Open Journal of Marine Science
Vol. 3 No. 2 (2013) , Article ID: 30842 , 9 pages DOI:10.4236/ojms.2013.32012
A Depth Wise Diversity of Free Living N2 Fixing and Nitrifying Bacteria and Its Seasonal Variation with Nitrogen Containing Nutrients in the Mangrove Sediments of Sundarban, WB, India
1Department of Marine Science, University of Calcutta, Kolkata, India
2Microbiology Laboratory, Department of Botany, Burdwan University, Burdwan, India
Email: *subhajit_310@yahoo.com, detarun@gmail.com
Copyright © 2013 Subhajit Das et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 18, 2012; revised February 26, 2013; accepted March 20, 2013
Keywords: Nitrifying Bacteria; N2 Fixing Bacteria; Bacterial Population; Mangrove Sediment
ABSTRACT
Mangrove provides a unique ecological niche to different microbes which play various roles in nutrient recycling as well as various environmental activities. The highly productive and diverse microbial community living in mangrove ecosystems continuously transforms dead vegetation and recycle nitrogen, phosphorus, sulphur and other nutrients that can later be used by the plants. Mangrove ecosystems are rich in organic matter, and however, in general, they are nutrient-deficient ecosystems, especially of nitrogen and phosphorus. The present study investigated depth wise variation of nitrifying bacteria, nitrogen fixing bacteria, total bacterial population along with nitrate-nitrogen, nitrite-nitrogen and other physicochemical parameters of soil during pre-monsoon, monsoon and post-monsoon periods at three different sampling stations of mangrove sediments viz. deep forest region, rooted region and un-rooted region. The microbial population was also found maximum in the deep forest sediment relative to the other two sites. Populations of cultureable microbes were found maximum in surface soil and decreased with increase in depth in Sundarban mangrove environment. A decreasing trend of total microbial load, nitrifying and nitrogen fixing bacteria with increase in depth were recorded throughout the year. Present study revealed the relationship among depth integrated variations of physicochemical components (viz. soil temperature, pH, salinity, nitrite-nitrogen and nitrate-nitrogen concentration) and total microbial load, nitrifying and nitrogen fixing bacteria microbial populations.
1. Introduction
Mangrove forests are woody plant communities in the intertidal regimes of tropical and subtropical coasts. Mangroves provide a unique ecological niche to different microbes which play various roles in nutrient recycling as well as various environmental activities. The highly productive and diverse microbial community living in mangrove ecosystems continuously transforms dead vegetation into sources of nitrogen, phosphorus, and other nutrients that can later be used by the plants and in turn, plant-root exudates serve as a food source for the microorganisms living in the ecosystem, with other plant material serving a similar role for larger organisms, such as crabs and detritus feeding fish.
The fallen mangrove leaves, after autolysis and microbial breakdown, produce detritus which is the most important source of energy for the estuarine food chain. Thus mangroves, by introducing substantial quantities of organic material to the community, play an important and essential role in supporting a wide range of offshore marine organisms in their early stages of development [1]. Biogeochemical nitrogen and other nutrient cycles within mangrove forests are potentially important processes for the development of the forests and other biota of the ecosystem [2]. It has been studied that N2 fixation by heterotrophic bacteria is generally regulated by specific environmental factors like oxygen, combined Nitrogen and the availability of carbon source for energy requirement [3]. Aerobic, autotrophic nitrifiers oxidize ammonia to nitrite and nitrate, with molecular oxygen as electron acceptor. Nitrite and nitrate are reduced to di-nitrogen gas by heterotrophic denitrifying bacteria that use NOx instead of oxygen as electron acceptor [4]. Nitrifying bacteria are considered to be a significant group of bacteria that facilitates in mineralization of molecular nitrogen or nitrous oxide from ammonia which is derived from protein of organic debris. The reliable balance between population of nitrifying bacteria and nitrogen fixing bacteria in mangrove sediment is quiet effective for maintaining the fertility of soil sediment with respect to nitrate-nitrogen. Nitrous oxide is an intermediate product of both nitrification, the aerobic oxidation of ammonium to nitrite and nitrate, and denitrification, the suboxic reduction of nitrate and nitrite, principally to molecular nitrogen [5].
Thus mangrove ecosystems are rich in organic matter, however, in general, they are nutrient-deficient ecosystems, especially of nitrogen and phosphorus [6,7], which are indispensable for plant growth. In spite of this, on a global scale, mangroves are among the most productive ecosystems. This paradox can be explained by a very efficient recycling that keeps the scarce nutrients within the system. Microbial activity is responsible for major nutrient transformations within a mangrove ecosystem [8].
The purpose of the present study was look into depth wise variation of nitrifying bacteria, free living nitrogen fixing bacteria, total bacterial population along with nitrate-nitrogen, nitrite-nitrogen and other physicochemical parameters of soil during pre-monsoon, monsoon and post-monsoon periods at three different sampling stations. We also tried to set up the correlation pattern between them regarding soil fertility.
2. Materials and Methods
2.1. Study Area
Sundarban Mangrove forest is located geographically in between 21˚31'N and 22˚30'N and longitude 88˚10'E and 89˚51'E along the North East coast of Bay of Bengal, India. This mangrove forest is a part of the estuarine system of the River Ganges, NE coast of Bay of Bengal, which covers 9630 km2, out of which 4264 km2 of intertidal area, covered with thick mangroves. Climate in the region is characterized by the southwest monsoon (JuneSeptember), northeast monsoon or post-monsoon (October-January), and pre-monsoon (February-May); 70% - 80% of annual rainfall occurs during the summer monsoon (southwest monsoon), The tide in this estuarine complex is semidiurnal in nature with spring tide range between 4.27 and 4.75 m and neap tide range between 1.83 and 2.83 m. It is a unique bioclimatic zone in land ocean boundaries of Bay of Bengal and the largest delta on the globe. The deltaic soil of Sundarban Biosphere Reserve comprises mainly with saline alluvial soil consisting of clay, silt, fine sand and coarse sand particles. It is described as very deep, poorly drained, fine soils occurring on level to nearly level lower delta with loamy surface, severe flooding and very strong salinity (extensive extent) associated with very deep, very poorly drained, fine loamy soil. Sediment samples were collected from three distinct zones namely Deep forest region (Station 1), Rooted region (Station 2) & Un-rooted region (Station 3) of Sundarban mangrove ecosystem (Figure 1).
2.2. Collections and Analyses
Sediment core was sectioned at 10 cm intervals in sterile conditions to obtain representative samples at 0 - 10, 10 - 20, 20 - 30, 30 - 40, 40 - 50, and 50 - 60 cm depth using a hand—held stainless steel core sampler (3.2 cm diameter, 100 cm long) from three distinct sampling zones of Sunderban mangrove ecosystem during January 2008 to December 2009. Samples were collected into sterilized container and were transferred to laboratory immediately for both chemical and microbiological assay. The different collection zones were: 1) dense or deep forest region; 2) rooted region (mid littoral zone) where pneumatophores were present; and 3) un-rooted region where pneumatophores were absent (lower littoral zone).
2.3. Quantification of Nitrifying Bacteria & Free Living N2 Fixing Bacteria
Sediment samples were stored at 4˚C immediately after
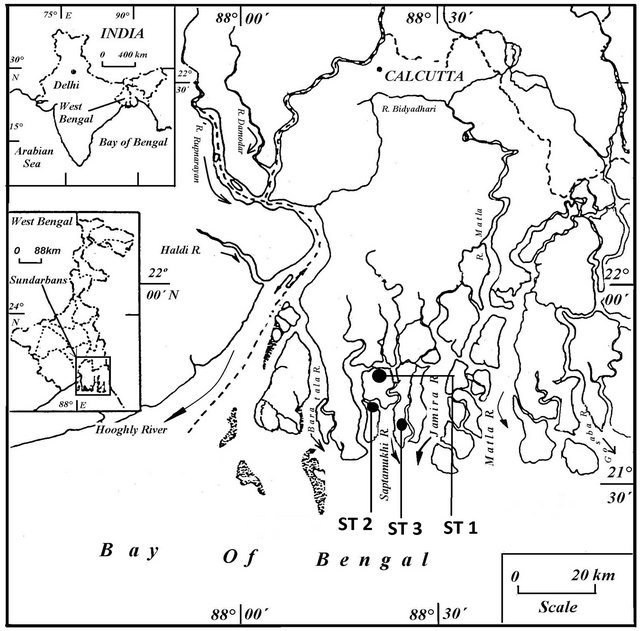
Figure 1. Map showing the study area.
collection and transported with adequate care to the laboratory for analysis. For quantification of bacteria we followed the procedure as described by [9]. We homogenized 10 g sample from different locations in sterile phosphate buffer. Serial dilutions up to 10-4 were made and inoculation was done with 0.1 ml. For quantification of free living nitrogen fixers inoculations from each zone were done in a selective medium, comprising Manitol (15.0 g), K2HPO4 (0.5 g), MgSO4·7H2O (0.2 g), CaSO4 (0.1 g), NaCl (0.2 g), CaCO3 (5.0 g), Agar (15.0 g), Isotonic solution with the soil prepared from NaCl and sterilized distilled water (1ltr) and pH was maintained at 8.3. The nitrifying bacteria were quantified on Winogardsky’s medium (g/l: K2HPO4 1, NaCl 2, MgSO4·7H2O 0.5, FeSO4·7H2O trace, CaCl2·2H2O 0.02, pH 8.5) containing 1.0 g/l either (NH4)2SO4 and the colonies were visualized (pink colour) by flooding the plates with sulphanillic acid reagent (sulphanillic acid 8 g/l acetic acid (5 M) and a-naphthayl amine 5 g/l acetic acid (5 M); 1:1, v/v) [10].
2.4. Nutrients Analysis of the Sediment
Nitrate-Nitrogen of the soil sediment samples were measured following standard procedure. We added 30 g of soil subsample to 75 ml of 2 M potassium chloride (KCl). The mixture was shaken until well mixed and allowed to stand overnight. After 24 h, 4 ml of the supernatant was collected for the estimation of nitrate-nitrogen using standard spectrophotometric methods [11]. The pH value was measured in a 1:5 (w/w) soil water suspension using an electric digital pH meter [12] and salinity of a soil saturation extract (ECe) was determined by measuring the electrical conductance of soil water saturation extract with the help of a conductivity meter (Richards LA (ed), Agriculture Hand Book No. 60, Oxford and IBH Publishing Company, New Delhi) [13].
3. Results
Mangrove sediment at Indian Sundarban showed seasonal variation with respect to nitrate-nitrogen, nitritenitrogen concentrations and population of nitrifying bacteria and free living nitrogen fixing bacteria. Beside monsoonal addition of nutrients to the system, mangrove litters also played a significant role in regulating the nutrient status that in turn controls the microbial population. Among several physical factors tidal inundation, wave action, presence of mangrove roots and bioturbation are the important factors considered for determining microbial abundance in the mangrove sediment from surface to depth of up to 60 cm. Similarly the pH, temperature and salinity are also important factors which regulate the microbial population in the mangrove sediments.
3.1. Seasonal and Depth Wise Variation of pH, Temperature and Salinity of Mangrove Sediments
In the deep forest region average pH value was found to show a decreasing trend from 10 cm depth to the 60 cm of depth, however, a reverse profile was found for salinity (psu) in depth during pre-monsoon, monsoon and post-monsoon. No such stratification was found for temperature in all cases (Table 1). The rooted region was found to show a decreasing trend of pH value from surface to 60 cm of depth during pre-monsoon and postmonsoon but no distinct stratification of pH was noted in monsoon. On contrary salinity of the sediment showed an increasing trend from surface to 60 cm of depth during pre-monsoon and monsoon but no such stratification was observed in post-monsoon rather it was fluctuated. Similarly, the temperature was did not show any distinct increasing or decreasing stratification with increasing depth in pre-monsoon and monsoon and it was fluctuated in post-monsoon (Table 2). However, the sediment tem
Table 1. Seasonal and vertical distribution of physical parameters in the deep forest region.

Table 2. Seasonal and vertical distribution of physical parameters in the rooted region.

perature, pH of un-rooted region did not show satisfactory stratification with increasing depth at un-rooted region during during pre-monsoon, monsoon and postmonsoon only salinity was fluctuated in all season (Table 3).
3.2. Seasonal and Depth Wise Variation of Nitrite Nitrogen, Nitrate Nitrogen, Nitrifying Bacteria, Free Living N2 Fixing Bacteria and Total Bacterial Population
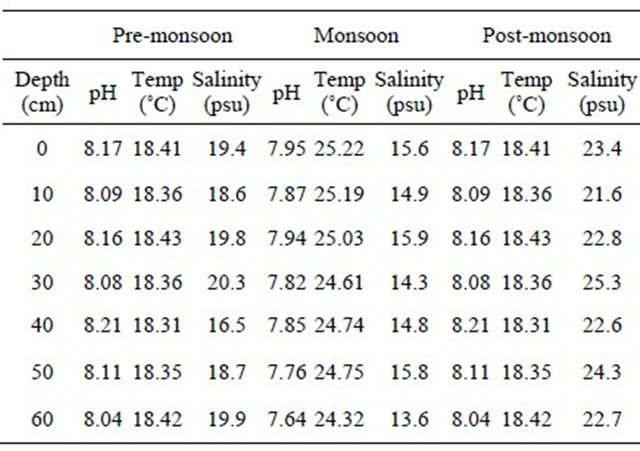
1) Deep forest sediments There were decreasing trend was found for nitrite nitrogen of soil but increasing trend of nitrate-nitrogen concentration with increase in depth of sediment at deep forest region during pre-monsoon. The population of nitrifying bacteria, free living nitrogen fixing bacteria and total bacterial population were found to a slight decreasing trend with gradual increase of the depth (Figure 2(a)).
During monsoon a slight fluctuating trend of nitritenitrogen concentration was found from surface to gradual increasing of depth of sediment but nitrate-nitrogen concentration was found to show a decreasing trend from surface to depth. The population of nitrifying bacteria, free living nitrogen fixing bacteria and total microbial population were found to show a decreasing trend from surface to 60 cm of depth (Figure 2(b)).
During post-monsoon decreasing trend for nitrate-ni trogen was found from surface to 60 cm of depth. A similar decreasing pattern of depth profile regarding the population of nitrifying bacteria, free living nitrogen fixing bacteria and total bacteria were found. No distinc stratification of nitrite-nitrogen concentration of sediment was found with increase in depth (Figure 2(c)).
2) Rooted sediment During pre-monsoon the nitrate-nitrogen concentration was found to show an increasing trend from surface to 30 cm of depth and below this 30 cm of depth its value was
Table 3. Seasonal and vertical distribution of physical parameters in the un-rooted region.
 (a)
(a) (b)
(b) (c)
(c)
Figure 2. Depth profile of population of nitrifying bacteria, free living nitrogen fixing bacteria, total microbial population along with the concentration of nitrate-nitrogen & nitrite-nitrogen of the sediment at the deep forest region during pre-monsoon (a), monsoon (b), post-monsoon (c).
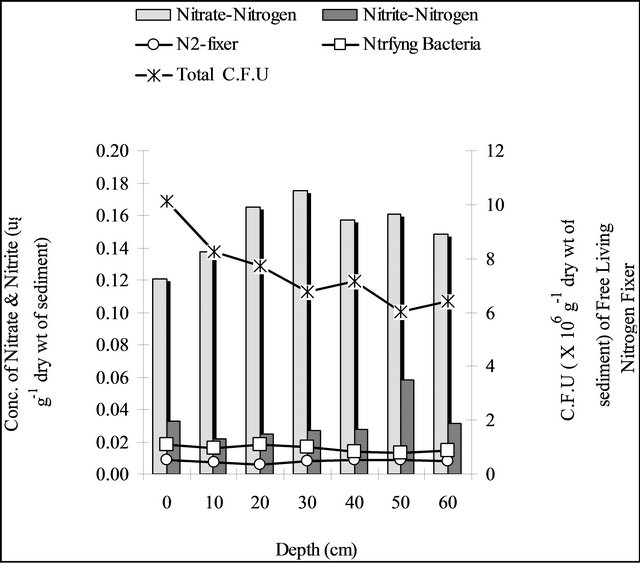
found to show a decreasing trend up to 60 cm of depth but no consistent stratification was found for nitrite-nitrogen concentration of the sediment. The population of nitrifying bacteria did not show any stratification from surface to 30 cm of depth, but their population showed highest at 50 cm of depth. The population of free living nitrogen fixing bacteria was found to show a decreasing trend from surface but after 30 cm depth to 60 cm of depth their population showed an increasing trend. The total microbial population showed a decreasing trend from surface with increasing depth (Figure 3(a)).
The concentration of nitrite-nitrogen of sediment did not show any remarkable stratification with increasing depth but nitrate-nitrogen concentration was found to show a decreasing trend from surface of sediment with ncreasing depth during monsoon. The population of nitrifying bacteria and total bacterial population were found to show a decreasing trend with gradual increase of depth. The population of free living nitrogen fixing bacteria showed a decreasing trend from surface to 30 cm of depth from 40 cm depth to next 60 cm of depth their population showed a little increasing trend with increasing depth (Figure 3(b)).
During post-monsoon the concentration of nitrate-nitrogen of the sediment showed a decreasing pattern with fluctuating concentration from surface to 60 cm of depth but the concentration of nitrite-nitrogen of the sediment was fluctuating at different depth of the sediments. Population of nitrifying bacteria and total bacteria were found to reveal a decreasing trend from surface. Quite surprising depth profile regarding the population of free living nitrogen fixing bacteria was found, from surface, their population was found to decrease with increase in depth up to 30 cm of depth but a sudden increase of their population was found from 40 cm to the next 60 cm of depth (Figure 3(c)).
3) Un-rooted sediments.
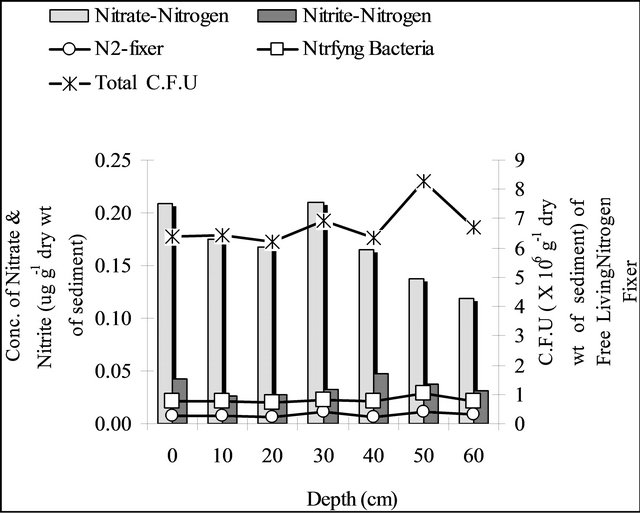
During pre-monsoon the concentration of nitrite nitrogen did not show any distinct stratification with increaseing depth but nitrate-nitrogen concentration was highest recorded at 30 cm depth and then it was gradually decreased with increasing depth. The nitrifying bacteria, free living nitrogen fixing bacteria and total bacteria populations were fluctuating at un-rooted regions (Figure 4(a)).
During monsoon the concentration of nitrite-nitrogen did not show any prominent gradation with increase in depth. But the nitrate-nitrogen concentration of sediments, the nitrifying bacterial population and the population of free living nitrogen fixing bacteria were fluctuating with gradual increase of depth. The population total bacterial load showed a decreasing trend from surface to 60 cm of depth of the sediment concerned (Figure 4(b)).
The concentration of nitrate-nitrogen showed an aver-
 (a)
(a) (b)
(b) (c)
(c)
Figure 3. Depth profile of population of nitrifying bacteria, free living nitrogen fixing bacteria, total microbial population along with the concentration of nitrate-nitrogen & nitrite-nitrogen of the sediment at the rooted region during pre-monsoon (a), monsoon (b), post-monsoon (c).
 (a)
(a) (b)
(b) (c)
(c)
Figure 4. Depth profile of population of nitrifying bacteria, free living nitrogen fixing bacteria, total microbial population along with the concentration of nitrate-nitrogen & nitrite-nitrogen of the sediment at the un-rooted region during pre-monsoon (a), monsoon (b), post-monsoon (c).
age decreasing trend from surface but suddenly increased at 30 cm of depth in post monsoon. The concentration of nitrite-nitrogen did not show any prominent stratification with increase in depth. The population of nitrifying bacteria, free living nitrogen fixing bacteria and total bacteria were found increasing trend but more fluctuating with increasing depth of sediments (Figure 4(c)).
4. Discussions
Nitrogen (N2) fixation is common in mangroves. High rates of nitrogen fixation were detected in association with dead and decomposing leaves, pneumatophores (aerial roots), the rhizosphere, tree bark, cyanobacterial mats covering the surface of sediments, and in the sediments themselves [14-16]. N2 fixing microorganisms can colonize in both terrestrial as well as marine environments. N2 fixation in mangrove sediments is likely to be limited insufficient energy sources. It has been studied that N2 fixation by heterotrophic bacteria are generally regulated by specific environmental factors like oxygen, combined Nitrogen and the availability of carbon source for energy requirement. Aerobic, autotrophic nitrifiers oxidize ammonia to nitrite and nitrate, with molecular oxygen as electron acceptor. Nitrite and Nitrate are reduced to dinitrogen gas by heterotrophic denitrifying bacteria that use NOx instead of oxygen as electron acceptor [17].
The microbial population was also found maximum in the deep forest sediment relative to the other two sites.  -N concentration was increased from surface to 40 cm of depth but decreased from 40 cm to 60 cm. Vertical movement of materials, nutrient cycling and reuse driven by various burrowing organisms could have an effect on this
-N concentration was increased from surface to 40 cm of depth but decreased from 40 cm to 60 cm. Vertical movement of materials, nutrient cycling and reuse driven by various burrowing organisms could have an effect on this  -N distribution along the depth profile up to 40 cm. less abundance of bioturbation below 40 cm could enhance the anoxic condition which in turn initiate denitrification causing sudden depletion of
-N distribution along the depth profile up to 40 cm. less abundance of bioturbation below 40 cm could enhance the anoxic condition which in turn initiate denitrification causing sudden depletion of  -N. The
-N. The  -N concentration showed no significant variation throughout depth but slight increased below 50 cm of depth which may be an indication of denitrification. The existing environmental conditions facilitated the growth of nitrifying bacteria, which explained the higher concentration nitrate in overlying water [18]. Decrease in nitrate-nitrogen concentration with increase in depth could be explained by the decrease in population of nitrifying bacteria with increase in depth as earlier study has showed active participation of nitrifying bacteria in bio-mineralization [19].
-N concentration showed no significant variation throughout depth but slight increased below 50 cm of depth which may be an indication of denitrification. The existing environmental conditions facilitated the growth of nitrifying bacteria, which explained the higher concentration nitrate in overlying water [18]. Decrease in nitrate-nitrogen concentration with increase in depth could be explained by the decrease in population of nitrifying bacteria with increase in depth as earlier study has showed active participation of nitrifying bacteria in bio-mineralization [19].
Population of free living nitrogen fixing bacteria and nitrifying bacteria are showed decreasing pattern with increase in depth in almost every cases. The  -N content and organic carbon content (data not shown) of soil showed decreasing patterns along with decrease in population of nitrifying bacteria with increase in depth. The total bacterial population also shown decreasing pattern but in rooted region it was slightly increased upto 20 cm of depth and then decreasing trend. All the bacteral population was highest in deep forest region, higher in rooted region and comeratively less in un-rooted region. The low rates of N2 fixation by heterotrophic bacteria detected in marine sediments are probably due to decrease abundance of microbial load and lack of energy sources which can be regulated by specific environmental factors such as oxygen and the availability of carbon source to support energy requirement. Energy for N2 fixation in mangrove sediments was derived from leaves and roots decomposed by non-diazitrophic microorganisms [20]. Nitrogen-Fixing bacteria identified as members of the genera Azospirillum, Azotobacter, Rhizobium, Clostridium and Klebsiella were isolated from the sediments, rhizosphere, and root surfaces of various mangrove species [21]. In an arid mangrove of Mexico, several strains of diazotrophic bacteria were isolated from the rhizosphere of the mangroves Rhizophora mangle, Avicennia germinans, and Laguncularia racemosa. Some of these strains were identified as Vibrio campbelli, Listonella anguillarum, Vibrio aestuarianus, and Phyllobacterium sp. [22]. The abundance of N2 fixing bacteria in mangrove sediments may be dependent on physical condition like light intensity, water temperature and mangrove community composition. When the non-N2 fixing bacteria were removed from the rhizosphere it was observed that N2 fixing activity decreased which indicated that these non-N2 fixing bacteria like Staphylococcus sp. [23] could also contributed N2 fixation process. N2 fixing Azotobacter was abundant in mangrove habitats of Pichavaram which can be used as biofertilizer [24]. Halotolerant N2 fixing Rhizobium sp. Isolated from root nodules of Derris scandens and Sesbania sp. growing in Sundarban [25].
-N content and organic carbon content (data not shown) of soil showed decreasing patterns along with decrease in population of nitrifying bacteria with increase in depth. The total bacterial population also shown decreasing pattern but in rooted region it was slightly increased upto 20 cm of depth and then decreasing trend. All the bacteral population was highest in deep forest region, higher in rooted region and comeratively less in un-rooted region. The low rates of N2 fixation by heterotrophic bacteria detected in marine sediments are probably due to decrease abundance of microbial load and lack of energy sources which can be regulated by specific environmental factors such as oxygen and the availability of carbon source to support energy requirement. Energy for N2 fixation in mangrove sediments was derived from leaves and roots decomposed by non-diazitrophic microorganisms [20]. Nitrogen-Fixing bacteria identified as members of the genera Azospirillum, Azotobacter, Rhizobium, Clostridium and Klebsiella were isolated from the sediments, rhizosphere, and root surfaces of various mangrove species [21]. In an arid mangrove of Mexico, several strains of diazotrophic bacteria were isolated from the rhizosphere of the mangroves Rhizophora mangle, Avicennia germinans, and Laguncularia racemosa. Some of these strains were identified as Vibrio campbelli, Listonella anguillarum, Vibrio aestuarianus, and Phyllobacterium sp. [22]. The abundance of N2 fixing bacteria in mangrove sediments may be dependent on physical condition like light intensity, water temperature and mangrove community composition. When the non-N2 fixing bacteria were removed from the rhizosphere it was observed that N2 fixing activity decreased which indicated that these non-N2 fixing bacteria like Staphylococcus sp. [23] could also contributed N2 fixation process. N2 fixing Azotobacter was abundant in mangrove habitats of Pichavaram which can be used as biofertilizer [24]. Halotolerant N2 fixing Rhizobium sp. Isolated from root nodules of Derris scandens and Sesbania sp. growing in Sundarban [25].
5. Conclusion
From the present study a conclusion can be drawn as a result of our research on depth profile exploration of nitrifying bacteria, nitrogen fixing bacteria, total bacterial population along with nitrate-nitrogen, nitrite-nitrogen and other physicochemical parameters of soil during premonsoon, monsoon and post-monsoon periods at three different sampling stations viz. deep forest region, rooted region and un-rooted region from the sediments of Sundarban mangrove forest, India. The microbial population was also found highest in the deep forest sediment relative to the other two sites. Present study revealed that there was a co-relationship exists among depth integrated variations of physicochemical components (viz. soil temperature, pH) salinity nitrite-nitrogen and nitrate-nitrogen concentration and total microbial load, nitrifying and nitrogen fixing bacteria microbial populations. Vertical decrease in nitrate and nitrite concentrations along microbial populations suggested that increasing depth caused unfavorable condition for microorganisms to carry out bio-mineralization processes. Energy for N2 fixation in mangrove sediments may be derived from leaves and roots decomposed by non-diazitrophic microorganisms, particularly cellulose degrading bacteria in the mangrove sediment is under investigation.
REFERENCES
- B. F. Clough, “Mangrove Ecosystems in Australia: structure, function and management,” Australian Institute of Marine Science in Association with Australian National University Press, 1982.
- A. Sengupta and S. Chaudhuri, “Ecology of Heterotrophic Dinitrogen Fixation in the Rhizosphere of Mangrove Plant Community at the Ganges River Estuary in India,” Oecologia, Vol. 87, No. 4, 1991, pp. 560-564. doi:10.1007/BF00320420
- T. C. Balser and M. K. Firestone, “Linking Microbial Community Composition and Soil Processes in a California Annual Grassland and Mixed Conifer Forest,” BiogeoChemistry, Vol. 73, No. 2, 2005, pp. 395-415. doi:10.1007/s10533-004-0372-y
- R. H. Riley, M. Peter and P. M. Vitousek Nutrient Dynamics and Nitrogen Trace Gas Flux during Ecosystem Development in Montane Rain Forest,” Ecology, Vol. 76, No. 1, 1995, pp. 292-304. doi:10.2307/1940650
- J. E. Corredor, J. M. Morell and A. J. Bauz, “Atmospheric Nitrous Oxide Fluxes from Mangrove Sediments,” Marine Pollution Bulletin, Vol. 38, No. 6, 1999, pp. 473- 478. doi:10.1016/S0025-326X(98)00172-6
- D. M. Alongi, P. Christoffersen and F. Tirendi, “The Influence of Forest Type on Microbial-Nutrient Relationships in Tropical Mangrove Sediments,” Journal of Experimental Marine Biology and Ecology, Vol. 171, No. 2, 1993, pp. 201-223. doi:10.1016/0022-0981(93)90004-8
- P. Vazquez, G. Holguin, M. E. Puente, A. Lopez-Cortes and Y. Bashan, “Phosphate-Solubilizing Microorganism Associated with the Rhizosphere of Mangroves in a Semiarid Coastal Lagoon,” Biology and Fertility of Soils, Vol. 30, No. 5-6, 2000, pp. 460-468. doi:10.1007/s003740050024
- G. Holguin, P. Vazquez and Y. Bashan, “The Role of Sediment Microorganisms in the Productivity, Conservation, and Rehabilitation of the Mangrove Ecosystems: An Overview,” Biology and Fertility of Soils, Vol. 33, No. 4, 2001, pp. 265-278. doi:10.1007/s003740000319
- A. L. Ramanathan, G. Singh, J. Majumder, A. C. Samal, R. Chowhan, R. K. Rayan, K. Roykumar and S. C. Santra, “A Study of Microbial Diversity and Its Interaction with Nutrients in the Sediments of Sundarban Mangroves,” Indian Journal of Marine Science, Vol. 37, No. 2, 2008, pp. 159-165.
- J. Das and T. K. Dangar, “Microbial Population Dynamics, Especially Stress Tolerant Bacillus thuringiensis, in Partially Anaerobic Rice Field Soils during Postharvest Period of the Himalayan, Island, Brackish Water and Coastal Habitats of India,” World Journal of Microbiology and Biotechnology, Vol. 24, No. 8, 2008, pp. 1403- 1410. doi:10.1007/s11274-007-9620-3
- K. Grasshoff, M. Ehrhardt and K. Kremling, “Standard Methods for Seawater Analysis,” 2nd Edition, John Wiley & Sons, Hoboken, 1983.
- S. C. Tiwari, B. K. Tiwari and R. R. Mishra, “Microbial Community, Enzyme Activity and CO2 Evolution in Pineapple Orchard Soil,” Journal of Tropical Ecology, Vol. 30, No. 2, 1989, pp. 265-273.
- L. A. Richards, “Agriculture Hand Book No. 60,” Oxford and IBH Publishing Company, New Delhi, 1954.
- G. Holguin, M. A. Guzman and Y. Bashan, “Two New Nitrogen-Fixing Bacteria from the Rhizosphere of Mangrove Trees: Their Isolation, Identification and in vitro Interaction with Rhizosphere Staphylococcus sp.,” FEMS Microbiology Ecology, Vol. 101, 1992, pp. 207-216.
- F. D. Mann and T. D. Steinke, “Biological Nitrogen Fixation (Acetylene Reduction) Associated with Decomposing Avicennia marinaleaves in the Beachwood Mangrove Nature Reserve,” South African Journal of Botany, Vol. 58, 1992, pp. 533-536.
- G. Toledo, Y. Bashan and A. Soeldner, “Cyanobacteria and Black Mangroves in Northwestern Mexico: Colonization, and Diurnal and Seasonal Nitrogen Fixation on Aerial Roots,” Canadian Journal of Microbiology, Vol. 41, No. 11, 1995, pp. 999-1011. doi:10.1139/m95-139
- J. H. Ahn, R. Yu and K. Chandran, “Distinctive Microbial Ecology and Biokinetics of Autotrophic Ammonia and Nitrite Oxidation in a Partial Nitrification Bioreactor. Biotechnology and Bioengineering, Vol. 100, No. 6, 2008, pp. 1078-1087. doi:10.1002/bit.21863
- R. K. Ranjan, A. L. Ramanathan and G. Singh, “Evaluation of Geochemical Impact of Tsunami on Pichavaram Mangrove Ecosystem, Southeast Coast of India,” Environmental Geology, Vol. 55, No. 3, 2007, pp. 687-697.
- B. B. Ward, “Nitrification and Denitrification: Probing the Nitrogen Cycle in Aquatic Environments,” Microbial Ecology, Vol. 32, No. 3, 1996, pp. 247-261. doi:10.1007/BF00183061
- D. A. Zuberer and W. S. Silver, “Biological Dinitrogen Fixation (Acetylene Reduction) Associated with Florida Mangroves,” Applied and Environmental Microbiology, Vol. 35, No. 3, 1978, pp. 567-575.
- B. Eckert, O. B. Weber, G. Kirchhof, A. Halbritter, M. Stoffels1 and A. Hartmann, “Azospirillum doebereinerae sp. nov., a Nitrogen-Fixing Bacterium Associated with the C4-Grass,” Miscanthus International Journal of Systematic and Evolutionary Microbiology, Vol. 51, No. 1, 2001, pp. 17-26.
- A. Rojas, G. Holguin, B. R. Glick and Y. Bashan, “Synergism between Phyllobacterium sp. (N2-Fixer) and Bacillus licheniformis (P-Solubilizer), Both from a Semiarid Mangrove Rhizosphere,” FEMS Microbiology Ecology, Vol. 35, No. 2, 2001, pp. 181-187. doi:10.1111/j.1574-6941.2001.tb00802.x
- G. Holguin, M. A. Guzman and Y. Bashan, “NitrogenFixation by Azospirillum brasilense is Promoted When Co-Cultured with a Mangrove Rhizosphere Bacterium (Staphylococcus sp.),” Soil Biology and Biochemistry, Vol. 28, No. 12, 1996, pp. 1651-1660. doi:10.1016/S0038-0717(96)00251-9
- S. Ravikumar, K. Kathiresan, S. Thadedus, M. Ignatiammal, M. B. Selvam and S. Shanthy, “Nitrogen-Fixing Azotobacters from Mangrove Habitat and Their Utility as Marine Biofertilizers,” Journal of Experimental Marine Biology and Ecology, Vol. 312, No. 1, 2004, pp. 5-17. doi:10.1016/j.jembe.2004.05.020
- A. Sengupta and S. Chaudhuri, “Halotolerant Rhizobium Strains from Mangrove Swamps of the Ganges River Delta,” Indian Journal of Microbiology, Vol. 30, No. 4, 1990, pp. 483-484.
NOTES
*Corresponding author.

