plant hormones comprise a structurally unrelated group of small molecules that are derived from various essential metabolic pathways. Recent studies have demonstrated the interaction of hormone signaling at multiple levels during plant growth and development. Cytokinins are a type of plant hormones that play many essential signaling roles in plant growth and development. The application of the cytokinin BAP to micropropagated M. piperita plants produced a three-fold increase in root dry weight and increases in shoot length, node number, and leaf number. Shoot fresh weight was significantly smaller in these plants than in controls, perhaps because the leaves were smaller and the shoots were thinner. Cytokinins are known to be involved in the regulation of cell division, shoot and root development, apical dominance, and growth of lateral buds [17]. The great increase in ramification number observed in BAP-treated plants reflected the suppression of apical dominance and was consistent with the finding of Hafiz et al. [18] that the exogenous application of cytokinin increased the lateral branching of Jatropha curcas plants maintained in greenhouses.
The treatment of plants with auxin (IBA 0.6 mg/L) increased root dry weight because of lateral root formation through repetitive cell division, similar to the finding of Liu et al. [19]. IBA treatment also increased shoot length and fresh weight because of increases in the numbers of leaves and nodes. The effective concentration of IBA in studies of this type is dependent on the pH of the medium [20]. At pH values of 5.6 - 8.8, the IBA concentrations in the medium were lower and insufficient to induce root and shoot development.
In regard to morphogenesis, the combination of BAP had the greatest overall effect on micropropagated plants, producing notable increases in the numbers of leaves, nodes and ramifications.
The response to particular elicitors may vary from plant to plant; a single plant hormone may regulate a wide range of physiological and growth processes. On the other hand, a particular process may be regulated by the action of many plant hormones. The effects of growth regulators on EO production in in vitro culture systems are highly variable. Changes were observed in the yield or content of EOs. Chemical changes were noted in some cases but not in others. In view of the fact that growth regulators influence plant growth and development through their effects on physiological and biochemical processes and even gene regulation, there are a great number of ways in which application of these compounds may alter secondary metabolite production [14,21,22]. Growth regulators can influence EO biosynthesis and the formation and development of storage structures. In Arabidopsis, cytokinins stimulate trichome initiation, with consequent increases in trichome numbers and densities [23]. The findings of the present study of M. piperita appear to be somewhat inconsistent with the previous findings of Farooqui and Sharma [24], who observed increases in the biomass and EO yield of M. arvensis L. var. piperascens Mal following cytokinin treatment. We observed an increase in EO yield, may be due to an increment in increases in trichome numbers [23], but not in fresh weight. In a study of Thymus mastichina by Fraternale et al. [25], BAP treatment resulted in increases in EO production and the density of glandular hairs on leaves. These authors suggested that cytokinins affect glandular hair development, although no direct correlation was demonstrated. In our experiments, cytokinin treatment caused a reduction in biomass even though the EO yield was increased. IBA-treated plants displayed a general increase in biomass because shoot length was greater and the number of leaves was approximately two-fold higher than in controls; however, these changes were not associated with increased EO production.
The combination of IBA + BPA did not greatly change the production of plant secondary compounds in com-


Figure 1. Effects of growth regulators on the concentrations of the four major EO components in micropropagated M. piperita plants. Vertical bars indicate mean values ± standard error (SE) of three replicates. Letters above bars indicate significant differences according to Fisher’s LSD test (P < 0.05).
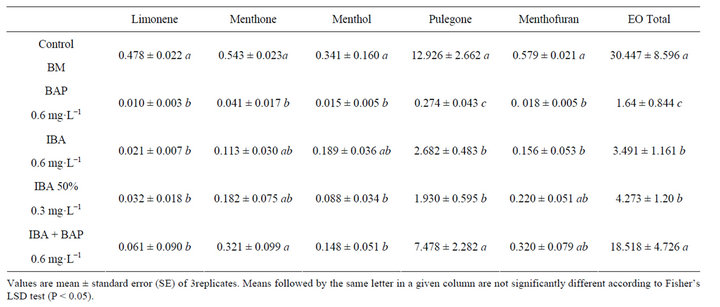
Table 2. Variation in the relative percentage (R%) of the four major EO components in micropropagated M. piperita plants treated with different combinations of growth regulators.
parison with controls. The major EO components of M. piperita (pulegone, menthone, menthol, and menthofuran) showed increases only in BAP-treated plants. The other treatments did not cause changes, suggesting that cytokinins influence pathway flux or specific steps of monoterpene metabolism in M. piperita.
The treatments did not cause changes in the qualitative chemical composition of EO components. The four major components accounted for ~60% of the total chemical components in EOs, similarly to controls. However, the relative percentages (R%) of the four major components were increased approximately two-fold by BAP treatment. Because the percentage compositions are relative values, it is often impossible to determine whether an increase in R% value is due to an increase in the absolute content of a particular component or to decreases in the contents of other components. In the present study, however, we were able to confirm that the increases in R% values of the four major EO components were due to decreased concentrations of minor compounds (α- and β- pinene, limonene, α-terpinene, p-cineole, α-terpineol, etc.) because the absolute contents of the major components were higher in treated plants.
Plant growth depends heavily on protein synthesis for the manufacture of photosynthetic, biosynthetic, and regulatory enzymes, and structural proteins [26]. Secondary metabolism (EO synthesis) competes with primary growth processes for common substrates such as sugars and proteins. When environmental conditions are favorable, vegetative growth (primary metabolism) generally displays resource priority over secondary metabolism [26]. In the present study, however, when abundant resources (BM + cytokinins) were provided, increased growth and morphogenesis responses occurred without any reduction in secondary metabolism. These findings appear to be inconsistent with the carbon nutrient theory, which states that in situations in which plants have access to excess carbon and nutrients, optimal growth occurs with a corresponding suppression of secondary metabolism [26,27]. Our findings indicate that the content of volatile EOs can be significantly altered in the leaves of plantlets using micropropagation systems with the addition of a growth regulator. The use of micropropagated plantlets for the production of secondary metabolites is a promising approach for future commercial applications.
Growth regulators have been used in agriculture for decades, but little is known regarding the effects of these compounds on the production of secondary metabolites [3,9,22,28]. In the present study, addition of BAP to the BM used for micropropagation of M. piperita effectively increased plant growth and EO production without altering EO composition. This approach increased the yield of important EO components, apparently by inducing the biosynthesis of secondary metabolites.
5. Acknowledgements
This research was supported by grants from the Secretaría de Ciencia y Técnica de la Universidad Nacional de Río Cuarto, Consejo Nacional de Investigaciones Cientí- ficas y Técnicas (CONICET), and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT). EB, JZ and WG are Career Members of the CONICET. FN has a fellowship from the CONICET and MS has a fellowship from the CONICET-MCyT Cba. The authors wish to thank Dra. Marcela Palacio for her help in the technical support in the GCMS analysis. The authors are grateful to Steve Anderson for English editing of the manuscript.
REFERENCES
- N. S. Sangwan, A. H. A. Farooqi, F. Shabih and R. S. Sangwan, “Regulation of Essential Oil Production in Plants,” Plant Growth Regulation, Vol. 34, No. 1, 2001, pp. 3-21. doi:10.1023/A:1013386921596
- D. Ram, M. Ram and R. Singh, “Optimization of Water and Nitrogen Application to Menthol Mint (Mentha arvensis L.) through Sugarcane Trash Mulch in a Sandy Loam Soil of Semi-Arid Subtropical Climate,” Bioresource Technology, Vol. 97, No. 7, 2006, pp. 886-893. doi:10.1016/j.biortech.2005.04.047
- W. Schwab, R. Davidovich-Rikanati and E. Lewinsohn, “Biosynthesis of Plant-Derived Flavor Compounds,” The Plant Journal, Vol. 54, No. 4, 2008, pp. 712-732. doi:10.1111/j.1365-313X.2008.03446.x
- R. Croteau, T. Kutchan and N. Lewis, “Natural Products (Secondary Metabolites),” In: B. Buchanan, W. Gruissem and R. Joneas, Eds., Biochemistry and Molecular Biology of Plants, American Society of Plant Biologists, Rockville, 2000, pp. 1250-1268.
- P. Harrewijn, A. M. Van Oosten and P. G. Piron, “Natural Terpenoids as Messengers. A Multidisciplinary Study of Their Production, Biological Functions and Practical Applications,” Kluwer Academic Publishers, London, 2001.
- F. Bourgaud, A. Gravot, S. Milesi and E. Gontier, “Production of Plant Secondary Metabolites: A Historical Perspective,” Plant Science, Vol. 161, No. 13, 2001, pp. 839- 851. doi:10.1016/S0168-9452(01)00490-3
- R. Verpoorte, A. Contin and J. Memelink, “Biotechnology for the Production of Plant Secondary Metabolites,” Phytochemistry Reviews, Vol. 1, No. 1, 2002, pp. 13-25. doi:10.1023/A:1015871916833
- M. Vanisree and H.-S. Tsay, “Plant Cell Cultures: Production of Biologically Important Secondary Metabolites from Medicinal Plants of Taiwan,” In: O. Kayser and W. Quax Eds., Medicinal Plant Biotechnology. From Basic Research to Industrial Application, Wiley-VCH Verlag GmbH & Co., KGaA, Weinheim, 2007, pp. 267-285.
- R. Verpoorte, R. van der Heijden and J. Memelink, “Engineering the Plant Cell Factory for Secondary Metabolite Production,” Transgenic Research, Vol. 9, No. 4-5, 2000, pp. 323-343. doi:10.1023/A:1008966404981
- R. Rao and G. A. Ravishankar, “Plant Cell Cultures: Chemical Factories of Secondary Metabolites,” Biotechnology Advances, Vol. 20, No. 2, 2002, pp. 101-153. doi:10.1016/S0734-9750(02)00007-1
- S. V. Tisserat and R. Silman, “Influence of Modified Oxygen and Carbon Dioxide Atmospheres on Mint and Thyme Plant Growth, Morphogenesis and Secondary Metabolism in Vitro,” Plant Cell Reports, Vol. 20, No. 10, 2002, pp. 912-916. doi:10.1007/s00299-001-0428-6
- G. W. Turner, J. Gershenzon and R. B. Croteau, “Distribution of Peltate Glandular Trichomes on Developing Leaves of Peppermint,” Plant Physiology, Vol. 124, No. 2, 2000, pp. 655-664. doi:10.1104/pp.124.2.655
- B. Tisserat and S. Vaughn, “Essential Oils Enhanced by Ultra-High Carbon Dioxide Levels from Lamiaceae Species Grown in Vitro and in Vivo,” Plant Cell Reports, Vol. 20, No. 4, 2001, pp. 361-368. doi:10.1007/s002990100327
- Y. N. Shukla and A. H. Farooqi, “Utilization of Plant Growth Regulators in Aromatic Plant Production,” Medicinal & Aromatic Plants, Vol. 12, No. 3, 1990, pp. 152- 157.
- T. Murashige and F. A. Skoog, “Revised Medium for Rapid Growth and Bio Assay with Tobacco Tissue Culture,” Plant Physiology, Vol. 15, No. 3, 1962, pp. 473-497. doi:10.1111/j.1399-3054.1962.tb08052.x
- M. Santoro, J. Zygadlo, W. Giordano and E. Banchio, “Volatile Organic Compounds from Rhizobacteria Increase Biosynthesis of Essential Oils and Growth Parameters in Peppermint (Mentha piperita),” Plant Physiology and Biochemistry, Vol. 49, No. 11, 2011, pp. 1177- 1182. doi:10.1016/j.plaphy.2011.07.016
- M. C. Mok, “Cytokinins and Plant Development—An Overview,” In: D. W. S. Mok and M. C. Mok, Eds., Cytokinins. Chemistry, Activity, and Function, CRC Press, Boca Raton, 1994, pp. 155-166.
- A. A. Hafiz, S. D. Johnson and J. V. Staden, “Promoting Branching of a Potential Biofuel crop Jatropha curcas L. by Foliar Application of PGR,” Plant Growth Regulation, Vol. 58, No. 3, 2009, pp. 287-295. doi:10.1007/s10725-009-9377-9
- C. Liu, J. Zhu, Z. Liu, L. Li, R. Pan and L. Jin, “Exogenous Auxin Effects on Growth and Phenotype of Normal and Hairy Roots of Pueraria lobata (Wild.) Ohwi,” Plant Growth Regulation, Vol. 38, No. 1, 2002, pp. 37-43. doi:10.1023/A:1020904528045
- J. F. Harbage and D. P. Stimart, “Effect of pH and 1HIndole-3-Butyric Acid (IBA) on Rooting of Apple Microcuttings,” Journal of the American Society for Horticultural Science, Vol. 121, No. 6, 1996, pp. 1049-1053.
- V. R. Affonso, H. R. Bizzo, C. L. Salguiero Lage and A. Sato, “Influence of Growth Regulators in Biomass Production and Volatile Profile of in Vitro Plantlets of Thymus vulgaris L.,” Journal of Agricultural and Food Chemistry, Vol. 57, No. 14, 2009, pp. 6392-6395. doi:10.1021/jf900816c
- P. Baskaran, B. Ncube and J. Van Staden, “In Vitro Propagation and Secondary Product Production by Merwilla plúmbea (Lindl.) Speta,” Plant Growth Regulation, Vol. 67, No. 13, 2012, pp. 235-245. doi:10.1007/s10725-012-9682-6
- L. Maes and A. Goossens, “Hormone-Mediated Promotion of Trichome Initiation in Plants Is Conserved but Utilizes Speciesand Trichome-Specific Regulatory Mechanisms,” Plant Signaling & Behavior, Vol. 5, No. 2, 2010, pp. 205-207. doi:10.4161/psb.5.2.11214
- H. A. Farooqi and S. Sharma, “Effect of Growth Retardants on Growth and Essential Oil Content in Japanese mint. P1,” Growth Regulation, Vol. 7, No. 1, 1988, pp. 39-45. doi:10.1007/BF00121688
- D. Fraternale, L. Giamperi, D. Ricci, M. B. L. Rocchi, L. Guidi, F. Epifanio and M. C. Marcotullio, “The Effect of TRIA on Micropropagation and on Secretory System of Thymus mastichina,” Plant Cell, Tissue and Organ Culture, Vol. 74, No. 1, 2003, pp. 87-97. doi:10.1023/A:1023321024040
- D. A. Herms and W. J. Mattson, “The Dilemma of Plants: to Grow or Defend,” The Quarterly Review of Biology, Vol. 67, 1992, pp. 283-335. doi:10.1086/417659
- R. Matyssek, R. Agerer, D. Ernst, J.-C. Munch, W. Osswald, H. Pretzsch, E. Priesack, H. Schnyder and D. Treutter, “The Plant’s Capacity in Regulating Resource Demand,” Plant Biology, Vol. 7, No. 1, 2005, pp. 560- 580. doi:10.1055/s-2005-872981
- S. S. Mahmoud and R. B. Croteau, “Strategies for Transgenic Manipulation of Monoterpene Biosynthesis in Plants—Review,” Trends in Plant Science, Vol. 7, No. 8, 2002, pp. 366-373. doi:10.1016/S1360-1385(02)02303-8
Abbreviations
EO, essential oil;
IBA, 4-indol-3-ylbutyric acid;
BAP, 6-benzylaminopurine;
BM, basal medium;
R%, relative percentage.
NOTES
*Corresponding author.

