 Open Journal of Respi ratory Diseases, 2011, 1, 1-13 doi:10.4236/ojrd.2011.11001 Published Online August 2011 (http://www.SciRP.org/journal/ojrd) Copyright © 2011 SciRes. OJRD H-FABP in Pre Capillary Pulmonary Hypertension: Comparison to Other Surrogate Parameters for Prediction of Severity and Outcome Hagen Schroetter1, Michael Halank2, Dirk Stolte1, Peggy Barthel1, Gert Hoeffken2, Ruediger C. Braun-Dulleaus3, Ruth H. Strasser1, Alexander Schmeisser3 1University of Techno logy Dresden , Medical Clinic and Cardiology, Heart Center Dresden, University Hospital, Germany 2University of Techno logy Dresden , Medical Clinic/Pneumology, University Hospital, Germany 3Otto-von-Guericke University Magdeburg, Medical Clinic/Cardiol ogy, Pneumology an d Angi ology, Germany E-mail: hagen.schroetter@mailbox.tu-dresden.de Received August 8, 2011; revised August 19, 2011; accepted August 23, 2011 Abstract Background: Heart-type fatty acid-binding protein (H-FABP) is a promising novel biomarker for risk strati- fication of patients with chronic thromboembolic pulmonary hypertension (CTEPH). Whether disease seve- rity in a group of patients with pre capillary pulmonary hypertension (PH) can be predicted by determination of plasma H-FABP levels remains unknown. Methods: 41 consecutive patients with a mean (±SD) age of 60.6 ± 2.1 years and severe PH were studied with a mean follow up to 541 days (95% CI: 420; 661 days). H-FABP, but also the biomarkers NT-Pro-BNP and big-endothelin (Big-ET1), were correlated to parameters derived from right sided cardiac catheterization and cardio-pulmonary exercise test. Results: At baseline H-FABP levels ranged from 620 to 15200 pg/ml (3648 502 pg/ml) and were weakly but significantly cor- related to VO2AT (r = −0.37, p = 0.04) and VO2 peak (r = −0.38, p = 0.03). However, invasively measured hemodynamic parameters of PH and right ventricular dysfunction did not correlate with H-FABP levels. In- terestingly, moderate to high correlations between H-FABP and NT-Pro-BNP (r = 0.51, p = 0.01) and Big-ET1 (r = 0.65, p = 0.01) and between Big-ET1 and NT-Pro-BNP (r = 0.5, p = 0.01) could be observed. In contrast to H-FABP, NT-Pro-BNP, and even more Big-ET1, showed significant correlations to different invasively measured hemodynamic parameters of the disease indicating severity and consequently prognosis of severe pre-capillary pulmonary hypertension. However, in a multivariate Cox regression analysis, PVR, mixed venous oxygen saturation and the VE/VCO2 slope (>60) as well as the heart rate recovery within one minute (HRR) but none of the biomarkers were identified as independent factors of poor prognosis. In con- trast, peak workload and mixed venous saturation were identified as risk markers in the idiopathic pulmonary arterial hypertension subgroup. Conclusions: In contrast to the predictive value of H-FABP in CTEPH H-FABP fails to be a reliable marker in PH or to be a novel predictor of mid and long term outcome. The data suggest that an increased slope of VE/VCO2 and a decreased extent of HRR within one minute represent more promising diagnostic non invasive parameters. Keywords: Human Fatty Acid Binding Protein, Biomarker, Pulmonary Hypertension, Prognosis 1. Introduction Pulmonary arterial hypertension (PAH) is a progressive disease with poor prognosis due to fatal right heart fail- ure. The accurate assessment of prognosis in these pa- tients is difficult. Moreover, the optimal timing for com- bining medical therapy or planning invasive procedures such as atrial septostomy or lung transplantation remains controversial [1]. Worsening of invasively determined hemodynamic indices of right ventricular dysfunction, a poor functional status, or a low distance in 6 minute walk test (6MWT) [2-4] may be indicative of severity and  2 H. SCHROETTER ET AL. prognosis of pulmonary hypertension. The estimate of right ventricular systolic pressure derived from Doppler sonography is helpful to screen patients with PH but was found not to be predictive for outcome [5,6]. A variety of atrial, ventricular, and Doppler parameters were sugges- ted to be associated with an increased risk of death or need for transplantation in patients with PAH [7,8]. However, the drawback of Doppler sonography is its operator-dependency. Therefore, simple, non invasive, easily accessible and quantitative parameters which are repeatedly available and possibly quantitative methods to detect PH patients are still needed. Such parameters are of utmost value for follow up during therapy. Cardiac biomarkers in plasma such as plasma brain natriuretic peptide (BNP) or the N-terminal part of its pro-hormone (NT-pro-BNP) appear to have promising potential [9] [10]. Thus, it has been demonstrated that plasma BNP levels increase in correlation with the degree of right ventricular (RV) dysfunction and are well predictive for the functional status of patients with idiopathic pulmo- nary hypertension (IPAH) [11,12]. Consequently, high levels of BNP at baseline, and in particular, a further rise in plasma BNP during follow up of 3 months are inde- pendent prognostic factors in these patients [13]. NT- pro-BNP could be related with cardiopulmonary hemo- dynamics in patients with idiopathic PAH and with poor long term prognosis in an unselected patient population with pre-capillary PH [14]. Recently, other heart bio- markers reflecting myocardial injury, such as troponin or H-FABP gained increasing interest. Fatty acid-binding proteins (FABPs) are relatively small (15 KDa) cytosolic proteins that are widely distributed and highly expressed in tissues undergoing active fatty-acid metabolism, such as the heart and the liver [15]. The H-FABP (heart-type fatty acid-binding protein) isoform is one of the most abundant proteins in myocardial tissue, representing 5% - 15% of the whole aqueous cytosolic protein pool of the heart. H-FABP is truly cytosolic and has not been found outside the cell or in plasma under normal conditions [16]. The fact that H-FABP is a small protein, allows rapid diffusion and release from the injured myocardium resulting in a rapid increase in the plasma which suggests the H-FABP may be a valuable and easily accessible biomarker. This has qualified H-FABP as a rapid marker for example in myocardial infarction [17]. Therefore, it is conceivable that H-FABP may also be a reliable bio- marker in other cardiac diseases with myocardial damage such as pulmonary hypertension or acute pulmonary embolism (PE). In fact, Puls et al. [18] and Kaczynsky et al. [19] identified H-FABP as an early, highly sensitive and specific indicator of death or serious complications in acute PE. Moreover, recently, Lankeit et al. [20] as- sessed the prognostic value of this biomarker in a large, single center population of 93 consecutive patients with CTEPH. The results indicate that H-FABP is more sensi- tive than cardiac troponins in predicting an adverse out- come in these settings. But, the prognostic value of H-FABP was not compared to that of BNP and NT- Pro-BNP, which are commonly used as biomarkers in pulmonary hypertension [21]. Moreover, it is unknown whether H-FABP is a parameter that can be applied as a prognostic marker not only in CTEPH patients but also in patients with chronic pulmonary artery hypertension (PH) due to various diseases. Therefore, in the present study, we addressed the question if H-FABP may be used as a prognostic indica- tor and marker for the severity of the disease with vari- ous forms of chronic pre-capillary PH. Therefore patients with different disease stages and on different therapeutic regimens were investigated. In addition, the predictive value of H-FABP was compared with other well estab- lished biomarkers such as NT-pro-BNP, invasive hemo- dynamic measures and the exercise tests CPET and 6 MWT in this patient group. 2. Materials and Methods 2.1. Inclusion and Exclusion Criteria 41 consecutive patients (18 male, 23 female), referred for invasive hemodynamic evaluation for chronic pre-capil- lary PH from June 2004 to February 2007, were evalu- ated. According to the revised classification of PH, 27 patients had pulmonary arterial hypertension (PAH), 23 IPAH and 4 were associated with systemic sclerosis. In addition, 8 patients had angiographically verified chronic thromboembolic PH (CTEPH), 5 patients interstitial lung disease associated pulmonary hypertension sarcoidosis associated fibrosis (n = 1), connective tissue disease (n = 2), idiopathic pulmonary fibrosis (n = 2) and 1 patient was in the miscellaneous group diagnosed as having sarcoidosis without fibrosis associated pulmonary hyper- tension. At baseline examination, 13 patients were treated with bosentan alone, 6 with sildenafil and 2 patients with both (Table 1). At the follow up visit, all but 2 patients were medicated with the endothelin receptor antagonists or sildenafil. In 2 patients, a combination therapy was initiated. In 2 patients a specific therapy could not be initiated due to various contra-indications, lack of com- pliance, or multimorbidity with a very short expected life time. Exclusion criteria were: Patients with pulmonary hy- pertension due to congestive left heart failure, hypertro- phic or restrictive cardiomyopathy or caused by severe left sided valvular heart diseases, patients with life limit- ing severe concomitant disea es such as malignant neo- s Copyright © 2011 SciRes. OJRD  H. SCHROETTER ET AL. Copyright © 2011 SciRes. OJRD 3 Table 1. Hemodynamic, biomarker and CPET baseline characteristics of 41 patients with pre-capillary pulmonary hyperten- sion of different cause regarding of adverse and not adverse outcome (medications: numbers within parenthesis indicates medication before baseline examination and outside the parenthesis after the examination); ETBR = endothelin receptor blockers. Variable All patients n = 41Adverses outcome n = 9No adverse outcome n = 32p value Female n 23 3 20 Age (years) years 60.6 ± 2.1 59.0 ± 3.8 61.0 ± 2.5 0.67 6MWT m 282 ± 26 277 ± 21 283 ± 31 0.88 VO2peak ml/kg*min 11.7 ± 0.6 11.5 ± 0.5 11.7 ± 0.7 0.81 Work load max Watt 57 ± 4 50 ± 4 59 ± 4 0.09 VE/VCO2Slope 55.2 ± 2.5 62.5 ± 4.9 53.1 ± 2.8 0.12 Heart rate recovery bpm 10.4 ± 0.8 5.7 ± 0.6 11.5 ± 0.9 0.004 Bosentan/Sitaxentan 11 5 6 Sildenafil 8 2 6 Hemodynamics PAmean mmHg 50.2 ± 1.9 46.0 ± 1.9 51.4 ± 2.4 0.24 PCWP mmHg 7.7 ± 1.0 5.8 ± 2.0 8.3 ± 1.2 0.3 TPG mmHg 42.6 ± 2.0 40.2 ± 2.0 43.4 ± 2.4 0.51 RAP mmHg 8.8 ± 0.9 8.0 ± 2.8 9.1 ± 0.9 0.63 RVEDP mmHg 13 ± 2 13 ± 1 13 ± 3 0.93 SaO2 % 90 ± 2 91 ± 2 88 ± 2 0.55 SvO2 % 55 ± 2 55 ± 2 54 ± 3 0.87 CO l/min 3.7 ± 0.2 3.7 ± 0.5 3.7 ± 0.2 0.99 PVR dyn*s*cm−5 1029 ± 91 1006 ± 78 1036 ± 117 0.83 Biomarker NTpro-BNP fmol/ml 1711 ± 198 1258 ± 242 1870 ± 248 0.18 Big-ET fmol/ml 0.84 ± 0.13 1.04 ± 0.41 0.79 ± 0.12 0.58 H-FABP pg/ml 3648 ± 502 4004 ± 1202 3548 ± 557 0.71 plasms, pregnancy or juvenile patients (<18 years). All patients were informed and signed the written informed consent. The study was approved by the local ethic committee. 2.2. Six Minute Walk Test (6-MWT), Cardiopulmonary Exercise Test (CPET) and Hemodynamic Evaluation For evaluation of endurance, all patients performed a 6-MWT according to the American Thoracic Society guidelines [22] at first. All patients underwent a cardio- pulmonary exercise test (using the incremental cycling test method, upright cycle ergometer, ramp rate 5 to 15 watts per min, Schiller co., Germany). Transnasal respi- ration was avoided using a nose clip resulting in inspira- tion of room air via a mouthpiece with a two way valve. Standard 12 channel ECG tracings were monitored at rest, at one minute intervals and during the exercise and the recovery period. After an adoption period of 3 minu- tes of unloaded cycling, patients cycled under an increas- ing load until a symptom-limited maximum was attained. Various parameters were measured continuously. Heart rate, systolic and diastolic blood pressure, respiratory  4 H. SCHROETTER ET AL. minute volume (VE in litre), ventilatory oxygen uptake (VO2) at anaerobic threshold (AT) and at exertion maxi- mum (peak), respiratory carbon dioxide delivery (VCO2) and calculated data such as respiratory quotient (RQ); respiratory efficacy (VCO2Slope), the oxygen pulse at anaerobic threshold, and peak load (O2HRAT, O2HR peak in ml) were monitored. Within 24 to 48 hours after CPET/6MWT the diagnostic right heart catheterization was performed while patients were in stable condition during hospitalization. Baseline hemodynamic variables including mean pulmonary arterial pressure (MPAP), mean right atrial pressure (RAP), pulmonary capillary wedge pressure (PCWP), and RV end-diastolic pressure (RVEDP) were measured. Cardiac output (CO) was measured by Fick’s method. Total pulmonary resistance (PVR) was calculated by dividing mean pulmonary arte- rial pressure by cardiac output, the resistance index (RI) was calculated by the ratio of PVR and SVR. 2.3. Biomarker Testing Central venous blood samples were collected at the time of the initial right heart diagnostic catheter. Samples were immediately stored at −80˚C until analysis ensuring a single thaw for all consecutive samples from a single patient. Human-FABP was measured in serum using an ELISA Test Kit based on the sandwich principle (HyCult Biotechnology, Uden the Nederlands). The minimum detection level for H-FABP was 250 pg/ml with mea- surable concentrations up to 25.000 pg/ml. Serum NT- Pro-BNP was determined, as well (Enzyme immunoas- say, Biomedica, Austria). The minimum detection level was 5 fmol/ml at 95% B/Bo. The absorbance was mea- sured at 450nm. Big endothelin (BigET1) was measured by an enzyme immunoassay for the quantitative deter- mination of human big endothelin in EDTA plasma. The detection limit was 0.025 - 0.05 fmol/ml and reached a range between 0.025 - 25 fmol/ml. The cross reactivity with ET1/2/3 and Big-ET2 is <1% (Enzyme immunoas- say, Biomedica, Austria, www.bmgrp.com). The invest- tigator who quantified the biomarker levels was blinded for hemodynamic parameters of the patient or the clinical course. Biomarker levels were not used to guide patient management or to monitor the effects of treatment during the initial hospital stay or at any time during the follow up period. 2.4. Survival Estimates Survival was estimated from the date of blood sampling to March, 31, 2008, or cardiopulmonary death or lung transplantation with a follow up rate of 100%. 2.5. Statistical Analysis Based on the test result of equal distribution by the Kol- mogorow-Smirnow test, the baseline characteristics of survivors and non survivors were compared with the execution of the two-sided t-test for all continuous, met- ric scaled variables. All data were described by arithme- tic mean SEM. Bivariate correlations between metric scaled groups were recorded by Pearson’s coefficient r. The Spearman rank correlation (rho) test was performed, if at least one of the variables was non parametric. A coefficient value between 0.2 and 0.5 [–0.2 and –0.5], respectively, was regarded as a weak, a r or rho between 0.5 and 0.7 (–0.5 and –0.7 respectively) as a moderate correlation. At march 31, 2008 data of vital status or date of death from any cause or heart-lung transplantation (HLTx), the combined end point, were assessed. Event (or censoring) times for all patients were measured from the time of study inclusion (time zero). All patients were evaluated at the end of the follow up. To estimate the correlation of biomarkers to survival, differences between groups with normal or low marker levels vs. elevated marker levels were determined by the log-rank statistic and the time to the end point plotted according to Kaplan-Meier method. First, all hazard ratios for death or HLTx were measured unadjusted for covariates, followed by the use of Cox proportional regression models for survival adjustment for age, gender, specific pulmonary vasoreactive medica- tion at baseline or specific therapy extension during the follow up period. Hazard ratios were reported with their associated 95% confidence interval. Hazard ratios were illustrated by Forest plots graphs. For all test results we considered a two tailed p 0.05 significant. Statistical analysis was performed with SPSS for Windows, release 11.5. 3. Results During a mean follow up period of 541 days (95% CI 420; 661) 9 of 41 consecutive patients (22%) died of cardiopulmonary cause or underwent lung transplanta- tion (n = 4). Most of the patients who died (n = 6, 15%) died in the first year after the baseline visit. (Figure 1). Thereafter, the Kaplan-Meier survival curve levelled off for the rest of the observation period. 3.1. Comparison of Characteristics of All Patients with Pre-Capillary Pulmonary Hypertension At baseline, the characteristics of the patients with ad- verse outcome compared to those without adverse out- Copyright © 2011 SciRes. OJRD  H. SCHROETTER ET AL. 5 Figure 1. Kaplan-Meier survival curve in PH patients. The graph represents the survival with a maximum observation time of 1159 days. come did not differ significantly. Also there was no sig- nificant difference in the use of medications. At baseline, neither the distance of the 6 minute walk test, VO2peak, VE/VECO2 Slope nor RA mean, the PVR or cardiac output were able to discriminate between the two groups (Table 1). Moreover, no difference was found in the se- rum levels of the biomarkers NT-pro BNP, Big-ET and H-FABP. Only the heart rate recovery within the first minute after stress test was significantly delayed in pa- tients with adverse outcome compared to patients with- out adverse outcome (5.7 ± 0.6 bpm vs. 11.5 ± 0.9 bpm, p = 0.004; Table 1). The maximal work load showed only a trend toward lower values in patients with adverse outcome (p = 0.09). Comparison of characteristics of the selected IPAH group of patients with adverse and without adverse out- come In the group of 23 IPAH patients 6 patients died (26%) of cardiopulmonary causes, and four patients un- derwent a lung transplantation during the follow up pe- riod. Patients with no adverse outcome had faster heart rate recovery within the first minute after stress test (11 ± 1 vs. 5.6 ± 0.7 bpm, p = 0.01) and reached a higher maximal work load (63 ± 0.5 vs. 45 ± 3 Watt, p = 0.01). In right heart catheter survivors had a PCWP within the normal range but significantly higher than patients with an adverse outcome (8 ± 1 vs. 3 ± 1 mmHg, p = 0.04). The arterial oxygen saturation was significantly higher in the survivor group, too (92 ± 1 vs. 87 ± 1 %, p = 0.03) (Table 2). Also in IPAH patients, V02peak, VE/VECO2 slope, RAP, PVR or cardiac output failed to discriminate between those with or without adverse outcome. Simi- larly, the serum levels of the biomarkers NT-pro BNP, Big-ET and H-FABP could not indicate the risk of pa- tients for worsening right heart failure or death (Table 2). 3.3. Correlation of the H-FABP with Exercise (CPET), Hemodynamic Parameters and with Other Biomarkers (NT-Pro BNP, Big-ET) in All Patients with Pre-Capillary PH 3.3.1. CPET At baseline evaluation, H-FABP levels in plasma ranged from 620 to 15200 pg/ml (3648 502 pg/ml) and were weakly correlated with VO2 AT (r = −0.37, p = 0.04) and VO2 peak (r = −0.38, p = 0.03) (Table 3(a)). 3.3.2. Hemodynamic Parameters Plasma H-FABP levels did not correlate with invasively measured hemodynamic parameters of pulmonary hy- pertension and right ventricular dysfunction. (Table 3(b)). 3.4. Bivariate Correlation of H-FABP with the Biomarkers NT-Pro-BNP and Big-ET1 At the time of baseline evaluation, mean NT-Pro-BNP levels were 1711 ± 198 fmol/ml and Big-ET 0.84 ± 0.13 fmol/ml (Table 1). Interestingly, we observed a moder- ate correlation between H-FABP and NT-Pro-BNP (r = 0.51, p = 0.01). The correlation between H-FABP and Big-ET was even closer (r = 0.65, p = 0.01). Big-ET and NT-Pro-BNP were moderately associated with each an- other (r = 0.5, p = 0.01; Table 3(a)). Like H-FABP, Big- ET and NT-Pro-BNP correlated weakly with VO2 peak (r = −0.39, p = 0.02 and r = −0.45, p = 0.03, respectively). But in contrast to H-FABP, NT-Pro-BNP, and even more Big-ET, showed significant correlations to invasively measured hemodynamic parameters with known prog- nostic significance (NT-Pro-BNP: RAP: r = 48, p = 0.02, mixed venous saturation (SVO2) r = −0.64, p =< 0.001; (Big-ET: MPAP: r = 0.41, p = 0.04; RI r = 0.53, p = 0.004; SVO2: r = −0.53, p = 0.002; RVEDP: r = 0.53, p = 0.001 and RAP: r = 0.73, p = 0.001). 3.5. Risk Estimates for Adverse Outcome 3.5.1. H-FABP in Comparison to NT-Pro-BNP and Big-ET The impact of these biomarkers on observed outcome was assessed by Kaplan-Meier Analysis according to median values of baseline. No relevant cut off values for a significant prediction of death or transplantation were dentified by ROC analysis of the analysed biomarkers. i Copyright © 2011 SciRes. OJRD  H. SCHROETTER ET AL. Copyright © 2011 SciRes. OJRD 6 Table 2. Hemodynamic, biomarker and CPET baseline characteristics of 23 patients with IPAH regarding of adverse and not adverse outcome (medications: numbers within parenthesis indicates medication before baseline examination and outside the parenthesis after the examination). Subgroup of IPAH patients (n = 23) Variable IPAH patients n = 23Adverses outcome n = 6No adverse outcome n = 17 p value Gender female 13 2 11 Age (years) years 61.3 ± 2.5 55.8 ± 5.0 63.1 ± 2.9 0.22 6MWT m 319 ± 27 298 ± 8 323 ± 30 0.73 VO2peak ml/kg*min 12.0 ± 0.8 11.0 ± 0.5 12.3 ± 1.0 0.24 Work load max. Watt 59 ± 5 45 ± 3 63 ± 5 0.01 VE/VCO2Slope 56.7 ± 3.3 66.7 ± 2.6 53.9 ± 5.0 0.12 Heart rate recovery bpm 9.8 ± 1.0 5.6 ± 0.7 11 ± 1.0 0.01 Bosentan/Sitaxentan 7 3 4 Sildenafil 4 2 2 Hemodynamics PAmean mmHg 48 ± 2 46 ± 2 49 ± 3 0.5 PCWP mmHg 7 ± 1 3 ± 1 8 ± 1 0.04 TPG mmHg 42 ± 2 43 ± 2 41 ± 3 0.69 RAP mmHg 7 ± 1 5 ± 2 8 ± 1 0.18 RVEDP mmHg 12 ± 2 10 ± 3 13 ± 2 0.34 SaO2 % 91 ± 1 87 ± 1 92 ± 1 0.03 SvO2 % 57 ± 2 54 ± 3 58 ± 2 0.38 CO l/min 3.7 ± 0.2 3.3 ± 0.2 3.8 ± 0.3 0.16 PVR dyn*s*cm−5 998 ± 91 1058 ± 73 977 ± 122 0.7 Biomarker NTpro-BNP fmol/ml 1667 ± 245 1272 ± 339 1799 ± 304 0.21 Big-ET fmol/ml 0.67 ± 0.14 0.84 ± 0.49 0.61 ± 0.12 0.84 H-FABP pg/ml 3010 ± 536 3259 ± 975 2928 ± 492 0.34 (Figure 2, Kaplan Meyer curve for NTproBNP not shown). The multivariate, logistic forward stepwise Cox regression analysis with death or HLTx as dependent factors was adjusted for age, gender, specific pulmonary vasoreactive medication at baseline or specific therapy extension during the follow up period. Neither in unse- lected patients with pre-capillary PH nor in the IPAH group, plasma H-FABP demonstrated a significant pre- dictive value for an adverse outcome (HR of 1,0 for an increase by 1 pg/ml; 95%CI 0,99; 1,01). Surprisingly, nearly the same results were obtained after analysis of NT-Pro-BNP in the unselected group but also in the IPAH group (Table 4). Only Big-ET showed a trend for a positive prediction of an adverse outcome for each in- crease of BigET1 by 1 fmol/ml (all patients: HR 1.88 (0.89 - 3.97), p = 0.09; IPAH: HR 5.01 (0.65 - 38.71), p = 0.12). 3.5.2. Outcome Estimates Based on Hemodynamic and Exercise Tested Variables: Multivariate Predictive Analysis In the multivariate Hazard ratio proportional test for hemodynamic factors of pulmonary hypertension and/or right ventricular dysfunction, only a PVR over 900 dyn*s*  H. SCHROETTER ET AL. 7 Table 3. Bivariate correlation of HFABP, Big-ET and BT-Pro-BNP with exercise parameters (CPET) (a) and invasive mea- sured parameters of pulmonary hypertension and right ventricular dysfunction (b) in 41 patients with pre-capillary pulmo- nary hypertension. VO2 = Oxygen consumption; AT = aerob/anaerob threshold; peak= maximum of exertion; 6MWD = 6 minute walk distance. MAP = mean arterial pressure; MPAP mean pulmonary artery pressure; PCWP = pulmonary capil- lary wedge pressure; TPG = trans pulmonary pressure gradient; mRAP=mean right atrial pressure; RVEDP = right end-diastolic filling pressure; PVR = pulmonary vascular resistance, SVR: systemic vascular resistance). (a) Bivariate correlation of CPET and biomarkers at baseline Parameter Unit H-FABPp BNP p BigET1 p VO2AT ml/min*kg −0.37 0.04 −0.21 0.34 −0.35 0.06 VO2peak ml/min*kg −0.38 0.03 −0.45 0.03 −0.39 0.02 VO2peak predicted % −0.32 0.06 −0.26 0.23 −0.33 0.05 VE/VCO2Slope 0.05 0.76 0.12 0.58 −0.16 0.42 Workload peak Watt −0.22 0.20 −0.30 0.15 −0.20 0.27 Workload peak predicted % −0.11 0.54 −0.39 0.06 −0.30 0.08 Oxygenpulse AT ml/beat −0.04 0.90 −0.11 0.76 −0.19 0.59 Oxygenpulse peak ml/beat −0.32 0.06 −0.35 0.10 −0.13 0.48 Oxygenpulse peak predicted % −0.19 0.27 −0.23 0.28 −0.24 0.17 6 MWD m 0.13 0.79 0.58 0.42 0.31 0.54 Heart rate recovery beats 1st min −0.03 0.85 0.18 0.43 0.18 0.42 NTproBNP fmol/ml 0.51 0.01 0.50 0.01 H-FABP pg/ml 0.51 0.01 0.65 0.01 BigET1 fmol/ml 0.65 0.01 0.50 0.01 (b) Bivariate correlation of hemodynamics and biomarkers at baseline Parameter Unit H−FABP p BNP p BigET1 p MAP mmHg 0.12 0.49 −0.10 0.61 −0.09 0.66 MPAP mmHg −0.14 0.39 −0.10 0.62 0.41 0.04 PCWP mmHg −0.01 0.96 0.01 0.97 0.27 0.15 TPG mmHg −0.13 0.45 −0.10 0.64 0.25 0.12 RAP mmHg 0.13 0.44 0.48 0.02 0.73 0.001 RVEDP mmHg −0.14 0.47 0.20 0.44 0.53 0.001 SaO2 % −0.04 0.83 −0.15 0.47 −0.11 0.51 SvO2 % −0.29 0.07 −0.64 0.00 −0.53 0.002 Cardiac Index l/min*m2 −0.14 0.40 −0.33 0.10 −0.25 0.13 PVR dyn*s*cm−5 −0.07 0.70 −0.13 0.54 0.14 0.40 SVR dyn*s*cm−5 0.11 0.55 0.10 0.64 0.07 0.69 Resistance Index −0.26 0.12 −0.17 0.42 0.53 0.004 Copyright © 2011 SciRes. OJRD 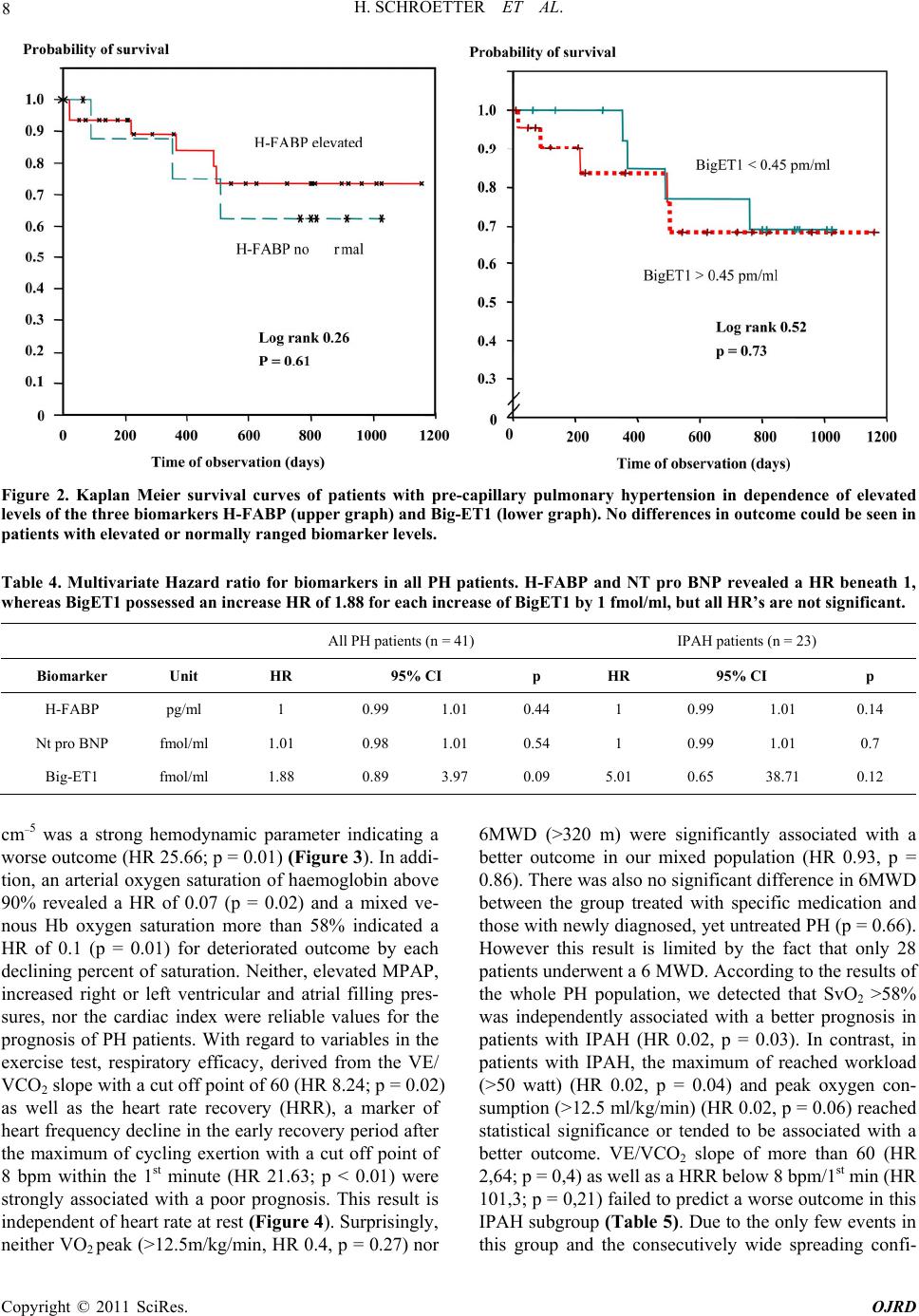 8 H. SCHROETTER ET AL. Figure 2. Kaplan Meier survival curves of patients with pre-capillary pulmonary hypertension in dependence of elevated levels of the three biomarkers H-FABP (upper graph) and Big-ET1 (lower graph). No differences in outcome could be seen in patients with elevated or normally ranged biomarker levels. Table 4. Multivariate Hazard ratio for biomarkers in all PH patients. H-FABP and NT pro BNP revealed a HR beneath 1, whereas BigET1 possessed an increase HR of 1.88 for each increase of BigET1 by 1 fmol/ml, but all HR’s are not significant. All PH patients (n = 41) IPAH patients (n = 23) Biomarker Unit HR 95% CI p HR 95% CI p H-FABP pg/ml 1 0.99 1.01 0.44 1 0.99 1.01 0.14 Nt pro BNP fmol/ml 1.01 0.98 1.01 0.54 1 0.99 1.01 0.7 Big-ET1 fmol/ml 1.88 0.89 3.97 0.09 5.01 0.65 38.71 0.12 cm−5 was a strong hemodynamic parameter indicating a worse outcome (HR 25.66; p = 0.01) (Figure 3). In addi- tion, an arterial oxygen saturation of haemoglobin above 90% revealed a HR of 0.07 (p = 0.02) and a mixed ve- nous Hb oxygen saturation more than 58% indicated a HR of 0.1 (p = 0.01) for deteriorated outcome by each declining percent of saturation. Neither, elevated MPAP, increased right or left ventricular and atrial filling pres- sures, nor the cardiac index were reliable values for the prognosis of PH patients. With regard to variables in the exercise test, respiratory efficacy, derived from the VE/ VCO2 slope with a cut off point of 60 (HR 8.24; p = 0.02) as well as the heart rate recovery (HRR), a marker of heart frequency decline in the early recovery period after the maximum of cycling exertion with a cut off point of 8 bpm within the 1st minute (HR 21.63; p < 0.01) were strongly associated with a poor prognosis. This result is independent of heart rate at rest (Figure 4). Surprisingly, neither VO2 peak (>12.5m/kg/min, HR 0.4, p = 0.27) nor 6MWD (>320 m) were significantly associated with a better outcome in our mixed population (HR 0.93, p = 0.86). There was also no significant difference in 6MWD between the group treated with specific medication and those with newly diagnosed, yet untreated PH (p = 0.66). However this result is limited by the fact that only 28 patients underwent a 6 MWD. According to the results of the whole PH population, we detected that SvO2 >58% was independently associated with a better prognosis in patients with IPAH (HR 0.02, p = 0.03). In contrast, in patients with IPAH, the maximum of reached workload (>50 watt) (HR 0.02, p = 0.04) and peak oxygen con- sumption (>12.5 ml/kg/min) (HR 0.02, p = 0.06) reached statistical significance or tended to be associated with a better outcome. VE/VCO2 slope of more than 60 (HR 2,64; p = 0,4) as well as a HRR below 8 bpm/1st min (HR 101,3; p = 0,21) failed to predict a worse outcome in this IPAH subgroup (Table 5). Due to the only few events in this group and the consecutvely wide spreading confi- i Copyright © 2011 SciRes. OJRD  H. SCHROETTER ET AL. 9 Figure 3. Multivariate Hazard ratio proportional test for hemodynamic factors predicting survival in PH patients. PVR > 900 dyn*s*cm−5 is associated with an increased, low arterial and mixed venous Hb oxygen saturation with a decreased risk of death/HLtx. The levels of significant association are indicated. Figure 4. Multivariate Cox regression proportional analysis with Hazard ratios for exercise parameters predicting worse outcome in PH. A VE/VCO2 Slope more than 60 contributes to an 8 fold, a HRR below than 8 beats within the first minute following peak exertion leads to a 22 fold increase in death/HLTx. dence interval no statistical significance was reached. 4. Discussion and Conclusions The prognostic assessment of pulmonary hypertension is difficult but important, particularly in the context of op- timal timing for invasive procedures such as atrial sep- tostomy or lung transplantation. Therefore, a simple, non-invasive, and repeatedly available method is desir- able. Promising and indicative are studies measuring cardiac biomarkers in plasma such as plasma brain nat- Table 5. Hazard ratio proportional test in the group of 23 IPAH patients. Displayed are only the 3 variables with the strongest interrelation to an adverse outcome. SvO2 and the maximum of workload in CPET show the strongest inter- relation in IPAH patients. Variable HR 95% CI p SvO2 > 58% 0.02 0.001 0.710.03 Workload peak > 50 watts 0.02 0.001 0.730.04 VO2 peak > 12,5 ml/kg*min0.03 0.001 1.230.06 Copyright © 2011 SciRes. OJRD  10 H. SCHROETTER ET AL. riuretic peptide (BNP) or the N-terminal part of its pro- hormone [23,24]. The stimuli for increased secretion of BNP are ventricular wall stretch and volume overload [25]. Recently, newer myocardial biomarkers reflecting myocardial injury, but not stretch per se, such as troponin or H-FABP, have gained interest. In this study, we inve- stigated the H-FABP to determine whether H-FABP is an indicator of disease severity and survival in patients with various forms of chronic pre-capillary PH within different stages of the disease and under different medi- cal therapies. The salient finding of our study is that baseline serum levels of H-FABP are not able to predict an adverse outcome. In a midterm follow up period of about two years, baseline H-FABP concentrations were not different between patients with an adverse outcome compared to those with a favourable course. These data indicate the low predictive value of the biomarker. In addition, the probability of event free survival (HR 1.0, CI 0.99 - 1.01, p = 0.44) was not predicted by increased levels of H-FABP. Similar results were obtained in the subgroup of 23 patients with IPAH. Only a weak correla- tion of H-FABP with the established prognostic parame- ter for CPET VO2 peak (r = −0.38, p = 0.03) could be established. The H-FABP plasma levels failed to show any correlations with the other known prognostic hemo- dynamic markers of PH and right ventricular dysfunction (such as RAP, PVR, SVO2, CO) [26] and exercise (6MWT, VE/VCO2) parameters. Our findings are in con- trast to the results of Lankeit et al. [28] who examined the prognostic value of H-FABP in 93 consecutive pa- tients with the diagnosis of PH, namely CTEPH. They found significantly higher H-FABP levels in patients with an adverse outcome, while a multivariate analysis revealed that H-FABP (HR 1.11, CI 1.02 - 1.22, p = 0.015) was an independent predictor of adverse outcome, along with RAP and pulmonary endarterectomy (PEA). Inter- estingly, in this selected study group baseline H-FABP levels were only weakly correlated with other prognostic relevant hemodynamic parameters (CO, PCWP, RAP and 6MWD). The exact reasons for these apparent discrepancies to our results are unclear, espe- cially in case of comparable median H-FABP levels be- tween the different study groups. The main difference between the study groups consisted in the different aeti- ologies of PH and consecutive treatment options during the follow up. Particularly, the very selected entity (CTEPH) and the longer observation time in the study of Lankeit et al. [29] could be one explanation. In fact, 56% of the CTEPH patients underwent surgical PEA and showed a drastic relieve of hemodynamic impact on their right atriums and ventricles. In addition, PEA was asso- ciated with a highly significant lower risk of an adverse outcome. In our unselected patient group with pulmonary hypertension of various aetiologies only 8 patients were diagnosed as CTEPH but without surgical accessibility of the thromboembolic lesions. During the observation period almost all patients received different medications for PH. In summary, these data indicated, that H-FABP even though representing an easily accessible biomarker, is not a reliable predictor for the outcome of these pa- tients and thus cannot be used reliably to develop thera- peutic strategies for individual patients with PH. In addi- tion we did compare the hemodynamic and prognostic value of H-FABP with Big-ET and with the natriuretic peptide NT-Pro-BNP, which has already been shown to have prognostic relevance in patients with PH. Surpris- ingly, we observed moderate correlations between H-FABP and NT-Pro-BNP (r = 0.51, p = 0.01) but higher correlations to Big-ET (r = 0.65, p = 0.01). Big-ET and NT-Pro- BNP were moderately correlated to each another (r = 0.5, p = 0.01), too. Like H-FABP, Big-ET and NT-Pro-BNP correlated weakly with VO2 peak (r = −0.39, p = 0.02 and r = −0.45, p = 0.03, respec- tively). But in contrast to H-FABP, NT-pro-BNP, and even more Big-ET showed weak to strong correlations to invasively measured he- modynamic parameters with known prognostic signifi- cance (NT-Pro-BNP: mRAP: r = 48, p = 0.02, mixed venous saturation (SVO2) r = −0.64, p < 0.001; (Big-ET: PAP: r = 0.41, p = 0.04; re- sistance index r = 0.53, p = 0.004; SVO2: r = −0.53, p = 0.002; RVEDP: r = 0.53, p = 0.001 and mRAP: r = 0.73, p = 0.001). Using the multi- variable Cox`s proportional hazard model NT-pro-BNP, comparable to H-FABP, had not emerged as an inde- pendent predictor of outcome in our study group of very unselected patients but also in the subgroup of IPAH patients. Only, increasing Big-ET levels, tended to be predictive for an adverse outcome in the whole group (HR 1.88, CI 0.89 - 3.97, p = 0.09) and in IPAH (HR 5.01, CI 0.65 - 38.7, p = 0.12). Accordingly, Big-ET, compared with H-FABP and NT-pro-BNP, showed the highest correlations with different prognostic relevant hemodynamic parameters of pulmonary hyper- tension and right ventricular dysfunction. In contrast to the biomarker H-FABP, hemodynamic and exercise test parameters were proved to have at least a good correla- tion with survival. In the multivariate Hazard ratio pro- portional test, the hemodynamic parameters PVR, SVO2 und SAO2 and the exercise parameters VE/VECO2 slope at AT and, sur- prisingly, the heart rate recovery (HRR) were independ- ently associated with an adverse vs. a favourable out- come. Most interestingly in this context is the non-inva- sive derived CPET parameter HRR (<8 beats within the first minute) with its very powerful pre- dictive value (HR 21.63, CI 2.85 - 321.21, p = 0.01) for adverse outcome in our patient group. The rise in heart rate during exercise is considered to be due to the com- Copyright © 2011 SciRes. OJRD  H. SCHROETTER ET AL. 11 bination of parasympathetic withdrawal and sympathetic activation [27]. The fall in heart rate immediately after exercise is considered to be a function of reactivation of the parasympathetic nervous system [28]. Increased va- gal activity has been associated with a reduction in the risk of death [29]. In contrast, a slow rate of heart rate recovery after exercise testing is a powerful and inde- pendent predictor of overall mortality. This easily avail- able marker is independent from workload, the presence or absence of myocardial perfusions defects, and changes in heart rate during exercise [30]. In patients with left heart failure, delayed heart rate recovery is a significant predictor of both sudden cardiac death and mortality due to pump failure [31]. With regard to pulmonary diseases, different observations are consistent with the existence of an important imbalance of autonomic dysfunction in pa- tients with various grades of lung disease. For example, Seshadri et al. [32] demonstrated that in COPD patients either obstructive or restrictive abnormalities found on spirometry are associated with abnormal heart rate re- covery. With re- gard to pulmonary hypertension, Velez-Roa et al. [33] reported that the activity of the sympathetic nervous sys- tem, as assessed by peroneal nerve microneuropathy, is markedly increased in patients with pulmonary arterial hypertension. This sympathetic overactivity appears to be related to right atrial distention and decreased cardiac output. Both may be improved by atrial septostomy, a live saving intervention for patients with severe PAH, to bridge for lung transplantation. In fact, Ciarka et al. [34] showed that atrial septostomy in PAH patients decreases sympathetic nerve hyperactivity, mostly related to de- creased right atrial distension. The specific role of an additionally modulated parasympa- thetic reactivation, reflected by a diminished HRR, was not examined in these studies. Recently, Swigris et al. [35] could show that in patients with idiopathic pulmo- nary fibrosis and pulmonary hypertension an abnormal heart rate recovery after 6MWT appeared to be the most potent predictor of mortality (HR 5,2). The findings of the present study, however, demonstrated that a dimin- ished HRR (<8 beat within the first minute) was able to indicate an adverse outcome in patients with pre-capil- lary pulmonary hyper-tension of different causes. Thus, the present study strongly supports the notion that in pa- tients with pre-capillary pulmonary hypertension the imbalance of the autonomic nerve system is of utmost importance for prognosis. Future studies with larger numbers of patients and potentially longer follow up have to define the exact role of such an autonomic dys- function in the pathophysiology and prognosis of pul- monary arterial hypertension. The main limitations of the present study are the population size of only 41 patients. This is due to the low prevalence of this disease. In an attempt to evaluate the different prognostic parameters in patient groups with distinct aetiologies of pre-capillary pulmonary hyperten- sion, we calculated the results at first for all patients, followed by a calculation for the IPAH patients only. But, the even smaller number of patients of the latter group (IPAH, n = 23) with only 6 major events in the follow up period does not allow any final conclusions and may only be considered as pilot data. One of the best evalu- ated prognostic predictors in patients with IPAH and other causes of pre-capillary hypertension is the 6MWD [36]. In our study only 28 patients underwent the 6MWD test. However, in the present study an independent prog- nostic value of this exercise parameter could not be achieved, neither in IPAH patients nor in the total, unse- lected PH hypertension group. Again, this may be due to the different severities and stages of the disease. Further studies have to address this valid point. In conclusions, the present study indicates that H- FABP is neither a reliable parameter of disease severity nor a novel predictor of mid and long term outcome in patients with various forms of pre-capillary pulmonary hypertension. Also, other biomarkers such as NT-Pro- BNP and Big-ET did not appear to be useful tools for outcome stratification in this patient group. They reflect the disease severity better, because of their moderate to high correlation to different hemodynamic and exercise parameters. Still, they did not prove to be independent predictors of event free survival. More promising diag- nostic tools with prognostic relevance for such typically unselected patients could be the well known and estab- lished exercise parameters of an increased slope of VE/VCO2, but also a delayed HRR, the latter indicative for a diminished vagal activity. Both are noninvasive and easily measurable parameters. Especially, the quite pow- erful and easily accessible predictive value of heart rate recovery (HRR) may provide the rationale for new stud- ies defining the temporal relation of autonomic nervous system distortion to right heart failure with bad prognosis in pulmonary arterial hypertension. 5. Acknowledgements H.S. thanks MH who participated in the design of the study and helped to perform right heart catheterization and CPET, DS who participated in study coordination, data acquisition and performing statistical analysis. Sin- cere thanks are given to PB carried out the immunoas- says, to GH, RCBD and RHS helped to draft the manu- script and revised it critically and to AS who conceived of the study, and participated in creating design, partici- pated in the sequence alignment, drafted the manuscript, performed right heart catheterization and gave final ap- Copyright © 2011 SciRes. OJRD  12 H. SCHROETTER ET AL. proval of the version to be published. All authors read and approved the final manuscript. 6. References [1] W. Klepetko, E. Mayer, J. Sandoval, E. P. Trulock, J. L. Vachiery, P. Dartevelle, J. Pepke-Zaba, S. W. Jamieson, I. Lang and P. Corris, “Interventional and Surgical Modali- ties of Treatment for Pulmonary Arterial Hypertension,” Journal of the American College of Cardiology, Vol. 43, No. 12, 2004, pp. 73S-80S. doi:10.1016/j.jacc.2004.02.039 [2] R. J. Barst, M. McGoon, A. Torbicki, O. Sitbon, M. J. Krowka, H. Olschewski and S. Gaine, “Diagnosis and Differential Assessment of Pulmonary Arterial Hyperten- sion,” Journal of the American College of Cardiology, Vol. 43, No. 12, 2004, pp. 40S-47S. doi:10.1016/j.jacc.2004.02.032 [3] V. V. McLaughlin, K. W. Presberg, R. L. Doyle, S. H. Abman, D. C. McCrory, T. Fortin and G. Ahearn, “Prog- nosis of Pulmonary Arterial Hypertension: ACCP Evi- dence-Based Clinical Practice Guidelines,” Chest, Vol. 126, No. 1, 2004, pp. 78S-92S. doi:10.1378/chest.126.1_suppl.78S [4] A. Torbicki, M. Kurzyna, P. Kuca, A. Fijalkowska, J. Sikora, M. Florczyk, P. Pruszczyk, J. Burakowski and L. Wawrzynska, “Detectable Serum Cardiac Troponin T as a Marker of Poor Prognosis among Patients with Chronic Precapillary Pulmonary Hypertension,” Circulation, Vol. 108, No. 7, 2003, pp. 844-848. doi:10.1161/01.CIR.0000084544.54513.E2 [5] S. B. Eysmann, H. I. Palevsky, N. Reichek, K. Hackney, P. S. Douglas, “Two-Dimensional and Doppler- Echo- cardiographic and Cardiac Catheterization Correlates of Survival in Primary Pulmonary Hypertension,” Circula- tion, Vol. 80, 1989, pp. 353-360. doi:10.1161/01.CIR.80.2.353 [6] R. J. Raymond, A. L. Hinderliter, P. W. Willis, et al., “Echocardiographic Predictors of Adverse Outcomes in Primary Pulmonary Hypertension,” Journal of the American College of Cardiology, Vol. 39, No. 7, 2002, pp. 1214-1219. doi:10.1016/S0735-1097(02)01744-8 [7] N. Galie, A. L. Hinderliter, A. Torbicki, et al., “Effects of the Oral Endothelin-Receptor Antagonist Bosentan on Echocardiographic and Doppler Measures in Patients with Pulmonary Arterial Hypertension,” Journal of the American College of Cardiology, Vol. 41, No. 8, 2003, pp. 1380-1386. doi:10.1016/S0735-1097(03)00121-9 [8] S. M. Kawut, E. M. Horn, K. K. Berekashvili, et al., “New Predictors of Outcome in Idiopathic Pulmonary Arterial Hypertension,” The American Journal of Cardi- ology, Vol. 95, No. 2, 2005, pp. 199-203. doi:10.1016/j.amjcard.2004.09.006 [9] N. Nagaya, T. Nishikimi, M. Uematsu, et al., “Plasma Brain Natriuretic Peptide as a Prognostic Indicator in Pa- tients with Primary Pulmonary Hypertension,” Circula- tion, Vol. 102, 2000, pp. 865-870. [10] H. H. Leuchte, N. M. El, J. C. Tuerpe, et al., “N-terminal Pro-brain Natriuretic Peptide and Renal Insufficiency as Predictors of Mortality in Pulmonary Hypertension,” Chest, Vol. 131, No. 2, 2007, pp. 402-409. doi:10.1378/chest.06-1758 [11] H. H. Leuchte, M. Holzapfel, R. A. Baumgartner, et al., “Clinical Significance of Brain Natriuretic Peptide in Primary Pulmonary Hypertension,” Journal of the American College of Cardiology, Vol. 43, No. 5, 2004, pp. 764-770. doi:10.1016/j.jacc.2003.09.051 [12] N. Nagaya, T. Nishikimi, Y. Okano, et al. , “Plasma Brain Natriuretic Peptide Levels Increase in Proportion to the Extent of Right Ventricular Dysfunction in Pulmonary Hypertension,” Journal of the American College of Car- diology, Vol. 31, No. 1, 1998, pp. 202-208. doi:10.1016/S0735-1097(97)00452-X [13] N. Nagaya, T. Nishikimi, M. Uematsu, et al., “Plasma Brain Natriuretic Peptide as a Prognostic Indicator in Pa- tients with Primary Pulmonary Hypertension,” Circula- tion, Vol. 102, 2000, pp. 865-870. [14] A. Fijalkowska, M. Kurzyna, A. Torbicki, et al., “N-Terminal Brain Natriuretic Peptide as a Prognostic Parameter in Patients with Pulmonary Hypertension,” Chest, Vol. 129, No. 5, 2006, pp. 1313-1321. doi:10.1378/chest.129.5.1313 [15] M. M. Pelsers, W. T. Hermens and J. F. Glatz, “Fatty Acid-Binding Proteins as Plasma Markers of Tissue In- jury,” Clinica Chimica Acta, Vol. 352, No. 1-2, 2005, pp. 15-35. doi:10.1016/j.cccn.2004.09.001 [16] H. A. Alhadi and K. A. Fox, “Do We Need Additional Markers of Myocyte Necrosis: The Potential Value of Heart Fatty-acid-binding Protein,” QJM, Vol. 97, No. 4, 2004, pp. 187-198. doi:10.1093/qjmed/hch037 [17] M. M. Pelsers, W. T. Hermens, J. F. Glatz, “Fatty Acid-Binding Proteins as Plasma Markers of Tissue In- jury,” Clinica Chimica Acta, Vol. 352, No. 1-2, 2005, pp. 15-35. doi:10.1016/j.cccn.2004.09.001 [18] M. Puls, C. Dellas, M. Lankeit, et al., “Heart-Type Fatty Acid-Binding Protein Permits Early Risk Stratification of Pulmonary Embolism,” European Heart Journal, Vol. 28, No. 2, 2007, pp. 224-229. doi:10.1093/eurheartj/ehl405 [19] A. Kaczynska, M. M. Pelsers, A. Bochowicz, et al., “Plasma Heart-Type Fatty Acid Binding Protein is Supe- rior to Troponin and Myoglobin for Rapid Risk Stratifi- cation in Acute Pulmonary Embolism,” Clinica Chimica Acta, Vol. 371, No. 1-2, 2006, pp. 117-123. doi:10.1016/j.cca.2006.02.032 [20] M. Lankeit, C. Dellas, A. Panzenbock, et al., “Heart- Type Fatty Acid-Binding Protein for Risk Assessment of Chronic Thromboembolic Pulmonary Hypertension,” Euro- pean Respiratory Journal, Vol. 31, No. 5, 2008, pp. 1024- 1029. doi:10.1183/09031936.00100407 [21] J. L. Snow and S. M. Kawut, “Surrogate End Points in Pulmonary Arterial Hypertension: Assessing the Re- sponse to Therapy,” Clinics in Chest Medicine, Vol. 28, No. 1, 2007, pp. 75-89. doi:10.1016/j.ccm.2006.11.005 [22] ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories, “ATS Statement: Guide- lines for the Six-Minute Walk Test,” American Journal of Copyright © 2011 SciRes. OJRD  H. SCHROETTER ET AL. Copyright © 2011 SciRes. OJRD 13 Respiratory and Critical Care Medicine, Vol. 166, No. 1, 2002, pp. 111-117. [23] N. Nagaya, T. Nishikimi, M. Uematsu, et al., “Plasma Brain Natriuretic Peptide as a Prognostic Indicator in Pa- tients with Primary Pulmonary Hypertension,” Circula- tion, Vol. 102, 2000, pp. 865-870. [24] H. H. Leuchte, N. M. El, J. C. Tuerpe, et al., “N-terminal Pro-brain Natriuretic Peptide and Renal Insufficiency as Predictors of Mortality in Pulmonary Hypertension,” Chest, Vol. 131, No. 2, 2007, pp. 402-409. doi:10.1378/chest.06-1758 [25] J. A. de Lemos, D. K. McGuire and M. H. Drazner, “B-type Natriuretic Peptide in Cardiovascular Disease,” Lancet, Vol. 362, No. 9380, 2003, pp. 316-322. doi:10.1016/S0140-6736(03)13976-1 [26] S. Rosenkranz, “Pulmonary Hypertension: Current Di- agnosis and Treatment,” Clinical Research in Cardiology, Vol. 96, No. 8, 2007, pp. 527-541. doi:10.1007/s00392-007-0526-8 [27] Y. Arai, J. P. Saul, P. Albrecht, et al., “Modulation of Cardiac Autonomic Activity during and Immediately af- ter Exercise,” American Journal of Physiology, Vol. 256, No. 1, 1989, pp. H132-H141. [28] K. Imai, H. Sato, M. Hori, et al., “Vagally Mediated Heart Rate Recovery after Exercise is Accelerated in Ath- letes but Blunted in Patients with Chronic Heart Failure,” Journal of the American College of Cardiology, Vol. 24, No. 6, 1994, pp. 1529-1535. doi:10.1016/0735-1097(94)90150-3 [29] P. J. Schwartz, M. T. La Rovere and E. Vanoli, “Auto- nomic Nervous System and Sudden Cardiac Death. Ex- perimental Basis and Clinical Observations for Post- Myocardial Infarction Risk Stratification,” Circulation, Vol. 85, 1992, pp. I77-I91. [30] C. R. Cole, E. H. Blackstone, F. J. Pashkow, et al., “Heart-Rate Recovery Immediately after Exercise as a Predictor of Mortality,” The New England Journal of Medicine, Vol. 341, 1999, pp. 1351-1357. doi:10.1056/NEJM199910283411804 [31] M. Guazzi, J. Myers, M. A. Peberdy, et al., “Heart Rate Recovery Predicts Sudden Cardiac Death in Heart Fail- ure,” International Journal of Cardiology, Vol. 144, No. 1, 2009, pp. 121-123. doi:10.1016/j.ijcard.2008.12.149 [32] N. Seshadri, T. R. Gildea, K. McCarthy, et al., “Associa- tion of an Abnormal Exercise Heart Rate Recovery with Pulmonary Function Abnormalities,” Chest, Vol. 125, No. 4, 2004, pp. 1286-1291. doi:10.1378/chest.125.4.1286 [33] S. Velez-Roa, A. Ciarka, B. Najem, et al., “Increased Sympathetic Nerve Activity in Pulmonary Artery Hyper- tension,” Circulation, Vol. 110, 2004, pp. 1308-1312. doi:10.1161/01.CIR.0000140724.90898.D3 [34] A. Ciarka, J. L. Vachiery, A. Houssiere, et al., “Atrial Septostomy Decreases Sympathetic Overactivity in Pul- monary Arterial Hypertension,” Chest, Vol. 131, No. 6, 2007, pp. 1831-1837. doi:10.1378/chest.06-2903 [35] J. J. Swigris, J. Swick, F. S. Wamboldt, et al., “Heart Rate Recovery after 6-Minute Walk Test Predicts Sur- vival in Patients with Idiopathic Pulmonary Fibrosis,” Chest, Vol. 136, No. 3, 2009, pp. 841-848. doi:10.1378/chest.09-0211 [36] S. Miyamoto, N. Nagaya, T. Satoh, et al., “Clinical Cor- relates and Prognostic Significance of Six-Minute Walk Test in Patients with Primary Pulmonary Hypertension: Comparison with Cardiopulmonary Exercise Testing,” American Journal of Respiratory and Critical Care Medi- cine, Vol. 161, No. 2, 2000, pp. 487-492.
|