 Surgical Science, 2011, 2, 232-241 doi:10.4236/ss.2011.25052 Published Online July 2011 (http://www.SciRP.org/journal/ss) Copyright © 2011 SciRes. SS The Evaluation of Refrigerated and Frozen Osteochondral Allografts in the Knee Albert Washington Pearsall IV1, Sudhakar Govindarajulo Madanagopal1, Joseph Allan Tucker2 1Department of Orthopaedic Surgery, College of Medicine, University of South Alabama, Mobile, US 2Departmen t o f Pathology, College of Medicine, University of South Alabama, Mobile, US E-mail: apearsal@usouthal.edu Received May 6, 2011; revised June 27, 2011; accepted July 10, 2011 Abstract Between 1998 and 2002, 25 patients who were treated with a refrigerated or frozen allograft were evaluated. The mean patient age was 48 years. The mean lesion size was 4.5 cm2. Validated outcome instruments [Knee Society Score, Western Ontario and McMaster University Score] were used. Clinical and radiographic eval- uations were performed pre-operatively and at the most recent follow-up. Histological and electron micro- scopic analysis was performed on grafts prior to implantation. Clinical follow-up averaged 46 months (range 24 - 60 months). The Western Ontario and McMaster University Score improved from 46 + 24 to 66 + 22 (p = 0.003). The Knee Society Score improved from 104 + 43 to 132 + 42 (p = 0.01). No correlation was noted between graft type and histological or electron microscopy scoring. Post-operative mechanical alignment was not correlated with an improvement in Western Ontario and McMaster University Score (p = 0.19) or Knee Society Score (0.27). Six patients (24%), all refrigerated allografts, were failures and underwent knee ar- throplasty. Seventy-six percent of implanted frozen and refrigerated osteochondral allografts are in place 4 years after surgery. Frozen allografts appear to be surviving as well as refrigerated grafts. The use of mag- netic resonance imaging may enable the evaluation of graft incorporation and articular cartilage integrity. Keywords: Allograft, Refrigerated, Frozen, Knee, Transplantation 1. Introduction Biologic treatment options for large full thickness osteo- chondral lesions include microfracture, autologous carti- lage transplantation, mosaicplasty and refrigerated or frozen osteochondral allograft transplantation [1-6]. The result of these treatments is partial defect filling with fibrocartilage consisting of predominately type I collagen. Fibrocartilage has diminished resilience and a predilec- tion for deterioration over time [2,3,5,7-10]. The use of fresh osteochondral allografts in the treat- ment of full thickness articular cartilage defects has been well documented, with success rates of 75% reported at 5 years, slightly deteriorating to 63% at 14 years [8,11-18]. The term “fresh” usually indicates graft harvest within 24 hours of the donor’s death and a time from graft har- vest to implantation of 7 days or less [2,9,10,12,13, 19-24]. Deep frozen allografts have also been used for reconstruction of osteoarticular defects. However, au- thors have cited the diminished cell viability and poten- tial matrix degeneration that can occur after freezing hyaline cartilage [11,25-28]. Previously, authors have published the viability results of stored refrigerated allografts [3,29,30]. However, the authors are unaware of data correlating patients’ func- tional outcome with radiographic evaluation and histo- logical/ electron microscopy grading of refrigerated and frozen allografts at the time of implantation. The purpose of the current study was to clinically and radiographi- cally evaluate patients who underwent refrigerated or frozen allograft transplantation. In addition, histologi- cal/electron microscopy grading of the allograft and ra- diographic evaluation of the affected knee was analyzed in relationship to functional outcome at an average of 46 months follow-up. 2. Materials and Methods 2.1. Patient Data The current study was approved by the Institutional Re- view Board at our institution prior to implementation. All  A. W. PEARSALL IV ET AL. 233 study patients gave informed consent prior to being en- rolled in the study. Between 1998 and 2002, 26 patients underwent osteoarticular transplantation of the femur and/or patella with a refrigerated or frozen allograft from which histologic and electron microscopic data was available at the time of implantation. Inclusion criteria were as follows: 1) Tegner 3 or greater activity level; and 2) a contained articular cartilage defect amenable to a non-structural osteoarticular graft; 3) articular cartilage damage limited to 1 or 2 compartments; 4) biomechani- cal knee alignment that was less than 5° of varus or val- gus or correctable with a distal femoral or proximal tibial osteotomy; and 5) failure of conservative measures in- cluding non-steroidal anti-inflammatory medications and physical therapy for a minimum of 3 months. Body mass index greater than 30 and chronological age were not used as exclusionary criteria from surgery. Patients whose lifestyle included less demanding activities were encouraged to undergo a unicondylar or total knee ar- throplasty. Prior to surgery, all patients underwent clinical evalu- ation, standing radiographic imaging of both knees, and magnetic resonance imaging of the affected knee. Spe- cific magnetic resonance imaging sequences (fast spin echo) were performed to assess the articular cartilage of the patella, femur and tibia. All images were reviewed by the senior author and a board certified, fellowship- trained radiologist. 2.2. Operative Technique All allograft transplants were performed through a mini-arthrotomy unless a tibial tubercle osteotomy was performed for a patellar and/or trochlear defect. After the recipient defect was measured, the bone was reamed to a depth of 8 and 12 mm. The donor allograft was cored with a coring device (Arthrex, Inc., Naples, Florida) with a minimum diameter of 18 mm. The graft was press-fit into the recipient site with the articular surfaces congru- ent. All grafts were placed with less than 1 mm of do- nor-recipient articular surface incongruity. Early in the study, 1 graft (patella) was secured with an intra-articular screw. No subsequent grafts underwent fixation. All tibial tubercle osteotomies were performed ac- cording to the technique described by Fulkerson and se- cured with two 3.5 mm or 4.5 mm screws [24] .All high tibial osteotomies were performed with a lateral closing or medial opening wedge. All distal femoral osteotomies were performed with a lateral opening wedge. For all opening wedge osteotomies, frozen allograft corticocan- cellous bone graft was used to fill the osteotomy defect. 2.3. Postoperative Treatment All patients undergoing an isolated mini-arthrotomy were discharged the following day. Patients undergoing a tibial tubercle osteotomy, high tibial osteotomy or distal femoral osteotomy remained in the hospital 48 to 72 hours. For all inpatients, continuous passive motion was instituted on the first post-operative day. Continuous passive motion was continued on an outpatient basis 3 to 6 hours per day for 3 weeks post-operatively. Patients undergoing isolated allograft transplantation were kept toe-touch weight bearing for 4 weeks and then pro- gressed to full weight bearing over 2 weeks. All patients undergoing a tibial tubercle osteotomy, high tibial os- teotomy or distal femoral osteotomy were kept non-weight bearing for 6 weeks and progressed to full weight- bear- ing over the next 2 weeks. Heavy labor and athletic ac- tivity were delayed until 6 months after surgery. 2.4. Clinical and Radiographic Assessment All patients underwent radiographic evaluation preopera- tively and at yearly intervals. Two standardized radio- graphic views (standing antero-posterior and skyline) were obtained by a trained radiological technician. In addition, standing long-leg antero-posterior radiographs were obtained pre-operatively and at the most recent follow-up. A trained research assistant blinded to the patient’s clinical data measured each film. Each film was assessed for the area of greatest joint space narrowing (Grade 3 = > 3mm joint space, Grade 2 = < 3mm of joint space but not bone on bone, Grade 1= bone on bone) in specific areas (Figure 1). Based upon these measure- ments, the patient was given a composite score ranging from 3 to 6 which was used for later analysis. Long leg weight-bearing radiographs were measured by one of the authors. The biomechanical axis was determined by the intersection of a line drawn from the center of the femo- ral head to the center of the tibial plateau and a line drawn from the center of the talus to the center of the tibial plateau. All patients underwent clinical evaluation including knee range of motion. Patients completed the Knee Soci- ety Score, Western Ontario and McMaster University (WOMAC) Score preoperatively and at yearly intervals after surgery. Failure was defined as conversion of a transplanted graft to a unicondylar or total knee arthro- plasty. All failures were included in the overall analysis and also analyzed separately. 2.5. Refrigerated Allografts All grafts were aseptically processed within 72 hours of Copyright © 2011 SciRes. SS  A. W. PEARSALL IV ET AL. 234 Figure 1. Knee radiographic grading form. A grade from one to three is given for the medial and lateral joint spaces within the knee and patellofemoral joint. the donor’s death and procedures were performed in ac- cordance with the guidelines established by the Ameri- can Association of Tissue Banks. Donors’ blood was screened for malignancies, autoimmune and certain neu- rological disorders, and any high-risk behavior. Donor blood and tissue was screened for human immunodefi- ciency virus-1 and human immunodeficiency virus-2 antibodies, Hepatitis B and C antibodies, human T-lym- photropic Virus I and II, syphilis, and polymerase chain reaction testing for human immunodeficiency virus-1. Harvested osteochondral grafts were aseptically clea- nsed with saline pulse lavage in order to remove blood and fat from the cancellous bone. All grafts were pre- -soaked in antibiotic storage medium. Grafts were trans- ferred to double sterile pouches containing storage media to maximize chondrocyte viability during storage (IN- CELL Corp., San Antonio, Texas). Only grafts demon- strating no growth after 7 day microbial culturing were released for distribution. Grafts were refrigerated and stored at 2° - 8° Celsius with a shelf life of 6 weeks from the date of processing (Regeneration Technologies Inc., Alachua, Florida). 2.6. Frozen Allografts All grafts were aseptically processed within 72 hours of the donor’s death and procedures were performed in ac- cordance with the guidelines established by the Ameri- can Association of Tissue Banks. Donors’ blood was screened for malignancies, autoimmune and certain neu- rological disorders, and any high-risk behavior. Donor blood and tissue was screened for human immunodefi- ciency virus-1 and human immunodeficiency virus-2 antibodies, Hepatitis B and C antibodies, human T- lymphotropic Virus I and II, syphilis, and polymerase chain reaction testing for human immunodeficiency vi- rus-1. All grafts were stored at –70° Fahrenheit until the day of use. 2.7. Cartilage Preparation and Histologic Scoring Immediately upon opening the allograft in the operating room, three 5 mm plugs were sterilely harvested from a peripheral area of articular cartilage with an Osteo- chondral Autograft Transfer System (OATS) harvester (Arthrex, Naples, Florida). The sample site was free from any visual cartilage defects. All plugs were placed in a sterile container with a small amount of normal saline and sealed for transport to the pathology and flow cy- tometry laboratories for evaluation. The time from plug harvest to processing was approximately 30 minutes for all specimens. For histology a portion of fresh cartilage was cut from the bone surface, fixed in 10% buffered formalin, proc- essed to paraffin, sectioned at 5 um, and stained with hematoxylin and eosin. Three slides were prepared from the plugs and sent for evaluation. The slides were scored blindly and the scores averaged. The average score was used for data analysis. The following scoring system was utilized for each slide: 0: all cells appeared lethally in- jured; 1+: majority of cells exhibited marked to lethal injury; 2+: minority of cells exhibited marked to lethal injury; 3+: all cells appear viable. Severe pyknosis and cell lysis were judged to represent marked to lethal in- jury. For electron microscopy, fresh tissue was placed in 3% glutaraldehyde (0.1 N sodium cacodylate buffer, pH 7.2), post-fixed in 1% osmium tetroxide, and embedded in epoxy resin. Sections (1 um) were stained with tolu- idine blue. Thin sections were stained with uranyl acetate and lead citrate and examined in a Phillips CM100 elec- tron microscope (FEI Company, Hillsboro, Oregon). The scoring scheme above was also applied to the ultrastruc- tural findings. Features such as severe pyknosis, ob- scured detail of the organelles, and rupture of the plasma membrane were considered indicators of lethal injury, and lesser degrees of cytoplasmic contraction, cytoplas- mic blebs, and accumulation of myelin figures were con- sidered indications of marked injury (Figures 2(a)-2(c)). Copyright © 2011 SciRes. SS 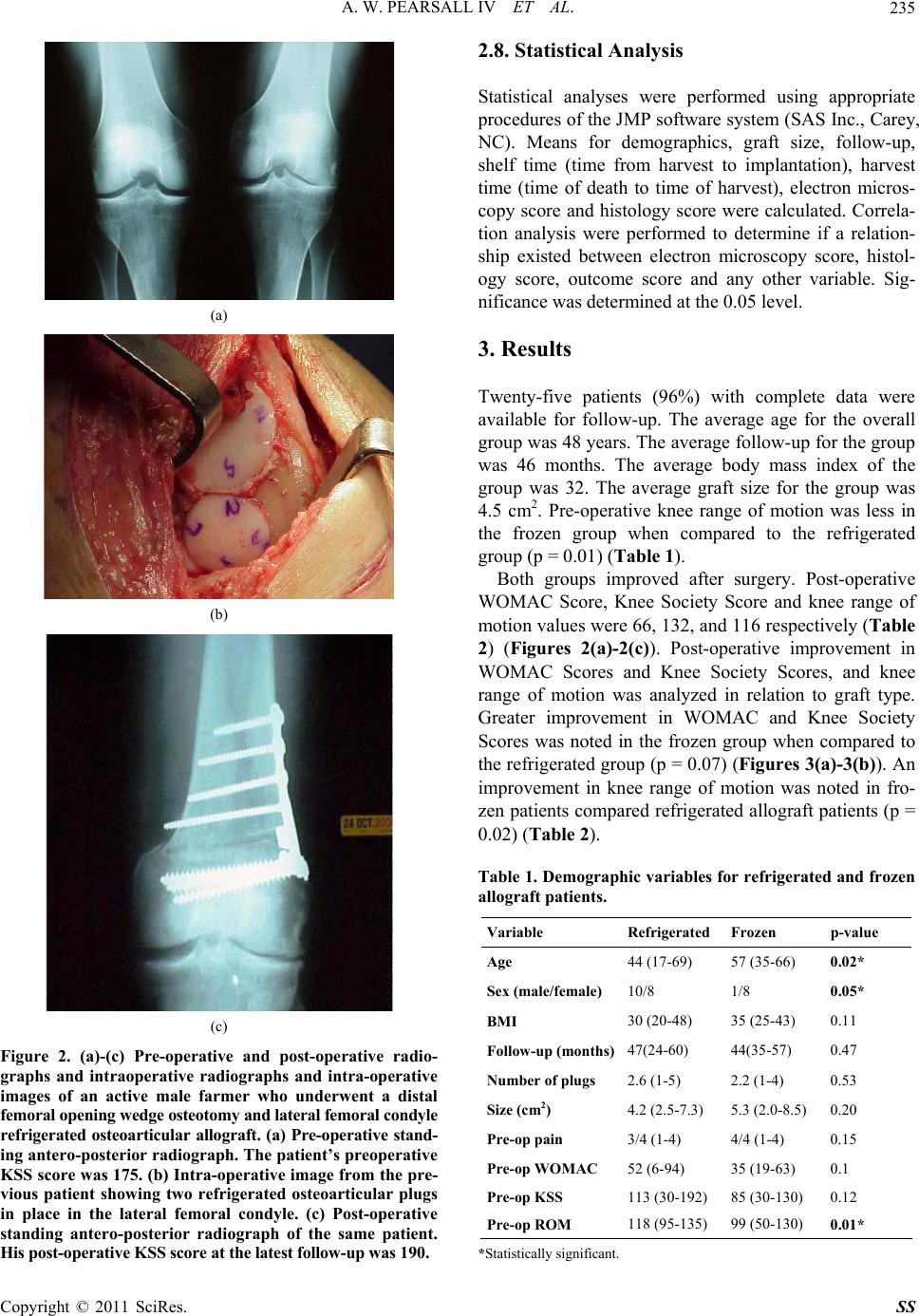 A. W. PEARSALL IV ET AL. 235 (a) (b) (c) Figure 2. (a)-(c) Pre-operative and post-operative radio- graphs and intraoperative radiographs and intra-operative images of an active male farmer who underwent a distal femoral opening wedge osteotomy and lateral femoral condyle refrigerated osteoarticular allograft. (a) Pre-operative stand- ing antero-posterior radiograph. The patient’s preoperative KSS score was 175. (b) Intra-operative image from the pre- vious patient showing two refrigerated osteoarticular plugs in place in the lateral femoral condyle. (c) Post-operative standing antero-posterior radiograph of the same patient. His p ost - oper a tive K S S sc ore at the l a test fo l l o w- u p was 190. 2.8. Statistical Analysis Statistical analyses were performed using appropriate procedures of the JMP software system (SAS Inc., Carey, NC). Means for demographics, graft size, follow-up, shelf time (time from harvest to implantation), harvest time (time of death to time of harvest), electron micros- copy score and histology score were calculated. Correla- tion analysis were performed to determine if a relation- ship existed between electron microscopy score, histol- ogy score, outcome score and any other variable. Sig- nificance was determined at the 0.05 level. 3. Results Twenty-five patients (96%) with complete data were available for follow-up. The average age for the overall group was 48 years. The average follow-up for the group was 46 months. The average body mass index of the group was 32. The average graft size for the group was 4.5 cm2. Pre-operative knee range of motion was less in the frozen group when compared to the refrigerated group (p = 0.01) (Table 1). Both groups improved after surgery. Post-operative WOMAC Score, Knee Society Score and knee range of motion values were 66, 132, and 116 respectively (Table 2) (Figures 2(a)-2(c)). Post-operative improvement in WOMAC Scores and Knee Society Scores, and knee range of motion was analyzed in relation to graft type. Greater improvement in WOMAC and Knee Society Scores was noted in the frozen group when compared to the refrigerated group (p = 0.07) (Figures 3(a)-3(b)). An improvement in knee range of motion was noted in fro- zen patients compared refrigerated allograft patients (p = 0.02) (Table 2). Table 1. Demographic variables for refrigerated and frozen allograft patients. Variable Refrigerated Frozen p-value Age 44 (17-69) 57 (35-66) 0.02* Sex (male/fem ale) 10/8 1/8 0.05* BMI 30 (20-48) 35 (25-43) 0.11 Follow-up (month s)47(24-60) 44(35-57) 0.47 Number of plugs 2.6 (1-5) 2.2 (1-4) 0.53 Size (cm2) 4.2 (2.5-7.3) 5.3 (2.0-8.5) 0.20 Pre-op pain 3/4 (1-4) 4/4 (1-4) 0.15 Pre-op WOMAC 52 (6-94) 35 (19-63) 0.1 Pre-op KSS 113 (30-192) 85 (30-130) 0.12 Pre-op ROM 118 (95-135) 99 (50-130) 0.01* *Statistically significant. Copyright © 2011 SciRes. SS  A. W. PEARSALL IV ET AL. 236 Table 2. Overall pre and post-operative outcome scores and outcome improvement in refrigerated and frozen allograft patients. Outcome Measure Pre-operative score Post-operative score P value WOMAC 46 +/– 24 66 +/– 22 0.003 * KSS 104 +/– 43 132 +/– 42 0.01* Range of motion 112 +/– 19 116 +/– 11 0.22 Outcome Measure RefrigeratedFrozen P value WOMAC improvement 10 +/– 21 28 +/– 21 0.07 KSS improvement 14 +/– 35 49 +/–52 0.07 ROM improvement –3 +/– 15 20 +/– 30 0.02* *Statistically significant. Radiographic measurements were made on standing antero-posterior (AP) and skyline views. Medial and lateral tibio-femoral joint spaces were given a score from 1 to 3, while medial and lateral patello-femoral joint spaces were given a score from 1 to 3. The baseline group AP radiographic score was 5.1 which increased at follow-up to 5.3 (p = 0.06). The baseline patella score for the group was 5.6 which increased at follow-up to 5.7 (Table 3). Mechanical axis was measured in all patients without a tibial tubercle osteotomy. The average pre-operative and post-operative mechanical axes were 174° and 177° respectively (p = 0.08). When post-operative alignment was analyzed in relation to outcome improvement, no correlation was noted (Table 4). Due to the small numbers, the 4 histological and elec- tron microscopy scores were combined (scores zero and one = Grade 1/scores two and three = Grade 2). When pre-operative WOMAC and Knee Society Scores were compared, there was no difference between groups. No correlation was noted between graft type and histological or electron microscopy scoring. Therefore, no difference in chondrocyte viability was noted on histological or electron microscopy between refrigerated and frozen allografts at the time of implantation. No correlation was noted between the histological or electron microscopy grading systems and any outcome tool (Table 5). Improvement in outcome was analyzed in relation to the number of sites undergoing grafting. No difference in outcome improvement was noted between single or mul- tiple site grafts (Knee Society Score p = 0.7; WOMAC Score p = 0.5; range of motion p = 0.9). No difference in post-operative scores was noted be- tween the 2 groups (p = 0.8, p = 0.7). However, poorer (a) (b) Figure 3. (a), (b) Pre-operative and post-operative radio- graphs of a homemaker with 22 degrees of combined femo- ral and tibial varus. She underwent combined distal femo- ral closing wedge and medial tibial opening osteotomies. A frozen osteoarticular allograft was used to reconstruct the medial femoral condyle. (a). Pre-operative stan- ding an- tero-posterior radiograph. The patient’s pre-ope- rative KSS score was 59. (b). Post-operative standing an- tero-posterior radiograph of the same patient. Her post-ope- rative KSS score at the latest follow-up was 185. Copyright © 2011 SciRes. SS  A. W. PEARSALL IV ET AL. 237 Table 3. Pre-operative and post-operative radiological scores for all patients (n = 25). Score Pre-operative Post-operative P value AP Score 5.1 +/– 0.96 5.3 +/– 1.18 0.6 Patella Score 5.6 +/– 1.12 5.7 +/– 1.36 0.5 Table 4. Analysis of post-operative mechanical alignment in relation to improvement in outcome score. Outcome Varus (n = 12) Neutral (n = 2) Valgus (n = 3) P value WOMAC 6 +/– 6 34 +/– 15 24 +/– 130.19 KSS 31 +/– 12 54 +/– 31 –9 +/– 250.27 Knee Range of motion 6.4 +/– 6.7 22.5 +/– 17 1.6 +/– 140.33 Table 5. Evaluation of histological and electron microscopy grades compared to outcome measures. Histology grading versus outcome measures Outcome measures Gr a de 1 Grade 2 P value WOMAC improvement 10.66 21.63 0.25 KSS improvement 24.41 24.81 0.9 ROM improvement –1.6 10.45 0.2 Electron microscopy grading versus outcome measures Outcome measures Grade 1 Grade 2 P value WOMAC improvement 13.73 21.75 0.5 KSS improvement 23.93 41.25 0.5 ROM improvement 1.4 15.25 0.29 pre-operative knee range of motion, Knee Society and WOMAC scores in patients receiving a frozen allograft (p = 0.01, p = 0.1, p = 0.1) resulted in a greater net change in outcome in the frozen allograft group. Six patients (24%) who underwent a knee arthroplasty were considered failures and analyzed separately. All failures were refrigerated allografts. We evaluated histo- logical and electron microscopy findings at the time of implantation with success or failure of the graft. No fail- ures were noted if the histology score was Grade 2 (p = 0.02) or if the electron microscopy score was Grade 2 (p = 0.4). 4. Discussion The use of fresh allografts for the treatment of full thickness articular defects is well documented [2,15, 31-34]. Fresh osteochondral shell allografts (<1 cm of subchondral bone) provide the greatest likelihood of chondrocyte survivability, while reducing immunogenic- ity by decreasing the exposure of white cells found in cancellous bone [1]. Various authors have indicated that the long-term survival of an allograft is dependent upon viable chondrocytes with a high level of donor deoxyri- bonucleic acid (DNA) to replenish the transplanted ma- trix [35]. Currently, fresh allografts provide the largest population of viable donor chondrocytes for transplanta- tion. Although fresh osteochondral allografts provide an excellent source of live chondrocytes, their use is not without drawbacks. Foremost is the need for the graft to be as fresh as possible to maintain chondrocyte viability, yet not expose the recipient to infection at the time of implantation. Currently, all published reports of osteo- chondral grafts using the term “fresh” have cited a time from graft harvest to implantation of 7 days or less [4,36-44]. In the current study, the time from graft har- vest to implantation was greater than 7 days for all non-frozen grafts. Noting this, we chose to label these grafts as “refrigerated”, rather than “fresh”. Recent data have indicated good results with the use of refrigerated osteochondral allografts. Emmerson et al. reported 72% good/excellent results at 7.7 years with refrigerated allografts averaging 7.5 cm in size implanted for osteochondritis dissecans of the femoral condyle. [45]. McCulloch et al. reviewed 25 consecutive patients who underwent refrigerated osteochondral allograft transplantation for defects in the femoral condyle. Over- all, patients reported 84% satisfaction with their results [46]. Williams et al. reported on 19 patients who were treated with “fresh stored” allografts maintained for an average of 30 days prior to implantation. Mean lesion size was 602 mm2. At the most recent follow-up, the mean Short Form-36 score improved to 66 (+/–24) and the mean score on the Activities of Daily Living Scale increased to 70 (+/–22) [47]. The outcome tools used in the current study were not identical to those used by other authors [46,47]. However, both the WOMAC and Knee Society Scores are vali- dated outcome instruments reported in the literature for the evaluation of patients with arthritic conditions [48- 50]. Indeed, previous studies of function in osteoarthritis have shown that WOMAC Score is more sensitive to change and has greater efficiency than other instruments used to assess osteoarthritis, including the SF-36 Health Survey [51,52]. In the current study we noted clinical improvement in patients implanted with a refrigerated or frozen allograft. Many of these patients had malalignment or patel- lofemoral disease requiring a concomitant osteotomy. Whe- ther the osteotomies themselves played a larger Copyright © 2011 SciRes. SS  A. W. PEARSALL IV ET AL. 238 role then the transplantation in improved patient outcome is unclear. Numerous animal studies have indicated that har- vested osteochondral allografts can be safely preserved up to 28 days with overall maintenance of biomechanical properties, cell matrix collagen content, and permeability [53-56]. Wayne et al. demonstrated that osteochondral shell grafts could be stored in culture medium at 4° Cel- sius for up to 60 days without deterioration of collagen content, proteoglycan amount, or histologic appearance [56]. Pearsall et al. reported on 16 refrigerated osteo- chondral allografts that underwent histologic and ultra- structural examination prior to implantation. The authors found an inverse correlation between matrix staining and time to implantation (p < 0.05) and reported an average time from harvest to implantation of 30 days (range: 17 to 44 days) [57]. Despite the aforementioned animal and histologic/electron microscopy data, the authors are un- aware of published data correlating pre-implantation histologic and electron microscopy findings with clinical outcome. The use of deep frozen allografts for the treatment of osteoarticular defects has also been reported, with cited failure rates as high as 25% [2,15,58]. Reasons cited for failure include slow incorporation, diminished chondro- cyte survival, and subsequent matrix degeneration [1,58]. Pritzker and others noted that freezing cartilage kills chondrocytes [4,23,59-61]. Despite reports of poor chondrocyte viability at the time of frozen allograft im- plantation, we detected no significant difference in out- come between our refrigerated and frozen allograft pa- tients. Moreover, when pre-operative scores were exam- ined, the frozen allograft group had significantly worse scores at the time of implantation, indicating that these patients were actually doing better at the latest follow-up. There are several weaknesses to the current study. The analysis included a limited number of patients. Therefore, statistical significance could not be determined for sev- eral variables. There was no follow-up magnetic reso- nance imaging of the study patients to assess incorpora- tion of the implanted graft. Previous authors have hy- pothesized that early magnetic resonance imaging ab- normalities represent non-specific post-operative change, whereas persistent abnormalities represent immune-related injury [62]. Further follow-up of the current patient co- hort will include magnetic resonance imaging to evaluate graft incorporation and analyze the findings in relation to clinical outcome. The histological and electron micros- copy evaluation was performed by a single pathologist. However, the evaluator has over 20 years of expertise in this area and interpreted all specimens blinded. Although several of the electron microscopy and histologic scores were low, these findings may be attributable to the need to refine the technique of electron microscopy and his- tologic assessment in articular cartilage. Finally, al- though the current study assessed chondrocyte viability, it did not evaluate chondrocyte function. Various authors have reported on the mechanical properties of articular cartilage, including an assessment of cartilage stiffness [63-65]. In conclusion, 76% of implanted frozen and refriger- ated osteochondral allografts in the current study are still in place at 4 years after surgery. At the latest follow-up, frozen allografts are surviving as well as refrigerated grafts. The reason for this finding is unclear, as the ma- jority of these grafts were used in salvage cases in older patients. Long term follow-up is needed to assess clinical outcome, while the use of magnetic resonance imaging may be beneficial to evaluate graft incorporation and articular cartilage integrity. 5. References [1] J. E. Browne and T. P. Branch, “Surgical Alternative for Treatment of Articular Cartilage Lesions,” Journal of the American Academy of Orthopaedic Surgeons, Vol. 8, No. 3, 2000, pp. 180-189. [2] F. F. Parrish, “Allograft Replacement of All or Part of the End of a Long Bone Following Excision of a Tumor,” The Journal of Bone and Joint Surgery, Vol. 55, No. 1, 1973, pp. 1-22. [3] A. W. Pearsall, J. A. Tucker, R. B. Hester and R. J. Heitman, “Osteochondral Transplantation of the Knee: An Assessment of Graft Viability, American Orthopaedic Society for Sports Medicine Specialty Day,” American Academy of Orthopaedic Surgeons, Dallas, 2002. [4] K. P. H. Pritzker, A. E. Gross, F. Langer, S. C. Luk and J. B. Houpt, “Articular Cartilage Transplantation,” Human Pathology, Vol. 8, No. 6, 1977, pp. 635-651. doi:10.1016/S0046-8177(77)80093-2 [5] T. Furakawa, D. R. Eyre, S. Koide and M. J. Glimcher, “Biochemical Studies on Repair Cartilage Resurfacing Experimental Defects in the Rabbit Knee,” The Journal of Bone and Joint Surgery, Vol. 62, No. 1, 1980, pp. 79- 89. [6] W. W. Curl, J. Krome, E. S. Gordon, J. Rushing, B. P. Smith and G. G. Poehling, “Cartilage Injuries: A Review of 31,516 Knee Arthroscopies,” Arthroscopy, Vol. 13, No. 4, 1997, pp. 456-460. doi:10.1016/S0749-8063(97)90124-9 [7] R. W. Jackson, “Arthroscopic Treatment of Degenerative Arthritis,” In: J. B. McGinty, Ed., Operative Arthroscopy, Raven Press, New York, 1991, pp. 319-323. [8] L. Hangody and P. Füles, “Autologous Osteochondral Mosaicplasty for the Treatment of Full-Thickness Defects of Weight-Bearing Joints. Ten Years of Experimental and Clinical Experience,” The Journal of Bone and Joint Surgery, Vol. 85-A, Supplement 2, 2003, pp. 25-32. [9] L. L. Johnson, “Surgical Arthroscopy: Principles and Prac- Copyright © 2011 SciRes. SS  A. W. PEARSALL IV ET AL. 239 tice,” Mosby, St Louis, 1986. [10] Y. Matsusue, T. Yamamuro and H. Hama, “Arthroscopic Multiple Osteochondral Transplantation to the Chondral Defect in the Knee Associated with Anterior Cruciate Ligament Disruption,” Arthroscopy, Vol. 9, No. 3, 1993, pp. 318-321. doi:10.1016/S0749-8063(05)80428-1 [11] V. Bobić, “Arthroscopic Osteochondral Autograft Trans- plantation in Anterior Cruciate Ligament Reconstruction: A Preliminary Clinical Study,” Knee Surgery, Sports Traumatology, Arthroscopy, Vol. 3, No. 4, 1996, pp. 262- 264. doi:10.1007/BF01466630 [12] H. Laprell and W. Peterson, “Autologous Osteochondral Transplantation Using the Diamond Bone-Cutting System (DBCS): 6-12 Years’ Follow-Up of 35 Patients with Osteochondral Defects at the Knee Joint,” Archives of Orthopaedic and Trauma Surgery, Vol. 121, No. 5, 2001, pp. 248-253. doi:10.1007/s004020000217 [13] D. Koulalis, W. Schultz, M. Heyden and F. König, “Au- tologous Osteochondral Grafts in the Treatment of Carti- lage Defects of the Knee Joint,” Knee Surgery, Sports Traumatology, Arthroscopy, Vol. 12, No. 4, 2004, pp. 329-334. doi:10.1007/s00167-003-0392-5 [14] H. L. Ma, S. C. Hung, S. T. Wang, M. C. Chang and T. H. Chen, “Osteochondral Autografts Transfer for Post- -Traumatic Osteochondral Defect of the Knee-2 to 5 Years Follow-Up,” Injury, Vol. 35, No. 12, 2004, pp. 1286-1292. doi:10.1016/j.injury.2004.02.013 [15] H. J. Mankin, F. S. Fogelson, A. Z. Thrasher and F. Jaffer, “Massive Resection and Allograft Transplantation in the Treatment of Malignant Bone Tumors,” The New Eng- land Journal of Medicine, Vol. 294, No. 23, 1976, pp. 1247-1255. doi:10.1056/NEJM197606032942301 [16] L. Hangody, G. Kish, Z. Kárpáti, I. Udvarhelyi, I. Szigeti and M. Bély, “Mosaicplasty for the Treatment of Articu- lar Cartilage Defects: Application in Clinical Practice,” Orthopedics, Vol. 21, No. 7, 1998, pp. 751-756. [17] L. Hangody, L. Sükösd, I. Szigeti and Z. Kárpáti, “Ar- throscopic Autogenous Osteochondral Mosaicplasty,” Hungarian Journal of Traumatology and Orthopaedics, Vol. 39, 1996, pp. 49-54. [18] L. Hangody, P. Feczkó, L. Bartha, G. Bodó and G. Kish, “Mosiacplasty for the Treatment of Articular Defects of the Knee and Ankle,” Clinical Orthopedics and Related Research, Vol. 391, Supplement l, 2001, pp. 328-336. doi:10.1097/00003086-200110001-00030 [19] T. Minas and S. Nehrer, “Current Concepts in the Treat- ment of Articular Cartilage Defects,” Orthopedics, Vol. 20, No. 6, 1997, pp. 525-538. [20] C. J. Campbell, “The Healing of Cartilage Defects,” Clinical Orthopedics and Related Research, Vol. 64, 1969, pp. 45-63. [21] D. W. Jackson, M. J. Scheer and T. M. Simon, “Cartilage Substitutes: Overview of Basic Science and Treatment Options,” Journal of the American Academy of Ortho- paedic Surgeons, Vol. 9, No. 1, 2001, pp. 37-52. [22] J. C. Garrett, “Fresh Osteochondral Allografts for Treat- ment of Articular Defects in Osteochondritis Dissecans of the Lateral Femoral Condyle in Adults,” Clinical Ortho- pedics and Related Research, Vol. 303, 1994, pp. 33-37. [23] T. Gibson, “The Transplantation of Cartilage,” Journal of Clinical Patholo g y , Vol. 20, 1967, p. 513. [24] J. P. Fulkerson, “Anteromedialization of the Tibial Tu- berosity for Patellofemoral Malalignment,” Clinical Or- thopedics and Related Research, Vol. 177, 1983, pp. 176- 181. [25] U. Horas, D. Pelinkovic, G. Herr, T. Aigner and R. Schnettler, “Autologous Chondrocyte Implantation and Osteochondral Cylinder Transplantation in Cartilage Re- pair of the Knee Joint. A Prospective, Comparative Tri- al,” The Journal of Bone and Joint Surgery. American Volume, Vol. 85-A, No. 2, 2003, pp. 185-192. [26] G. N. Homminga, S. K. Bulstra, P. S. M. Bouwmeester and A. J. van der Linden, “Perichondral Grafting for Car- tilage Lesions of the Knee,” The Journal of Bone and Joint Surgery. British Volume, Vol. 72, No. 6, 1990, pp. 1003-1007. [27] R. Lorentzon, H. Alfredson and C. Hildingsson, “Treat- ment of Deep Cartilage Defects of the Patella with Perio- steal Transplantation,” Knee Surgery, Sports Traumatol- ogy, Arthroscopy, Vol. 6, No. 4, 1998, pp. 202-208. doi:10.1007/s001670050100 [28] F. R. Noyes, R. W. Bassett, E. S. Grood and D. L. Butler, “Arthroscopy in Acute Traumatic Hemarthrosis of the Knee: Incidence of Anterior Cruciate Tears and Other Injuries,” The Journal of Bone and Joint Surgery. Amer- ican Volume, Vol. 62, No. 5, 1980, pp. 687-695. [29] S. K. Williams, D. Amiel, S. T. Ball, R. T. Allen, V. W. Wong, A. C. Chen, R. L. Sah and W. D. Bugbee, “Pro- longed Storage Effects on the Articular Cartilage of Fresh Human Osteochondral Allografts,” The Journal of Bone and Joint Surgery. American Volume, Vol. 85-A, No. 11, 2003, pp. 2111-2120. [30] W. D. Bugbee and B. Khadivi, “Fresh Osteochondral Allografting in the Treatment of Osteonecrosis of the Knee, Paper No. 108,” American Academy of Orthopae- dic Surgeons, 71st Annual Meeting, San Francisco, Mar- ch 2004. [31] A. E. Gross, F. Langer, J. Houpt, K. Pritzker and G. Friedlaender, “Allotransplantation of Partial Joints in the Treatment of Osteoarthritis of the Knee,” Transplant Proceedings, Vol. 8, Supplement 1, 1976, pp. 129-132. [32] C. H. Herndon and S. W. Chase, “The Fate of Massive Autogenous and Homogenous Bone Grafts Including Ar- ticular Surfaces,” Surgery, Gynecology & Obstetrics, Vol. 98, No. 3, 1954, pp. 273-290. [33] F. Langer, A. E. Gross, M. West and E. P. Urovitz, “The Immunogenicity of Allograft Knee Joint Transplants,” Clinical Orthopedics and Related Research, Vol. 132, 1978, pp. 155-162. [34] H. J. Mankin, S. Doppelt and W. Tomford, “Clinical Experience with Allograft Implantation. The First Ten Years,” Clinical Orthopedics and Related Research, Vol. 174, 1983, pp. 69-86. [35] D. W. Jackson, J. Halbrecht, C. Proctor, et al. “Assess- Copyright © 2011 SciRes. SS  A. W. PEARSALL IV ET AL. 240 ment of Donor Cell and Matrix Survival in Fresh Articu- lar Cartilage Allografts in a Goat Model,” Journal of Or- thopaedic Research, Vol. 14, No. 2, 1996, pp. 255-264. doi:10.1002/jor.1100140214 [36] F. R. Convery, M. H. Meyers and W. H. Akeson, “Fresh Osteochondral Allografting of the Femoral Condyle,” Clinical Orthopedics and Related Research, Vol. 273, 1991, pp. 139-145. [37] F. R. Convery, W. H. Akeson and M. H. Meyers, “The Operative Technique of Fresh Osteochondral Allografting of the Knee,” Operative Techniques in Orthopaedics, Vol. 7, No. 4, 1997, pp. 340-344. doi:10.1016/S1048-6666(97)80038-9 [38] J. C. Garrett, “Osteochondral Allografts for Reconstruc- tion of Articular Defects of the Knee,” Instructional Course Lectures, Vol. 47, 1998, pp. 517-522. [39] A. E. Gross, “Fresh Osteochondral Allografts for Post-Tra- umatic Knee Defects: Surgical Technique,” Operative Techniques in Orthopedics, Vol. 7, No. 4, 1997, pp. 334-339. [40] G. L. Bonney and M. Laurence, “Allograft Arthroplasty of the Knee,” Proceedings of the Royal Society of Medi- cine, Vol. 62, No. 6, 1969, pp. 583-585. [41] R. C. Locht, A. E. Gross and F. Langer, “Late Osteo- chondral Allograft Resurfacing for Tibial Plateau Frac- tures,” The Journal of Bone and Joint Surgery. American Volume, Vol. 66, No. 3, 1984, pp. 328-335. [42] M. H. Meyers and S. N. Chatterjee, “Osteochondral Transplantation,” The Surgical Clinics of North America, Vol. 58, No. 2, 1978, pp. 429-434. [43] M. H. Meyers, R. E. Jones, R. W. Bucholz, et al., “Fresh Autogenous Grafts and Osteochondral Allografts for the Treatment of Segmental Collapse in Osteonecrosis of the Hip,” Clinical Orthopedics and Related Research, Vol. 174, 1983, pp. 107-112. [44] K. Pap and S. Krompecher, “Arthroplasty of the Knee: Experimental and Clinical Experiences,” The Journal of Bone and Joint Surgery. American Volume, Vol. 43, No. 4, 1961, pp. 523-537. [45] B. C. Emmerson, S. Görtz, A. A. Jamali, C. Chung, D. Amiel and W. D. Bugbee, “Fresh Osteochondral Al- lografting in the Treatment of Osteochondritis Dissecans of the Femoral Condyle,” The American Journal of Sports Medicine, Vol. 35, No. 6, 2007, pp. 907-914. doi:10.1177/0363546507299932 [46] P. C. McCulloch, R. W. Kang, M. H. Sobhy, J. K. Hay- den and B. J. Cole, “Prospective Evaluation of Prolonged Fresh Osteochondral Allograft Transplantation of the Femoral Condyle: Minimum 2-Year Follow-Up,” The American Journal of Sports Medicine, Vol. 35, No. 3, 2007, pp. 411-420. doi:10.1177/0363546506295178 [47] R. J. Williams, A. S. Ranawat, H. G. Potter, T. Carter and R. F. Warren, “Fresh Stored Allografts for the Treatment of Osteochondral Defects of the Knee,” The Journal of Bone and Joint Surgery. American Volume, Vol. 89, No. 4, 2007, pp. 718-726. doi:10.2106/JBJS.F.00625 [48] N. Bellamy and W. W. Buchanan, “Outcome Measure- ment in Osteoarthritis Clinical Trials: The Case for Stan- dardisation,” Clinical Rheumatology, Vol. 3, No. 3, 1984, pp. 293-303. doi:10.1007/BF02032334 [49] A. Pace, N. Orpen, H. Doll and E. J. Crawfurd, “Outcome Scoring System Evaluation of Knee Osteoarthritis in Pa- tients Awaiting TKA,” The Journal of Knee Surgery, Vol. 19, No. 2, 2006, pp. 85-88. [50] M. A. Ritter, A. E. Thong, K. E. Davis, M. E. Berend, J. B. Meding and P. M. Faris, “Long-Term Deterioration of Joint Evaluation Scores,” The Journal of Bone and Joint Surgery. British Volume, Vol. 86, No. 3, 2004, pp. 438- 442. doi:10.1302/0301-620X.86B3.14243 [51] N. Bellamy, W. W. Buchanan, C. H. Goldsmith, J. Cam- pbell and L. W. Stitt, “Validation Study of WOMAC: A Health Status Instrument for Measuring Clinically Im- portant Patient Relevant Outcomes to Antirheumatic Drug Therapy in Patients with Osteoarthritis of the Hip or Knee,” Journal of Rheumatology, Vol. 15, No. 12, 1988, pp. 1833-1840. [52] N. Bellamy, W. F. Kean, W. W. Buchanan, E. Ge- recz-Simon and J. Campbell, “Double Blind Randomized Controlled Trial of Sodium Meclofenamate (Meclomen) and Diclofenac Sodium (Voltaren): Post Validation Re- application of the WOMAC Osteoarthritis Index,” Jour- nal of Rheumatology, Vol. 19, No. 1, 1992, pp. 153- 159. [53] D. Amiel, F. L. Harwood, J. A. Hoover, et al., “A Histo- logical and Biomechanical Assessment of the Cartilage Matrix Obtained from in Vitro Storage of Osteochondral Allografts,” Connective Tissue Research, Vol. 23, No. 1, 1989, pp. 89-99. doi:10.3109/03008208909103906 [54] M. K. Kwan, J. S. Wayne, et al., “Histological and Bio- mechanical Assessment of Articular Cartilage from St- ored Osteochondral Shell Allografts,” Journal of Ortho- paedic Research, Vol. 7, No. 5, 1989, pp. 637-644. doi:10.1002/jor.1100070503 [55] K. M. Oates, A. C. Chen, E. P. Young, et al., “Effects of Tissue Culture Storage on the in Vivo Survival of Canine Osteochondral Allografts,” Journal of Orthopaedic Re- search, Vol. 13, No. 4, 1995, pp. 526-569. doi:10.1002/jor.1100130411 [56] J. S. Wayne, D. Amiel, M. K. Kwan, et al., “Long-Term Storage Effects on Canine Osteochrondral Allografts,” ACTA Orthopaedica Scandinavian, Vol. 61, No. 6, 1990, pp. 539-545. doi:10.3109/17453679008993578 [57] A. W. Pearsall, J. A. Tucker, R. B. Hester and R. J. Heit- man, “Chondrocyte Viability in Refrigerated Osteo- chondral Allografts Used for Transplantation within the Knee,” The American Journal of Sports Medicine, Vol. 32, No. 1, 2004, pp. 125-131. doi:10.1177/0095399703258614 [58] V. M. Goldberg and S. Stevenson, “Natural History of Autografts and Allografts,” Clinical Orthopedics and Re- lated Research, Vol. 225, 1987, pp. 7-16. [59] T. Gibson, “Viability of Cartilage after Freezing,” Pro- ceedings of the Royal Society B: Biological Sciences, Vol. 147, No. 929, 1957, pp. 528-529. [60] W. H. Simon and W. T. Green, “Experimental Production Copyright © 2011 SciRes. SS  A. W. PEARSALL IV ET AL. Copyright © 2011 SciRes. SS 241 of Cartilage Necrosis by Cold Injury: Failure to Cause Degenerative Joint Disease,” The American Journal of Pathology, Vol. 64, No. 1, 1971, pp. 145-154. [61] W. H. Simon, S. Richardson, W. Herman, et al., “Long- -Term Effects of Chondrocyte Death on Rabbit Articular Cartilage in Vivo,” Journal of the American Academy of Orthopaedic Surgeons, Vol. 58, No. 4, 1976, pp. 517- 526. [62] C. B. Sirlin, J. Brossmann, R. D. Boutin, M. N. Pathria, F. R. Convery, W. Bugbee, R. Deutsch, L. K. Lebeck and D. Resnick, “Shell Osteochondral Allografts of the Knee: Comparison of MR Imaging Findings and Immunologic Responses,” Radiology, Vol. 219, No. 1, 2001, pp. 35-43. [63] J. H. Dashefsky, “Arthroscopic Measurement of Chon- dromalacia of Patella Cartilage Using a Microminiature Pressure Transducer,” Arthroscopy, Vol. 3, No. 2, 1987, pp. 80- 85. doi:10.1016/S0749-8063(87)80021-X [64] G. E. Kempson, H. Muir, S. A. Swanson and M. A. Free- man, “Correlations between Stiffness and the Chemical Constituents of Cartilage on the Human Femoral Head,” Biochimica et Biophysica Acta, Vol. 215, No. 1, 1970, pp. 70-77. [65] T. Lyyra, J. Jurvelin, P. Pitkänen, U. Väätäinen and I. Kviranta, “Indentation Instrument for the Measurement of Cartilage Stiffness under Arthroscopic Control,” Medical Engineering & Physics, Vol. 17, No. 5, 1995, pp. 395- 399. doi:10.1016/1350-4533(95)97322-G
|