Paper Menu >>
Journal Menu >>
 Chinese Medicine, 2009, 1, 1-6 Published Online September 2009 in SciRes (www.SciRP.org/journal/cm) Copyright © 2009 SciRes CM Midterm Outcomes of Prospective, Randomized, Single-Center Study of the Janus Tacrolimus-Eluting Stent for Treatment of Native Coronary Artery Lesions ABSTRACT From February 20, 2006 to August 26, 2006, a total of 200 patients were enrolled and randomly assigned to receive either Janus stent (n=100) or bare metal stent (Tecnic Carbostent, n=100). All patients were administered with clopi- dogrel for 4 months and aspirin for life long after stenting. Baseline clinical and angiographic characteristics were comparable between the two groups. AMI was present in 37% of patients with Janus and 36% with Tecnic Carbostent. At an average of 246-day follow-up, major adverse cardiac events (MACE) was 6% with the Janus stent and 15% with the Tecnic Carbostent (P=0.038). Primary events included 1 cardiac death, 1 myocardial infarction (MI) due to subacute stent thrombosis and 13 target lesion revascularizations (TLR) due to restenosis in patients with Tecnic Car- bostent and 6 TLR due to restenosis in patients with Janus stent. Although all patients had discontinued clopidogrel for an average of 126 days, there was no additional thrombotic event in the two groups. Keywords: drug-eluting stent, acute myocardial infarction, angioplasty, transluminal, percutaneous coronary 1. Introduction Drug-eluting stent (DES) has been reported to dramati- cally reduce the incidence of restenosis and target lesion revascularization (TLR), and is widely used in clinical practice in recent years. However, controversies remain with regard to the long-term efficacy and safety for the first generation DES1. Pathological findings had indi- cated that polymer based DES delayed vessel healing, which might lead to late severe adverse events such as in-stent thrombosis. The permanent existence of non- degradable polymer coatings, which promote local vessel inflammation, is considered as one of the leading causes reducing vessel healing. The Janus tacrolimus-eluting stent (SORIN, Italy), a novel DES without polymer coating, is deemed capable to eliminate the adverse ef- fects of non-degradable polymer coatings of DES. Pre- liminary clinical outcomes from Jupiter I and II studies demonstrated that Janus stent was as safe as bare metal stent (BMS) and tended to reduce restenosis rate. How- ever, the study population in Jupiter I and II comprised of only low to mid-risk patients and lesions. We con- ducted a prospective, randomized, single-center study aiming at evaluating the safety and efficacy of Janus stent for treating coronary artery disease in “real world” clinical practice of percutaneous coronary intervention (PCI) for the first time. 2. Methods 2.1. Study Population Between February 20, 2006 and August 26, 2006, a total of 200 patients with symptomatic or documented myo- cardial ischemia, including acute myocardial infarction (AMI), were enrolled in this prospective, randomized study. Patients were considered eligible for enrollment if they were: fit for coronary stent implantation; to be treated exclusively with one kind of stent, no more than 3 stents for one target vessel (or total length of stents ≤85 mm), and providing written informed consent. The major exclusion criteria were in-stent restenosis lesion, graft lesion, not eligible for DES implantation, such as intol- erant of anti-platelet treatment or planned to undergo surgery, and administration of IIb/IIIa antagonist. 2.2. Study Protocols Patients were randomly assigned to receive Janus stent 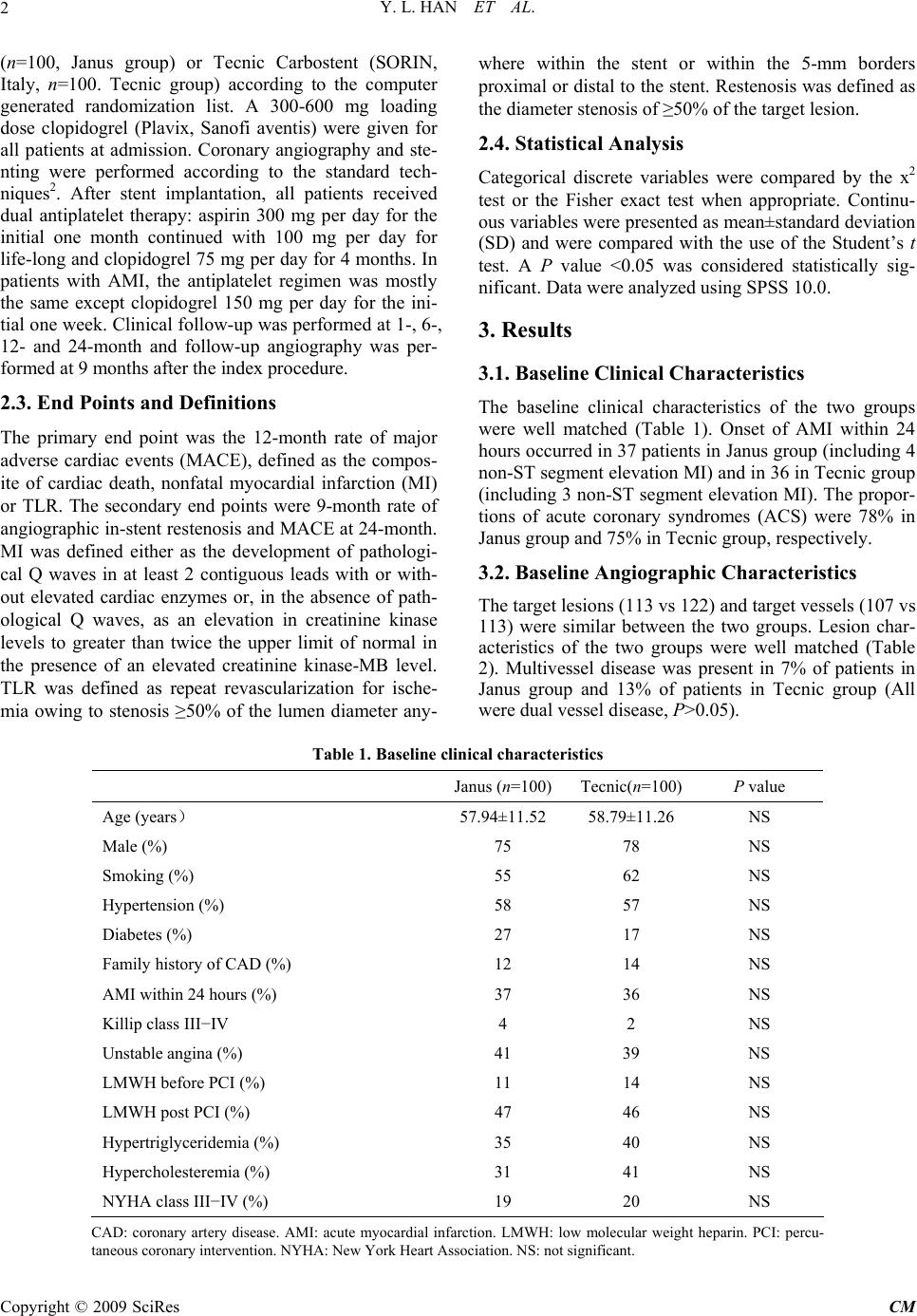 Y. L. HAN ET AL. 2 (n=100, Janus group) or Tecnic Carbostent (SORIN, Italy, n=100. Tecnic group) according to the computer generated randomization list. A 300-600 mg loading dose clopidogrel (Plavix, Sanofi aventis) were given for all patients at admission. Coronary angiography and ste- nting were performed according to the standard tech- niques2. After stent implantation, all patients received dual antiplatelet therapy: aspirin 300 mg per day for the initial one month continued with 100 mg per day for life-long and clopidogrel 75 mg per day for 4 months. In patients with AMI, the antiplatelet regimen was mostly the same except clopidogrel 150 mg per day for the ini- tial one week. Clinical follow-up was performed at 1-, 6-, 12- and 24-month and follow-up angiography was per- formed at 9 months after the index procedure. 2.3. End Points and Definitions The primary end point was the 12-month rate of major adverse cardiac events (MACE), defined as the compos- ite of cardiac death, nonfatal myocardial infarction (MI) or TLR. The secondary end points were 9-month rate of angiographic in-stent restenosis and MACE at 24-month. MI was defined either as the development of pathologi- cal Q waves in at least 2 contiguous leads with or with- out elevated cardiac enzymes or, in the absence of path- ological Q waves, as an elevation in creatinine kinase levels to greater than twice the upper limit of normal in the presence of an elevated creatinine kinase-MB level. TLR was defined as repeat revascularization for ische- mia owing to stenosis ≥50% of the lumen diameter any- where within the stent or within the 5-mm borders proximal or distal to the stent. Restenosis was defined as the diameter stenosis of ≥50% of the target lesion. 2.4. Statistical Analysis Categorical discrete variables were compared by the x2 test or the Fisher exact test when appropriate. Continu- ous variables were presented as mean±standard deviation (SD) and were compared with the use of the Student’s t test. A P value <0.05 was considered statistically sig- nificant. Data were analyzed using SPSS 10.0. 3. Results 3.1. Baseline Clinical Characteristics The baseline clinical characteristics of the two groups were well matched (Table 1). Onset of AMI within 24 hours occurred in 37 patients in Janus group (including 4 non-ST segment elevation MI) and in 36 in Tecnic group (including 3 non-ST segment elevation MI). The propor- tions of acute coronary syndromes (ACS) were 78% in Janus group and 75% in Tecnic group, respectively. 3.2. Baseline Angiographic Characteristics The target lesions (113 vs 122) and target vessels (107 vs 113) were similar between the two groups. Lesion char- acteristics of the two groups were well matched (Table 2). Multivessel disease was present in 7% of patients in Janus group and 13% of patients in Tecnic group (All were dual vessel disease, P>0.05). Table 1. Baseline clinical characteristics Janus (n=100) Tecnic(n=100) P value Age (years) 57.94±11.52 58.79±11.26 NS Male (%) 75 78 NS Smoking (%) 55 62 NS Hypertension (%) 58 57 NS Diabetes (%) 27 17 NS Family history of CAD (%) 12 14 NS AMI within 24 hours (%) 37 36 NS Killip class III−IV 4 2 NS Unstable angina (%) 41 39 NS LMWH before PCI (%) 11 14 NS LMWH post PCI (%) 47 46 NS Hypertriglyceridemia (%) 35 40 NS Hypercholesteremia (%) 31 41 NS NYHA class III−IV (%) 19 20 NS CAD: coronary artery disease. AMI: acute myocardial infarction. LMWH: low molecular weight heparin. PCI: percu- taneous coronary intervention. NYHA: New York Heart Association. NS: not significant. Copyright © 2009 SciRes CM 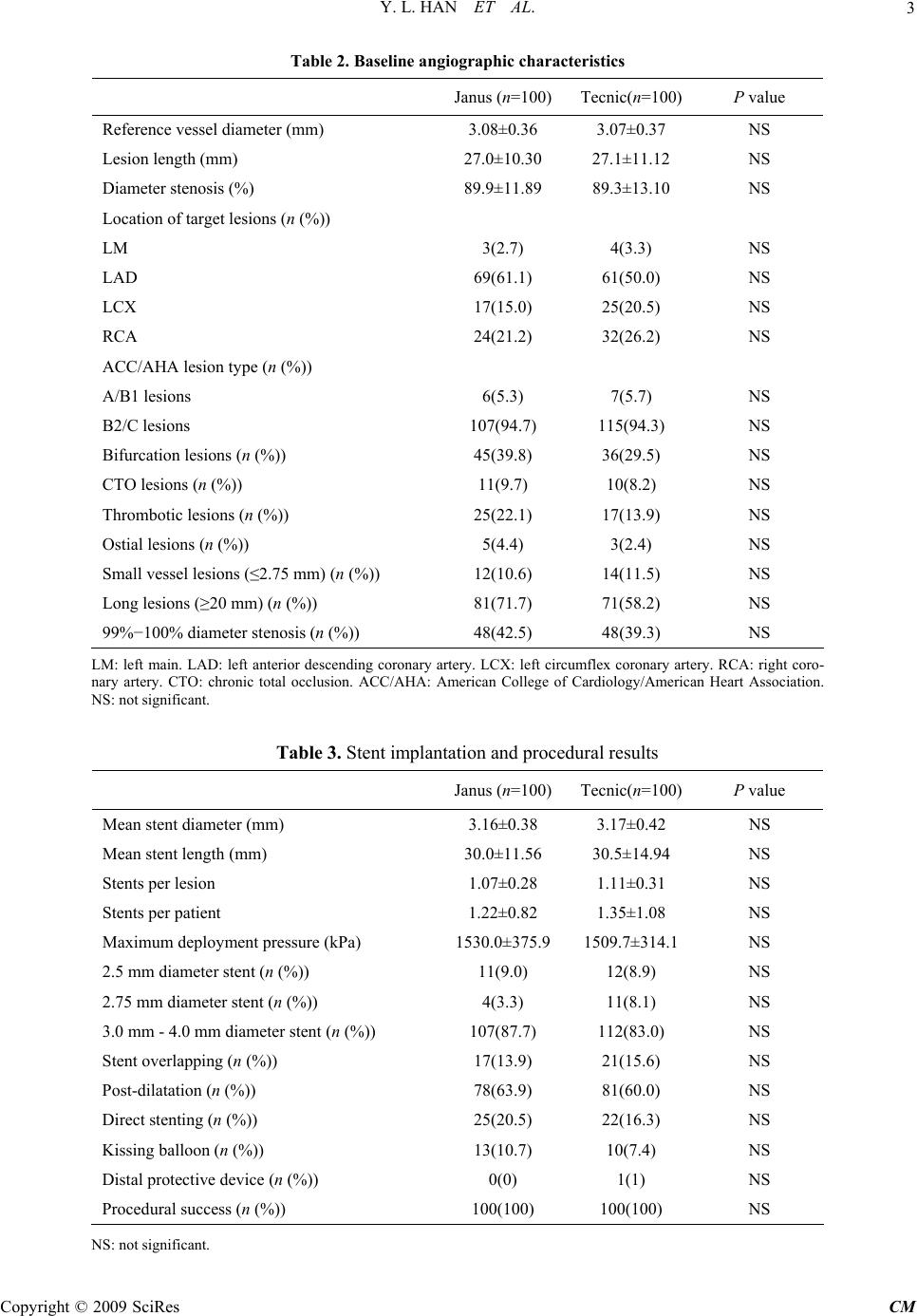 Y. L. HAN ET AL.3 Table 2. Baseline angiographic characteristics Janus (n=100) Tecnic(n=100) P value Reference vessel diameter (mm) 3.08±0.36 3.07±0.37 NS Lesion length (mm) 27.0±10.30 27.1±11.12 NS Diameter stenosis (%) 89.9±11.89 89.3±13.10 NS Location of target lesions (n (%)) LM 3(2.7) 4(3.3) NS LAD 69(61.1) 61(50.0) NS LCX 17(15.0) 25(20.5) NS RCA 24(21.2) 32(26.2) NS ACC/AHA lesion type (n (%)) A/B1 lesions 6(5.3) 7(5.7) NS B2/C lesions 107(94.7) 115(94.3) NS Bifurcation lesions (n (%)) 45(39.8) 36(29.5) NS CTO lesions (n (%)) 11(9.7) 10(8.2) NS Thrombotic lesions (n (%)) 25(22.1) 17(13.9) NS Ostial lesions (n (%)) 5(4.4) 3(2.4) NS Small vessel lesions (≤2.75 mm) (n (%)) 12(10.6) 14(11.5) NS Long lesions (≥20 mm) (n (%)) 81(71.7) 71(58.2) NS 99%−100% diameter stenosis (n (%)) 48(42.5) 48(39.3) NS LM: left main. LAD: left anterior descending coronary artery. LCX: left circumflex coronary artery. RCA: right coro- nary artery. CTO: chronic total occlusion. ACC/AHA: American College of Cardiology/American Heart Association. NS: not significant. Table 3. Stent implantation and procedural results Janus (n=100) Tecnic(n=100) P value Mean stent diameter (mm) 3.16±0.38 3.17±0.42 NS Mean stent length (mm) 30.0±11.56 30.5±14.94 NS Stents per lesion 1.07±0.28 1.11±0.31 NS Stents per patient 1.22±0.82 1.35±1.08 NS Maximum deployment pressure (kPa) 1530.0±375.9 1509.7±314.1NS 2.5 mm diameter stent (n (%)) 11(9.0) 12(8.9) NS 2.75 mm diameter stent (n (%)) 4(3.3) 11(8.1) NS 3.0 mm - 4.0 mm diameter stent (n (%)) 107(87.7) 112(83.0) NS Stent overlapping (n (%)) 17(13.9) 21(15.6) NS Post-dilatation (n (%)) 78(63.9) 81(60.0) NS Direct stenting (n (%)) 25(20.5) 22(16.3) NS Kissing balloon (n (%)) 13(10.7) 10(7.4) NS Distal protective device (n (%)) 0(0) 1(1) NS Procedural success (n (%)) 100(100) 100(100) NS NS: not significant. Copyright © 2009 SciRes CM  Y. L. HAN ET AL. Copyright © 2009 SciRes CM 4 3.3. Procedural Results There were totally 235 target lesions in 220 target vessel of 200 observed patients underwent coronary stenting. The procedure and device-deployment success rates achieved 100% in the two groups. Procedural results were similar for the two groups (Table 3). The maximum total stent length in one vessel was 81 mm in patients implanted with the Janus stent and 71 mm in patients implanted with Tecnic carbostent (overlapped by 3 stents). Stents with small diameter (2.5-2.75 mm) ac- counted for 12.3% in Janus group and 17.0% in Tecnic group (P>0.05). 3.4. Overall Clinical Outcomes Up to January 30, 2007, all patients were clinically fol- lowed up for an average of (246±48) days (ranged 150 to 340 days). All the patients discontinued clopidogrel at the end of the fourth month after PCI according to study protocol. The mean interval from the discontinuation of clopidogrel was (126±46) days (ranged 34 to 220 days). Primary events occurred in 15 patients in Tecnic group, including 1 cardiac death due to cardiac rupture secon- dary to anterior AMI, 1 AMI caused by subacute in-stent thrombosis, 12 repeat PCI and 1 CABG due to in-stent restenosis, so the overall and stent-related MACE rates were 15% and 14%, respectively. In Janus group, there were 6 primary events of repeat PCI due to in-stent restenosis, so the overall and stent-related MACE rates were all 6%. The overall MACE rate was significantly lower in Janus group as compared with Tecnic group (6% vs 15%, P=0.038). For the TLR and stent-related MACE, there was an obvious tendency of lower inci- dence associated with the Janus stent but not statistically significant (6% vs 14%, P=0.059). 3.5. Clinical Outcomes of AMI Subgroup For the subgroup of AMI patients who underwent emer- gent PCI within 24 hours of symptom onset, the mean clinical follow-up was (250±46) days (ranged 154 to 323 days) in Janus group and (243±52) days (ranged 150 to 340 days) in Tecnic group, respectively. There was no death or any thrombotic event in 37 patients in Janus group except 3 repeat PCI due to in-stent restenosis. In 36 patients in Tecnic group, there were 4 primary end points, including 1 cardiac death, 1 MI caused by subacute in-stent thrombosis and 2 repeat PCI due to in- stent restenosis. The incidence of death or MI was simi- lar between the two groups (0 vs 2%, P=0.24). Although all patients had discontinued clopidogrel, there was no additional death or thrombotic event in the two groups. 4. Discussion Controversies about DES regarding its late clinical out- comes were emerged and spread after the initial years of inspiritment. Because of the growing concern that de- layed endothelialization after implantation of a DES may cause late stent thrombosis, prolonged dual antiplatelet therapy with clopidogrel and aspirin is currently recom- mended after DES implantation. Unfortunately, even with 6 months or longer period of dual antiplatelet ther- apy, the incidence of late thrombosis or deadly cardiac events after DES implantation were still higher than those after BMS implantation3. Therefore, it is important to develop a new generation of DES which might de- crease the restenosis not at the expense of safety. The Janus tacrolimus-eluting stent is one of such new generation DES. As the platform of Janus stent, the Tec- nic Carbostent is coated with Carbofilm to increase the biocompatibility and hemocompatibility4,5. Phantom IV study demonstrated the efficacy and safety of Tecnic Carbostent. In that study, there was no death or MI at 6-month clinical follow-up after Tecnic Carbostent im- plantation, and the angiographic restenosis rate was 14%6. Of the 100 patients received the Tecnic Carbostent in the present study, the stent related thrombotic events rate was only 1% at an average of 8-month follow-up, which confirms that the Tecnic Carbostent is a safe BMS platform. Having kept the structural features of the Tec- nic Carbostent, the Janus stent is coated with tacrolimus, an immunosuppressant, which was demonstrated to be efficient in inhibiting neointima hyperplasia of porcine coronary artery7,8. Differed from the first generation DES, i.e. Cypher and TAXUS, Janus stent has some unique features. First, drugs are loaded in the embedded reser- voirs on the outer stent surface, which enables drugs re- leasing directly to vessel wall without being washed away in the bloodstream. Second, there is no polymer coating in Janus stent, which may decrease the potential risks of late in-stent thrombosis caused by unabsorbable polymer coatings of traditional DES9. Jupiter I study, the first-in-man registry of Janus stent, enrolled 58 patients. Of whom, 19% were ACS (includ- ing 6.9% of AMI). The target lesions included only 27% complex lesions of type B2/C with (11.5±5.9) mm of lesion length and (70.3±14.9)% of stenosis diameter. The randomized, controlled Jupiter II study presented at 2005 TCT showed that the total rate of MACE in the 157 pa- tients of Janus group was 7.6%, in which 6.4% of MACE was related to the stent, suggesting Janus stent having better clinical effects and safety. However, ACS ac- counted for only 27.6% of patients in Janus group in Jupiter II. Furthermore, ST segment elevation MI (STEMI) within 7 days and non-ST segment elevation MI (NSTEMI) within 3 days were all excluded in Jupiter II. It also excluded those with complicated lesions such as ostial lesions, left main diseases, chronic total occlu- sions (CTO), bifurcations, lesion in small vessels (refer- ence diameter ≤ 2.75 mm), long lesions (lesion length  Y. L. HAN ET AL.5 >20 mm) and thrombotic lesions. Complex lesions of Type B2/C took only 34.9%. Because of the relatively simple lesion type, 75.9% of patients underwent suc- cessful direct stenting. Compared with Jupiter I and II studies, the population in the present study included 78% of ACS patients. The target lesions included many com- plicated lesions of high risk, such as left main or ostial lesions, thrombotic lesions, CTO, bifurcations, long le- sions, small vessel lesions with 2.5 mm in diameter. Some of the patients were highly endangered by some risk factors of coronary heart disease, for instance, dia- betes, smoking and hypertension, which generally re- flected the “real world” of clinical PCI daily practice. Despite of the higher risk for clinic and restenosis in subjects of present study, the incidence of MACE was 6% without occurrence of death or thrombosis in Janus group during the mean 8-month follow-up. Besides, as compared with the Tecnic group, incidence of MACE was dramatically reduced (P=0.038). The rate of stent related MACE had an obvious lower tendency in Janus group but no statistical significance (P=0.059). The above result was similar to that of Jupiter I and II, and further confirms the safety and clinical efficacy of Janus stent. Different from the present study, Jupiter II did not achieve statistical differences in the total rate of MACE, which might mainly due to the differences in study population. The enrolled patients in Jupiter II were mostly at lower risk for thrombosis. It has been reported that the effect of DES in reducing restenosis is not supe- rior to that of BMS for lesions with length <15 mm and vessel diameter >2.8 mm10. Moreover, Tecnic stent itself has certain antithrombotic function which may reduce the thrombosis related events in the control group in Jupiter II, in which late lumen lost was only 0.64 mm, and the incidence of in-stent restenosis was 14.8% in patients implanted with Tecnic stent. This was probably the reason for Jupiter II to come to the conclusion that the total incidences of MACE and stent related MACE in Janus group were not significantly lower than those in Tecnic group. Up to now, the safety of DES implantation in primary PCI is uncertain. The randomized Typhoon trial showed that sirolimus eluting stent for treatment of STEMI was efficient in reducing MACE and TLR compared with BMS, but not beneficial for death, MI or in-stent throm- bosis11. In the present study, more than a third of study population were those with STEMI or NSTEMI under- going emergent PCI within 24 hours of symptom onset, which were excluded in Jupiter I and II study. For 37 AMI patients (including 33 STEMI) in Janus group, no acute, subacute or late thrombosis occurred at an average 8-month follow-up, suggesting Janus stent is safe in treating AMI. It was reported that incomplete endothelialization even appeared 1-2 years after implantation of Cypher or TAXUS in some cases12. The reports of very late in-stent thrombosis (>1 year) after DES implantation were also not rare13. Long-term dual antiplatelet therapy may re- duce the risk of thrombosis, but simultaneously accom- panies the increased adverse effects, such as hemorrhage, and the financial burden. Therefore, it is always confused to decide how long patients should take dual antiplatelet therapy after DES implantation. In Jupiter II study, about 60% patients took clopidogrel for over 6 months. Al- though patients in present study took clopidogrel for 4 months and then aspirin alone, no thrombosis was found after discontinuation of clopidogrel for 126 days on av- erage, preliminarily proving the feasibility and safety of this antiplatelet regimen, which provides an opportunity of receiving DES for patients who are intolerable of long-term dual antiplatelet therapy. The relatively shorter dual antiplatelet regimen may also associate with a de- crease of the hemorrhage risk, adverse effects and medi- cal expenses. The main limitation of our study lies in that the mean follow-up period is only 8 months, which does not reach the designed observation time and only reflects the mid-term clinical result. Because of the limited observa- tion time, angiographic follow-up rate is low as well (19%). In conclusion, for the first time we investigated the ef- ficacy and safety of the Janus stent, a novel DES without polymer coating, in “real world” PCI practice in this randomized single-center study. At our mean 8-month follow-up, Janus stent is efficient in reducing MACE compared with BMS, and is safe and efficient for treat- ing AMI patients. Discontinuation of clopidogrel at 4-month after Janus implantation is safe. References [1] Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003; 349: 1315-1323. [2] Han YL, Wang G, Jing QM, Wang SL, Wang ZL, Wang DM, et al. Percutaneous coronary intervention in acute coronary syndrome: single center experience from 4670 patients. Natl Med J China (Chin) 2005; 85: 1040-1044. [3] Pfisterer M, Brunner-La Rocca HP, Buser PT, Ricken- bacher P, Hunziker P, Mueller C, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents. J Am Coll Cardiol 2006; 48: 2584-2591. [4] Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Ha- yashi E, Perin M, et al. A randomized comparison of a Copyright © 2009 SciRes CM  Y. L. HAN ET AL. Copyright © 2009 SciRes CM 6 sirolimus-eluting stent with standard stent for coronary revascularization. N Engl J Med 2002; 346: 1773-1780. [5] Sousa JE, Costa MA, Abizaid AC, Rensing BJ, Abizaid AS, Tanajura LF, et al. Sustained suppression of neoin- timal proliferation by sirolimus-eluting stents: one-year angiographic and intravascular ultrasound follow-up. Circulation 2001; 104: 2007-2011. [6] Danzi GB, Capuano C, Sesana M, Baglini R, Bartorelli AL, Trabattoni D, et al. Six-month clinical and an- giographic outcomes of the tecnic carbostentTM coronary system: the phantom IV study. J In vasasive Cardiol 2004; 11: 641-644. [7] Kollum M, Farb A, Schreiber R, Terfera K, Arab A, Geist A, et al. Particle debris from a nanoporous stent coating obscures potential antiproliferative effects of tac- rolimus-eluting stents in a porcine model of restenosis. Catheter Cardiovasc Interv 2005; 64: 85-90. [8] Scheller B, Grandt A, Wnendt S, Lorenz G, Bohm M, Nickenig G. Comparative study of tacrolimus and pacli- taxel stent coating in the porcine coronary model. Z Kar- diol 2005; 94: 445-452. [9] Bartorelli A, Trabattoni D, Fabbiocchi F, Montorsi P, Martini SD, Calligaris G, et al. Synergy of passive coat- ing and targeted drug delivery: the tacrolimus-eluting Janus Carbostent. J Interven Cardiol 2003; 16: 499-505. [10] Pache J, Dibra A, Mehili J, Dirschinger J, Schomig A, Kastrati A. Drug-eluting stents compared with thin-strut bare stents for the reduction of restenosis: a prospective, randomized trial. Eur Heart J 2005; 26: 1262-1268. [11] Spaulding C, Henry P, Teiger E, Beatt K, Bramucci E, Carre D, et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med 2006; 355: 1093-1104. [12] Kotani J, Awata M, Nanto S, Uematsu M, Oshima F, Minamiquchi H, et al. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol 2006; 47: 2108-2111. [13] Takahashi S, Kaneda H, Tanaka S, Miyashita Y, Shiono T, Taketani Y, et al. Late angiographic stent thrombosis after sirolimus-eluting stent implantation. Circ J 2007; 71: 226-228. |

