Paper Menu >>
Journal Menu >>
 International Journal of Organic Chemistry, 2011, 1, 67-70 doi:10.4236/ijoc.2011.13011 Published Online September 2011 (http://www.SciRP.org/journal/ijoc) Copyright © 2011 SciRes. IJOC Thermochemistry of Heteroatomic Compounds: the Heats of Combustion and Formation of Glyc oside and Adenosine Phosphates Vitaly Ovchinnikov, Ludmila Lapteva National Researching University, Tupolev Kazan State Technical University, Kazan, Russian Federation E-mail: chem_vvo@mail.ru Received April 22, 2011; revised June 2, 2011; accepted July 13, 2011 Abstract The heats of combustion of 4-th glycoside in the condensed state with the use of the equation 15.7 117.2 comb H Ng , in which N is a number bond-forming (valence) electrons less the number (g) of lone electron pairs of nitrogen (g = 1) and oxygen (g = 2), have been determined. Such dependence is de- duced previously joint for the description of the combustion enthalpies of 17 simple ethers of a cyclic struc- ture and different sugars. The heats of formation (f H ) of the mentioned above glycosides were calculated according to the Hess law via two ways: 1) through the use their heats of hydrolysis (hydr H ), which have been investigated earlier experimentally, 2) with the use the calculated the heats of combustion. The last pro- cedure has been used also for the calculation of the heats of formation of the adenosine tri(ATP)-, di(ADP)- and mono(AMP)phosphates because of such thermochemical parameter is often hard achieved experimen- tally. The heats of hydrolysis () of ATP into ADP and ADP into AMP were calculated on the basis of their heats of formation in water ( hydr fH faq H ). The free energies of the same process (hy ere known in lit- erature. Last circumstance give us a possibility to calculate the hydrolysis entropies (hydrSg the Gibbs equation. The entropy values are a large negative, that pointed on the preliminary complex formation be- tween adenosine phosphates and water before the breaking of P-O bonds or P-O-C fragments in its. dr G) w ) usin Keywords: Glycoside Phosphates, Adenosine Phosphates, Heat of Combustion, Heat of Formation 1. Introduction Molecules, which contain cyclic phosphoric fragments, represent the big interest within many decades as a bio- chemical objects [1,2]. Nucleosides and phosphates of sugars are representative of the important classes of es- ters of a phosphorous acid. However their thermochem- istry, having essential value for the much deeper, de- tailed understanding of their important biochemical func- tion, is poorly investigated. For this reason we have un- dertaken the theoretical calculation of the heats of com- bustion (comb H ) and then on this basis the heats of formation (f H 2. Results and Dicussions The heats of hydrolysis (hydr H ) of methyl-α-D-glyco- pyranoside-4’,6’-cyclophosphate (1), methyl-β-D-ribofu- ranoside-3’,5’-cyclo-phosphate (2), adenosine(Ade)-3’,5’- mono-cyclophosphate(3), adenosine-2’,3’-mono- cyc-lo- phosphate (4) were founded previously (Table 1) [3]. The products of these interactions with water are methyl- ά-D-glucopyranoside (5,aq), methyl-β-D-ribofuranoside (6,aq) and 1-adenosine ribofuranoside (7,aq) are pre- sented in Equation (1) 2 34 -, 2 -, hydr liqH Oliq aqH POaqH 14 57 (1) ) in the condensed state of organic phosphor-nucleotides of a cyclic structure, kinetics and thermochemistry of hydrolysis of which has been inves- tigated earlier experimentally [3]. To calculate the heats of formation (f H ) of the molecules (1-4) and glycosides (5-7) in condensed state 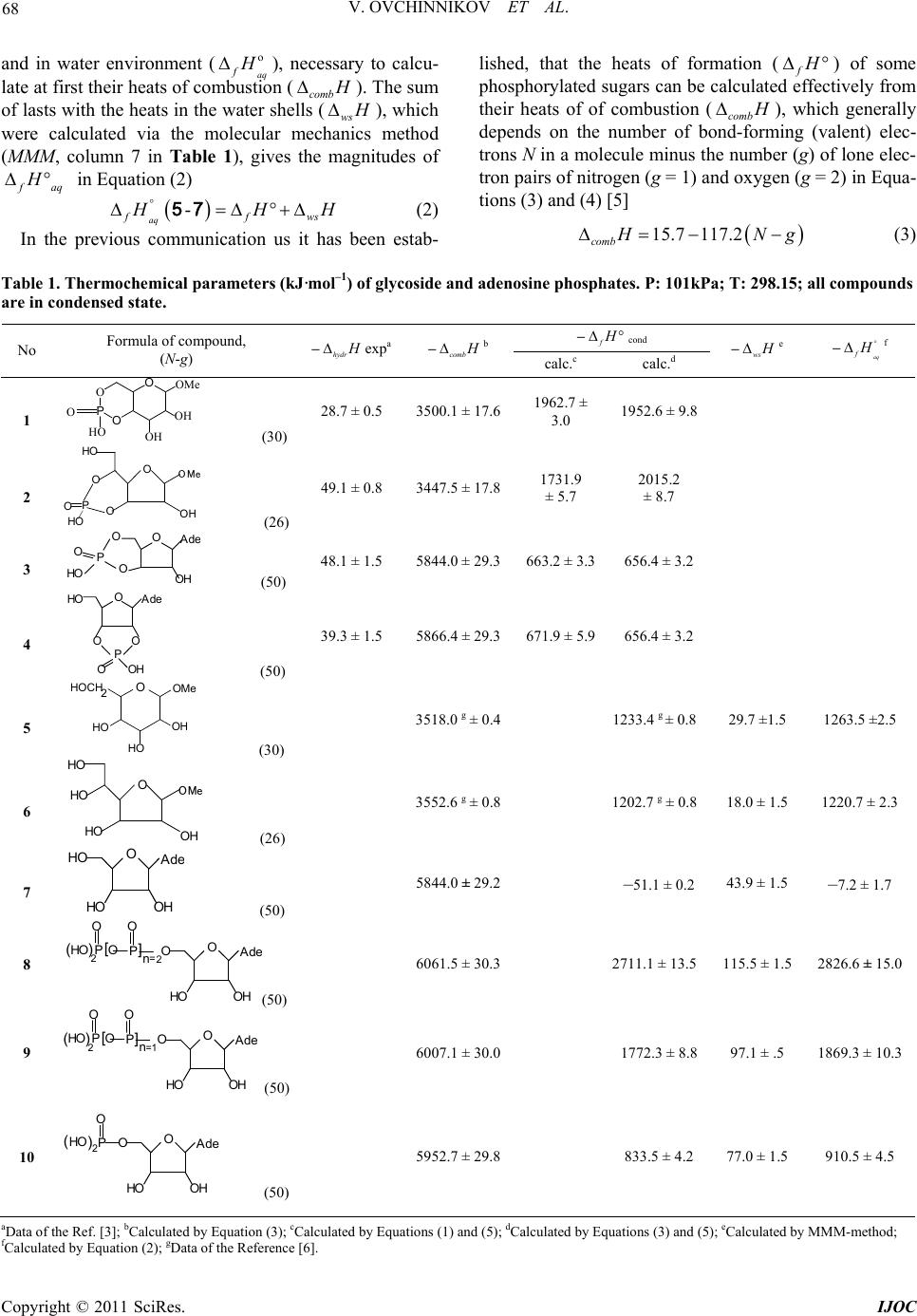 V. OVCHINNIKOV ET AL. 68 and in water environment (aq f o H ), necessary to calcu- late at first their heats of combustion (comb H ). The sum of lasts with the heats in the water shells (ws H ), which were calculated via the molecular mechanics method (MMM, column 7 in Table 1), gives the magnitudes of f aq H in Equation (2) - aq ffws H H 57 H (2) In the previous communication us it has been estab- lished, that the heats of formation (f H ) of some phosphorylated sugars can be calculated effectively from their heats of of combustion (comb H ), which generally depends on the number of bond-forming (valent) elec- trons N in a molecule minus the number (g) of lone elec- tron pairs of nitrogen (g = 1) and oxygen (g = 2) in Equa- tions (3) and (4) [5] 15.7 117.2 comb H Ng (3) Table 1. Thermochemical parameters (kJ·mol–1) of glycoside and adenosine phosphate s. P : 101kP a ; T: 298. 15; all compounds are in condensed state. –f H cond No Formula of compound, (N-g) –hydr H expa–comb H b calc.c calc.d –ws H e –aq f H f 1 O PO OOMe HO OH OH O (30) 28.7 ± 0.5 3500.1 ± 17.61962.7 ± 3.0 1952.6 ± 9.8 2 O HO OH HO O PO O (26) 49.1 ± 0.8 3447.5 ± 17.81731.9 ± 5.7 2015.2 ± 8.7 3 OAd e HO P OH O O O (50) 48.1 ± 1.5 5844.0 ± 29.3663.2 ± 3.3656.4 ± 3.2 4 O HO Ad e OH PO O O (50) 39.3 ± 1.5 5866.4 ± 29.3671.9 ± 5.9656.4 ± 3.2 5 OOMe HOCH HO HO OH 2 (30) 3518.0 g ± 0.4 1233.4 g ± 0.8 29.7 ±1.5 1263.5 ±2.5 6 HO HO OH HO O (26) 3552.6 g ± 0.8 1202.7 g ± 0.8 18.0 ± 1.5 1220.7 ± 2.3 7 O OAd e HO OH H (50) 5844.0 ± 29.2 –51.1 ± 0.2 43.9 ± 1.5 –7.2 ± 1.7 8 O HO )( OP O PAd e HO OH O O [] n =2 2 (50) 6061.5 ± 30.3 2711.1 ± 13.5 115.5 ± 1.5 2826.6 ± 15.0 9 O HO )( OP O PAd e HO OH O O [] n =2 1 (50) 6007.1 ± 30.0 1772.3 ± 8.8 97.1 ± .5 1869.3 ± 10.3 10 O HO ) ( O O PAd e HO OH 2 (50) 5952.7 ± 29.8 833.5 ± 4.2 77.0 ± 1.5 910.5 ± 4.5 aData of the Ref. [3]; bCalculated by Equation (3); cCalculated by Equations (1) and (5); dCalculated by Equations (3) and (5); eCalculated by MMM-method; lculated by Equation (2); gData of the Reference [6]. fCa Copyright © 2011 SciRes. IJOC  69 V. OVCHINNIKOV ET AL. 2 22 234 pqrst comb CHONPcondensO uNgasxCO gas y HOliqz HPOsolH (4) where p, q, r, s, t, u, x, y, z are stoichiometric factors. This approach was applied to all biomolecules (1-7) and the calculated comb H , f H and aq f H magnitudes through combination of the Equations (1-5) presented in Table 1. /() hydr combffreag prod HH H (5) Necessary values f H (kJ·mol–1) for СО2(gas): –395.5, H2O(liq): –285.8 and Н3РО4(aq, sol): –1289.9, –1279.0 are taken from the monograph [4]. If it can be seen from the data of Table 1, the calcu- lated the heats of formation in the condensed state with the use of the experimental heats of hydrolysis [3] and the same values obtained via Equations (3)-(5) [5] are good correspond each other in the range of the assumed in Table 1 (columns 5, 6) indefinites 2% - 3%, which expressed in Equation (6) 1 1 kJ mol90.9159.3 0.91.1kJmol hydr calc H H r = 0.985, so = 143.2, n = 4 (6) It gives a real basis for the application of the men- tioned above approach to the calculation of the thermo- chemical parameters of such important bioorganic sub- stances as adenosine tri(ATP, 8)-, di(ADP, 9)- and mono(AMP, 10) phosphates. It is known [1,2], that at each step of the hydrolysis reaction of adenosine phosphates in the water shell, as it shown on Equations (7)-(9), the essential quantity of energy due to the breaking of P-O bonds in P-O-P frag- ments is allocated accordingly to Hess law in Equation (5). 34 2 ,, ,, hydr ff ff H Haq HHPOaq H aqHHO liq 8 9 (7) 34 2 ,, ,, hydr ff ff H Haq HHPOaq H aqHHOliq 9 10 (8) 34 2 ,, ,, hydr ff ff H Haq HHPOaq HaqHHOliq 10 7 (9) In last Equation (9) the dissociation to the same bond in AMP leads to formation of a water 1-adenosine ribo- furanoside (7) and a water phosphorus acid. It is neces- sary to note, that into the structure of a listed adenosine phosphates enter the some hydroxyl groups at phospho- rus, the energy of each is estimated as –54.4 kJ·mol–1; this factor is considered at calculations of comb H for compounds (8-10). Despite of the approached calculations of the last thermochemical parameter, it is possible to note, that the hydrolysis of ATP and ADP is carried out with identical (about –45, –46 kJ·mol–1) energy while the hydrolysis AMP up to adenosine furanoside (7) and a phosphorus acid is characterized noticeably by more greater the heats of about –86 kJ mol-1, which presented in Table 2. Tak- ing into account the published in the monograph [1] the free energies of hydrolysis (hydr ) for ATP, ADP and AMP and obtained in this work hydr G H S magnitudes, the values of the entropies (hydr ) according to the Gibbs Equation (10) were calculated and shown in Table 2. hydr hydrhydr GHTS (10) Necessary to note, that the calculated the entropy magnitudes are a large negative, especially for the hy- drolysis process of AMP, that can pointed on the pre- liminary complex formation between AMP and a water molecules before the breaking of P-O bond in P-O-C fragment. 3. Conclusions Thus, as it has been shown during this work the Equation (3), worked up to the correlation between of the heats of combustion of 17 sugars of the different space structure and the number of the valent electrons in its, excluding of the lone electron pairs of heteroatoms, can be useful applied to the calculation of the same thermochemical parameters of the different bioorganic molecules, in partly glycosides and adenosine phosphates. Table 2. Thermodynamic parameters (J·mol–1 and J·mol–1·K–1) of the hydrolysis reaction of adenosine phos- phates at 298.15 K. Hydrolysis reaction –hydrG a –hydrH –hydrS Equation (7) 30543.2 46800 54.5 Equation (8) 27196.0 45300 60.7 Equation (9) 9204.8 86400 259.0 aData of the Reference [1]. Copyright © 2011 SciRes. IJOC  V. OVCHINNIKOV ET AL. 70 4. References [1] D. E. C. Corbribge, “Phosphorus: An Outline of Its Chemistry, Biochemistry, and Technology,” 2nd Edition, Elsevier, Amsterdam-Oxford-New York, 1980. [2] А. White, Ph. Handler, E. Smith, R. Hill and I. R. Leman, “Principles of Biochemistry,” McGraw-Hill, New York, 1978. [3] J. A. Gerlt, F. H. Westheimer and J. M. Sturtevant, “The Enthalpies of Hydrolysis of Acyclic, Monocyclic, and Glycoside Cyclic Phosphate Diesters,” The Journal of Biological Chemistry, Vol. 250, 1975, pp. 5059-5067. [4] J. D. Cox and G. Pilcher, “Thermochemistry of Organic and Organometallic Compounds,” Academic Press, New York, 1970. [5] V. V. Ovchinnikov and N. R. Muzafarov, “Thermo- Chemistry of Heteroatomic Compounds. Calculation of the Formation Enthalpy for Methyl-α-D-4’,6’-Cyclophos- Phate on the Basis of His Enthalpy of Combustion,” Rus- sian Journal of Organic Chemistry, Vol. 45, No. 1, 2009, pp. 318-319. [6] S. M. Skuratov, A. A. Strepikheev and M. P. Kozina, “About the Combustion of Five and Six-Membered Het- erocyclic Compounds,” Dokl. Acad. Sci. SSSR, Vol. 117, 1957, pp. 452-454. Copyright © 2011 SciRes. IJOC |

