Open Journal of Obstetrics and Gynecology
Vol.3 No.9(2013), Article ID:40061,5 pages DOI:10.4236/ojog.2013.39122
Laparoscopic diagnosis of endometriosis—Clinical study*
![]()
Department of Obstetrics and Gynecology, Oulu University Hospital, Oulu, Finland
Email: #markku.ryynanen@oulu.fi
Copyright © 2013 Henri Lahdemaki et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 24 April 2013; revised 22 May 2013; accepted 30 May 2013
Keywords: Endometriosis; Diagnostic Laparoscopy; Abdominal Pain; Pelvic Pain
ABSTRACT
Objective: To examine the laparoscopic findings in patients with suspicion of endometriosis. Study Design: Retrospective study. Setting: Department of Obstetrics and Gynecology, University of Oulu, Finland. Sample: First-time laparoscopy, without any known surgical diagnosis, was made in 53 consecutive patients between January 2006 and November 2011. Main Outcome Measures: The laparoscopic findings, staging of endometriosis, the percentages of different symptoms linked with endometriosis. Results: Laparoscopy revealed endometriosis in 40% of cases. Most frequent symptoms were dysmenorrhea (86%) and dyspareunia (81%) followed with vibration pain (71%), urinary symptoms (29%) and lowered fertility (24%). Only 5% of patients with endometriosis complained of bowel symptoms, which were significantly more common in patients without endometriosis (28%) (p = 0.034). The median interval between the onset of symptoms and laparoscopic diagnosis was 1.9 years (SD 3.2, range 0.6 - 11). Conclusions: The interval between the onset of symptoms and laparoscopic diagnosis is short reflecting the prompt availability of the necessary facilities in specialist health care. Finally laparoscopy seems to be safe in cases of endometriosis suspicion.
1. INTRODUCTION
Endometriosis is defined as the presence of endometrial-like tissue outside of the uterus, which includes a chronic, inflammatory reaction. The condition is predominantly found in women of reproductive age, from all ethnic and social groups. It affects 6% to 10% of women of reproductive age, 50% to 60% women and teenage girls with pelvic pain, and up to 50% of women with infertility [1,2]. Some women may have no symptoms at all—so surgical diagnosis of endometriosis may be coincidental. The associated symptoms can have negative impacts on general physical, mental and social wellbeing. Endometriosis has also been reported in postmenopausal women as well as in men [3,4]. A positive family history represents a six-fold greater risk of having the disorder. Also there is an association between endometriosis and uterine fibroids [5].
The most affected sites are the pelvic organs and peritoneum. The appearance of endometriosis is very variable. It can present as small lesions or implants in peritoneal and/or the ovarian surface. Endometrial implants may appear in a number of different ways, including subtle red or white lesions, clear “bubble” lesions, small hemorrhagic cysts (powder-burn, dark brown or bluish or red flame like), or white fibrotic lesions (like scarring) [6]. Endometriosis can present as large ovarian endometriomas.
Based on clinical and patient experience, the typical symptoms include severe dysmenorrhea, deep dyspareunia, chronic pelvic pain, ovulation pain, or vibration pain. Pelvic pain can occur independently on menstruation. Pain may be unior bilateral and may radiate to the lower back and down the legs. Some women with confirmed endometriosis in our area complain of pain or discomfort when bicycling (vibration pain). There can also be cyclical or peri-menstrual symptoms such as bowel and bladder symptoms, with or without abnormal bleeding or pain. 15% - 20% of women report abnormal uterine bleeding. Furthermore, infertility, chronic fatigue and pain on defecation belong to the clinical picture of endometriosis. In retrospect, more questions should be asked about bowel and bladder symptoms when interviewing patients. Clinical experience has shown that 17% - 29% of lesions resolve spontaneously, 24% to 64% progress, and 9% to 59% are stable during a oneyear period [2].
Diagnosis of endometriosis is surgical and it is based on laparoscopy. Visual inspection is usually adequate to diagnose endometriosis but histological confirmation of at least one lesion is ideal as well as to record laparoscopic findings on video. If symptoms fit endometriosis, it is first recommended to treat symptoms with hormonal contraceptives and non-steroidal anti-inflammatory drugs (NSAIDs) [6]. If these measures are not enough, then, according to guidelines given by European Society of Human Reproduction and Embryology (ESHRE), a diagnostic laparoscopy should be performed to confirm the diagnosis [6]. Surgical ablation of endometriotic implants should also be done at the same time, which has been shown to reduce pain [7].
Our objective was to study diagnostic laparoscopy findings of patients suspected to have endometriosis.
2. MATERIAL AND METHODS
The study group comprised 53 consecutive clinically suspected endometriosis cases in the University Hospital of Oulu during the study period, between January 1, 2006 and December 31, 2011. The median maternal age of all patients was 31 years (SD 8.1, range 15 - 47) and the median maternal age of patients with surgically confirmed endometriosis was 31 years (SD 5.1, range 21 - 36). All patients were Caucasian. The catchment area of the region is 160,000 people and the records of all patients were systematically examined. The stage of endometriosis was determined retrospectively by surgical reports using the classification of the American Society of Reproductive Medicine [8].
3. RESULTS
Laparoscopy revealed endometriosis in 40% (21/53) of cases. The median interval between the onset of symptoms and laparoscopic diagnosis was 1.9 years (SD 3.2, range 0.6 - 11). In 24 (45%) cases there was not any abnormal finding from laparoscopy, in six cases there was adhesions, in two cases pelvic varicosis. In endometriosis cases, most frequent symptoms described by patients were dysmenorrhea and dyspareunia followed by vibration pain, urinary symptoms and lowered fertility (Table 1). Only 5% of patients with endometriosis complained of bowel symptoms. On the other hand, in cases with no endometriosis the most frequent symptoms were dysmenorrhea, dyspareunia and vibration pain, but there was markedly less infertility and fewer urinary symptoms. Bowel symptoms were significantly more common in patients with no endometriosis (28%) (p = 0.034).
In all cases the stage of the endometriosis was grade I (N = 15) or grade II (N = 6), which indicates minimal disease. The surgeon was confident with the diagnosis of
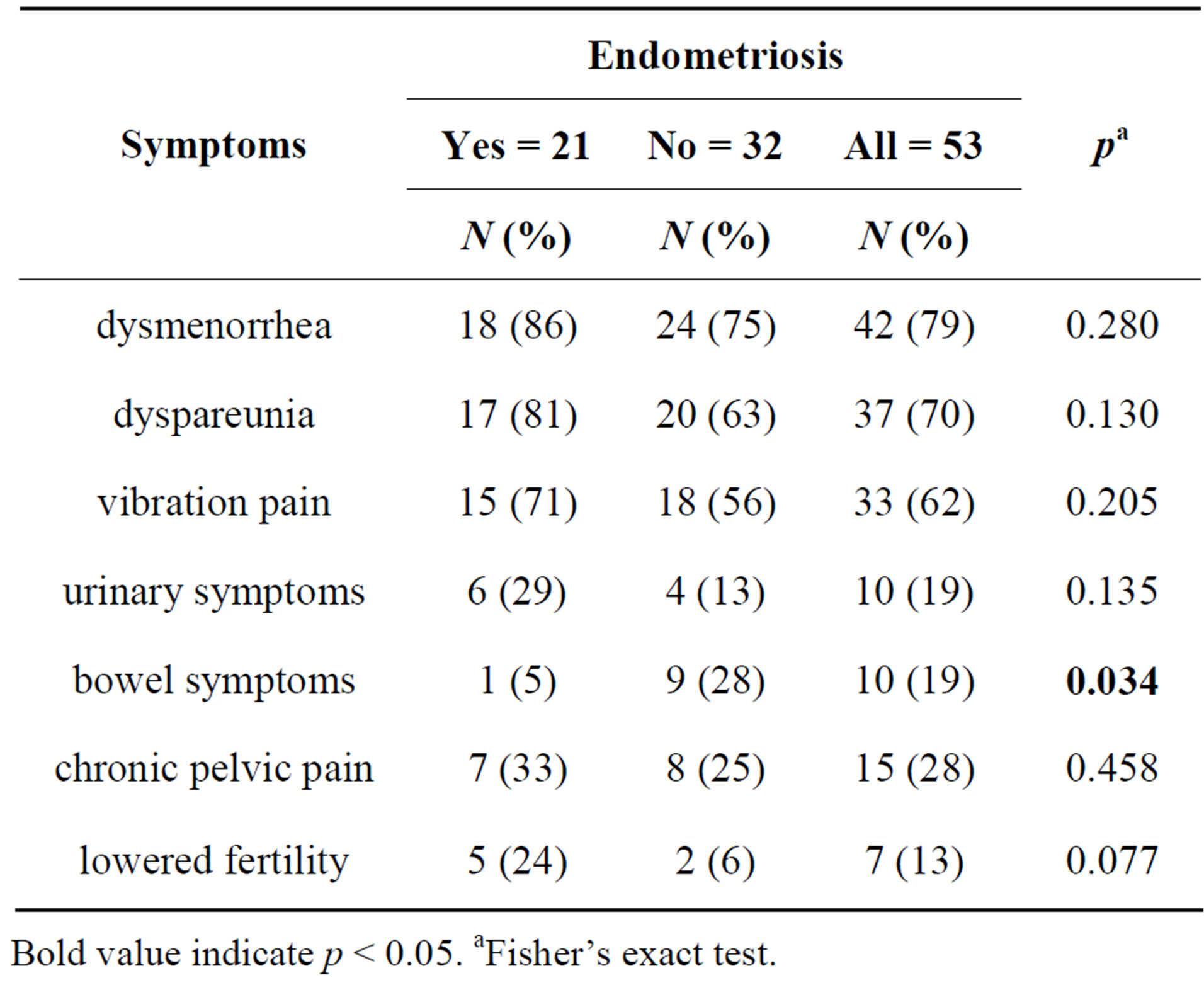
Table 1. The frequencies of symptoms described by patients suspected to have endometriosis.
Bold value indicate p < 0.05. aFisher’s exact test.
endometriosis in nearly all cases, but in seven cases tissue samples for histopathological diagnosis were taken. Endometriosis was confirmed in four cases out of seven, and in three cases the pathologist thought that the suspected endometriotic implant was not endometriosis (normal tissue, scar or unspecific fibrosis).
Endometriosis was treated before operation with combined oral contraceptives in nine cases, with an intrauterine hormonal device (IUD) in three cases, with NSAIDs or other painkillers in three cases, with antibiotics in two cases and no treatment was prescribed in four cases (Table 2). There was no mention in the records whether laparoscopy was done during hormonal therapy. During the laparoscopic procedure, endometriosis was treated in 13 cases (62%). Diathermic ablation to remove endometriotic implants and/or adhesions was used in eight patients and surgical excision in five cases. Due to close proximity to the ureter, urinary bladder or blood vessels the endometriotic implants were not treated in eight cases.
Hormonal post-operative treatment was prescribed to 15 patients with endometriosis and painkillers to six patients (Table 3). Combined oral contraceptives were prescribed to eight (38%) patients; it was the most common single choice of treatment. Hormonal intrauterine device (IUD) was prescribed to five (24%) patients. Progestogen was prescribed for two patients. GnRH-analogs were not primarily used in any case. Painkillers were prescribed to six patients; three of them were referred to the infertility clinic. There was no complication during operation in 53 laparoscopies. During the follow-up period of one year, no serious complications were recorded.
4. DISCUSSION
There are three important points to stress when con-

Table 2. Treatment before diagnostic laparoscopy.

Table 3. Primary treatment after definitive diagnosis of endometriosis.
sidering laparoscopic diagnosis of endometriosis: 1) what looks like endometriosis may or may not be endometriosis, 2) the disease may be invisible and 3) access to the tissues affected by the endometriosis may require further and extended surgery and removal of adhesions [9]. For years laparoscopy has been the gold standard in diagnosis of endometriosis. In clinical practice visual inspection is usually adequate for diagnosis. However, surgical diagnosis may either overestimate or underestimate the diagnosis of endometriosis since lesions are variable in size, color and location [10,11]. Whether or not histology should be obtained in cases of peritoneal disease alone is controversial, since positive histology confirms the diagnosis while, negative histology does not exclude it [6]. However, in several studies, visual diagnosis of endometriosis has been demonstrated to be unreliable. Only 54% - 67% of suspected endometriotic lesions are confirmed histologically, and 18% of patients clinically suspected to have endometriosis have no evidence of endometriosis upon pathological examination of tissue samples [12]. Indeed, a 2004 meta-analysis which assumed a 20% prevalence of endometriosis found that “a positive finding from laparoscopy will be incorrect in half of the cases [13].”
Also Brosens & Brosens suggest that the visual concept of endometriosis is no longer reliable and that the place of laparoscopy in the diagnosis should be reassessed [14]. Stegmann et al. showed that the surgeon’s impression of whether a mix of colors and textures of lesions indicates endometriosis has only a 65% positive predictive value of actual histology-confirmed endometriosis [15]. Wood et al. (2002) investigated the laparoscopic diagnosis of endometriosis in 215 patients suspected to have endometriosis [9]. In the first laparoscopy endometriosis was verified in 130 (60%) of patients and in the second laparoscopy (within 12 months) a further 38 patients were verified. All cases were confirmed with histological biopsy. Furthermore, in patients with clinical symptoms of endometriosis but no visual sign, a tissue biopsy was taken in areas usually affected and endometriosis was found via histology in 13% of normal-looking peritoneum in women with clinical signs of endometriosis. Furthermore, scanning electron microscopy diagnosed endometriosis in up to 25% of women with visually normal peritoneum [16]. In summary, laparoscopic visualization of peritoneal lesions alone is of limited accuracy, and if a diagnostic laparoscopy is performed, confirmatory biopsies of peritoneal lesions, even atypical ones, will be of value.
Based on previous reports we can speculate that also for the patients included in our study, endometriosis was not found in every case and the incidence of endometriosis in our patients may have been underestimated. One reason for underestimation might be that possibly some laparoscopies was done during hormonal therapy and endometriosis may shrink and not be visible during hormonal therapy [9]. The following important questions could be asked: Should we be more aggressive in looking for endometriosis using laparoscopy? Should we consider that women with a persistent history consistent with endometriosis should have laparoscopic peritoneal biopsy in common sites of the pelvis, the lateral pelvic walls and possibly the bladder and rectum?
The staging in all our patients was classified as mild. This might due to a short delay between symptoms and laparoscopy. Unfortunately, all classifications are subjective and correlate poorly with symptoms and fertility outcomes. Ablation of endometriotic lesions during the operation is a standard procedure and reduces endometriosis-associated pain [6]. In our study endometriosis was treated during the operation in 13 patients (62%), in others the localization of the disease prevented surgical attempts.
Symptoms linked to endometriosis were found to be variably during pre-surgical visit. Most commonly associated pain symptoms were best found out, while questions about the less common bladder and bowel symptoms were not asked. In total the preoperative history taking left room for improvement, in particular in light of differential diagnostics, which is an essential part of clinical assessment with pelvic pain.
The symptoms overlap with other conditions such as irritable bowel syndrome and pelvic inflammatory disease. Also, pelvic pain can be caused by a variety of different diseases of different organs (gynaecological conditions, gastrointestinal and urinary tract diseases and even psychological conditions), which makes the diagnosis medically challenging and requires a broad perspective. In our study six patients with adhesions and two with pelvic varicosis were found. It is possible, but not proven, that those conditions are responsible for pelvic pain.
According to this study, endometriosis seems to cause more bladder symptoms than experienced by women not having endometriosis. This suggests that when suspecting endometriosis bladder symptoms should be asked about more frequently. Conversely, bowel symptoms were more common in the group that did not have endometriosis confirmed by laparoscopy, although it was suspected. Lowered fertility was more common in women who had endometriosis.
The delay between the onset of symptoms and definitive diagnosis is long, up to 12 years [17]. According to the research by Hadfield’s et al. (1996), the mean interval between the onset of symptoms and laparoscopic diagnosis of endometriosis is on average 10.4 years in the United Kingdom and in the United States [18]. In the study by Dmowski, the delay in the diagnosis of endometriosis in patients with infertility and chronic pelvic pain was 3.5 and 11.7 years, respectively [19]. The mean interval between the onset of symptoms and laparoscopically confirmed endometriosis in this study (3.3 years) seems rather short when compared internationally perhaps suggesting that there is an eagerness on our part to perform diagnostic procedures. On the other hand, the short interval in our study may also reflect sufficient available resources for women’s medical care in our hospital.
In our study, no serious complications were seen in the patient surveillance record within one year after laparoscopy, which refers to the safety of laparoscopy as a diagnostic procedure.
5. CONCLUSION
With regard to the results of this study, we should improve the interview process with patients suspected to have endometriosis. We may also consider consulting pathologists more often in minor findings indicating endometriosis. The interval between the onset of symptoms and laparoscopic diagnosis is short, reflecting the prompt availability of necessary facilities in specialist health care and/or the enthusiasm of the surgeons for laparoscopy. Finally, laparoscopy seems to be safe in cases of endometriosis suspicion.
6. ACKNOWLEDGEMENTS
We are grateful to Ms Ritva Annala and Ms Ireene Hienonen at the Department of Obstetrics and Gynecology for collecting the data.
REFERENCES
- B. Eskenazi and M. Warner, “Epidemiology of endometriosis,” Obstetrics & Gynecology Clinics of North America, Vol. 24, 1997, pp. 235-258. http://dx.doi.org/10.1016/S0889-8545(05)70302-8
- L. C. Giudice, “Clinical Practice—Endometriosis,” The New England Journal of Medicine, Vol. 362, No. 25, 2010, pp. 2389-2398. http://dx.doi.org/10.1056/NEJMcp1000274
- J. D. Martin Jr. and A. E. Hauck, “Endometriosis in the Male,” AmSurg, Vol. 51, No. 7, 1985, pp. 426-430.
- R. F. Valle and J. J. Sciarra, “Endometriosis: Treatment Strategies,” Annals of the New York Academy of Sciences, Vol. 997, No., 2003, pp. 229-39. http://dx.doi.org/10.1196/annals.1290.026
- O. Uimari, I. Järvelä and M. Ryynänen, “Do Symptomatic Endometriosis and Uterine Fibroids Appear Together?” Journal of Human Reproductive Sciences, Vol. 4, No. 1, 2011, pp. 34-38. http://dx.doi.org/10.4103/0974-1208.82358
- S. Kennedy, A. Bergqvist, C. Chapron, et al., (ESHRE Special Interest Group for Endome-Triosis and Endometrium Guideline Development Group) “ESHRE Guideline for the Diagnosis and Treatment of Endometriosis,” Human Reproduction, Vol. 20, No. 10, 2005, pp. 2698- 2704. http://dx.doi.org/10.1093/humrep/dei135
- C. J. Sutton, A. S. Pooley, S. P. Ewen and P. Haines, “Follow-Up Report on a Randomized Controlled Trial of Laser Laparoscopy in the Treatment of Pelvic Pain Associated with Minimal to Moderate Endometriosis,” Fertility and Sterility, Vol. 68, No. 6, 1997, pp. 1070-1074. http://dx.doi.org/10.1016/S0015-0282(97)00403-2
- American Society for Reproductive Medicine, “Revised American Society for Reproductive Medicine Classification of Endometriosis: 1996,” Fertility and Sterility, Vol. 67, No. 5, 1997, pp. 817-821. http://dx.doi.org/10.1016/S0015-0282(97)81391-X
- C. Wood, R. Kun and J. Tsaltas, “Laparoscopic Diagnosis of Endometriosis,” Australian and New Zealand Journal of Obstetrics and Gynaecology, Vol. 42, No. 3, 2002, pp. 277-280. http://dx.doi.org/10.1111/j.0004-8666.2002.00277.x
- D. C. Martin, G. D. Hubert, R. Vander Zwaag, F. A. el-Zeky, “Laparoscopic Appearances of Peritoneal Endometriosis,” Fertility and Sterility, Vol. 51, No. 1, 1989, pp. 63-67.
- P. Stratton, C. Winkel, A. Premkumar, et al., “Diagnostic Accuracy of Laparoscopy, Magnetic Resonance Imaging, and Histopathologic Examination for the Detection of Endometriosis,” Fertility and Sterility, Vol. 79, No. 5, 2003, pp. 1078-1085. http://dx.doi.org/10.1016/S0015-0282(03)00155-9
- A. J. Walter, J. G. Hentz, P. M. Magtibay, J. L. Cornella, J. F. Magrina, “Endometriosis: Correlation between Histologic and Visual Findings at Laparoscopy,” American Journal of Obstetrics & Gynecology, Vol. 184, No. 7, 2001, pp. 1407-1413. http://dx.doi.org/10.1067/mob.2001.115747
- C. B. Wykes, T. J. Clark, K. S. Khan, “Accuracy of Laparoscopy in the Diagnosis of Endome-Triosis: A Systematic Quantitative Review,” BJOG: An International Journal of Obstetrics & Gynaecology, Vol. 111, No. 11, 2004, pp. 1204-1212. http://dx.doi.org/10.1111/j.1471-0528.2004.00433.x
- I. A. Brosens and J. J. Brosens, “Is Laparoscopy the Gold Standard for the Diagnosis of Endome-Triosis?” The European Journal of Obstetrics & Gynecology and Reproductive Biology, Vol. 88, No. 2, 2000, pp. 117-119. http://dx.doi.org/10.1016/S0301-2115(99)00184-0
- B. J. Stegmann, N. Sinaii, S. Liu, et al., ”Using Location, Color, Size, and Depth to Characterize and Identify Endometriosis Lesions in a Cohort of 133 Women,” Fertility and Sterility, Vol. 89, No. 6, 2008, pp. 1632-1636. http://dx.doi.org/10.1016/j.fertnstert.2007.05.042
- A. A. Murphy, W. R. Green, D. Bobbie, Z. C. dela Cruz and J. A. Rock, “Unsuspected Endometriosis Documented by Scanning Electron Microscopy in Visually Normal Peritoneum,” Fertility and Sterility, Vol. 46, 1986, pp. 522-524.
- G. K. Husby, R. S. Haugen and M. H. Moen, “Diagnostic delay in Women with Pain and Endome-Triosis,” Acta Obstetricia et Gynecologica Scandinavica, Vol. 82, No. 7, 2003, pp. 649-653. http://dx.doi.org/10.1034/j.1600-0412.2003.00168.x
- R. Hadfield, H. Mardon, D. Barlow and S. Kennedy, “Delay in the Diagnosis of Endometriosis: A Survey of Women from the USA and the UK,” Human Reproduction, Vol. 1, 1996, pp. 878-880. http://dx.doi.org/10.1093/oxfordjournals.humrep.a019270
- W. P. Dmowski, R. Lesniewicz, N. Rana, P. Pepping, M. Noursalehi, “Changing Trends in the Diagnosis of Endometriosis: A Comparative Study of Women with Pelvic Endometriosis Presenting with Chronic Pelvic Pain or Infertility,” Fertility and Sterility, Vol. 67, No. 2, 1997, pp. 238-243. http://dx.doi.org/10.1016/S0015-0282(97)81904-8
ABBREVIATIONS
ESHRE: European Society of Human Reproduction and Embryology;
GnRH agonist: gonadotropin releasing hormone agonist; GI-tract: gastrointestinal tract;
NSAID: non-steroidal anti-inflammatory drug;
IUD: Intrauterine device.
NOTES
*Conflicts of interests: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. No specific funding was received.
#Corresponding author.

