Agricultural Sciences
Vol. 3 No. 8 (2012) , Article ID: 25591 , 4 pages DOI:10.4236/as.2012.38123
Decumbenones A–C from marine fungus Aspergillus sulphureus as stimulators of the initial stages of development of agricultural plants
![]()
1G. B. Elyakov Pacific Institute of Bioorganic Chemistry Far Eastern Branch, Russian Academy of Sciences, Vladivostok, Russian Federation; *Corresponding Author: anisimov@piboc.dvo.ru
2Primorsky Scientific Research Institute of Agriculture, Russian Academy of Agricultural Sciences, Primorsky Krai, Ussuriisk, Russian Federation
Received 13 September 2012; revised 22 October 2012; accepted 28 November 2012
Keywords: Decumbenones; Marine Fungus Aspergillus Sulphureus; Plant Growth Regulators; Buckwheat; Wheat; Barley and Corn; Kinetin
ABSTRACT
The effect of decumbenones A (1), B (2) and C (3) from the marine-derived strain of the fungus Aspergillus sulphureus on the growth of seedling roots of buckwheat, wheat, barley and corn at the concentration range 10−5 - 10−18 M was studied. It was shown that decumbenone B had a stimulatory effect on the growth of seedling roots of buckwheat, decumbenone A—on the growth of seedling roots of spring soft wheat, decumbenone C—on the growth of seedling roots of spring barley, decumbenone A, B and C —on the growth of seedling roots of corn. The stimulatory effect for some substances was shown at ultra-low concentrations 10−12 - 10−18 M. It is possible to recommend decumbenones A, B and C for studying in field conditions as growth factors of buckwheat, wheat, barley and corn.
1. INTRODUCTION
Researches of last years show, that marine fungi are promising sources of new structural and biologically active secondary metabolites [1-5]. As a part of our previous search for secondary metabolites from marine fungi we have revealed that diterpene glycosides viresсenosides A, B, G and Q, isolated from marine fungus Acremonium striatisporum, stimulate the growth of seedling roots of maize. Viresсenoside B showed stimulating effect at ultra low concentration (10–14 M) [6]. Later, it was shown that the alkaloids isolated from marine fungus Aspergillus fumigatus had a stimulatory effect on the
growth of seedling roots of maize [7] and buckwheat [8]. A new spirocyclic diketopiperazine alkaloid spirotriprostatin F isolated from a marine fungus Aspergillus fumigatus at low and ultra-low doses (10−6 - 10−17 M) stimulated the growth of seedling roots of soybean, buckwheat and maize [9].
In our ongoing search for secondary metabolites from marine fungal isolates we have investigated the fungus Aspergillus sulphureus KMM 4640, obtained from the sediment of the Sakhalin Bay, Okhotsk Sea, Russia. Prior chemical studies of A. sulphureus have been limited, but it has been reported that this fungus produces ochratoxin A and penicillic acid when grown in liquid culture on a yeast extract-sucrose medium [10]. Chromatographic separation of the ethyl acetate extract of the strain KMM 4640 gave known decaline derivatives decumbenones A (1) and B (2), together with a new compound, named decumbenone C (3). Earlier decumbenones A and B have been isolated from the soil fungus Penicillium decumbens [11] and it was shown that decumbenone A inhibited the growth of the pathogen of rice Magnaporihe grisea. Decumbenone C shows potent activity against SK-MEL-5 human melanoma cells [12].
The purpose of the present work is to study the influence of decumbenones A (1), B (2) and C (3) on the growth of seedling roots of buckwheat (Fagopyrum esculentum Мoench) variety of Izumrud, wheat (Triticum aestivum L.) variety of Primorskay 40, barley (Hordeum vulgare L.) variety of Primorskii 44 and corn (Zea mays L.) variety of Slavynka at low and ultralow concentrations (10–5 - 10–18 M).
2. MATERIALS AND METHODS
The fungus Aspergillus sulphureus was isolated from sediments of Sakhalin Bay (Okhotsk Sea, Russia, depth of 24 m) and identified based on morphology by Dr. N. N.
Slinkina (Pacific Institute of Bioorganic Chemistry, PIBOC). Voucher specimens are stored in the Collection of Marine Microorganisms, PIBOC, Vladivostok, Russia with the code KMM 4640.
Cultivation of the fungus was carried out stationary at 22˚C for 21 days in 10 Erlenmeyer flasks, each containing 20 g of rice, yeast extract 20 mg, 10 mg KH2PO4, and 40 ml of sea-water.
At the end of the incubation period, the mycelium and medium were homogenized and extracted with EtOAc (2 L). The extract was concentrated to dryness in vacuo. The residue (2 g) was dissolved in 20% MeOH-H2O (0.1 L) and consequentially extracted with n-hexane (0.05 L × 3) and EtOAc (0.1 L × 3). After evaporation in vacuo of the EtOAc layer, the residual material (1.6 g) was passed over normal-phase silica, which was eluted first with n-hexane (1 L) followed by a step gradient from 5% to 100% EtOAc in n-hexane (total volume 10 L). Fractions of 200 mL were collected and combined by TLC examination (Si gel, toluene—isopropanol 6:1, v/v). The nhexane-EtOAc (1:1) fraction (590 mg) was purified by reverse-phase HPLC on a Supelco Discovery C-18 column using MeOH–H2O (50:50) as eluent to yield 1 (60 mg), 2 (36 mg) and 3 (6 mg). The compounds 1 and 2 were identified as decumbenones A and B based on LRHRESI-MS and comparison of their NMR data with literature values [11]. The structure of decumbenone C (3) was determined based on HRESI-MS and 1D and 2D (COSY, HSQC, HMBC, NOESY) NMR spectra [12].
The roots of seedlings of buckwheat (Fagopyrum esculentum Moench) variety of Izumrud, wheat (Triticum aestivum L.) variety of Primorskii 40, barley (Hordeum vulgare L.) variety of Primorskii 44 and corn (Zea mays L.) variety of Slavynka were object of study. Seeds from the 2011 harvest were obtained from Primorsky Research Institute of Agricultural, (Ussuriisk, Russia). We used the scheme for seed germination in rolls of filter paper. Dry seeds were spread on strips of filter paper (12 × 42 cm) that were previously moistened with test solution, rolled, placed into beakers with a small amount of test solution
(100 ml), and left for 3 days in an incubator at 26˚C - 27˚C. The length of the main root seedlings was measured after incubation. The controls were seedling of the same culture grown in distilled H2O. Kinetin was used as a positive control. Test results were estimated as the arithmetic mean of three repeated tests (20 seeds in each) and were expressed in percent of the controls (M ± se). Results were processed statistically using the ORIGIN 8.0 computer program.
3. RESULTS AND DISCUSSION
The results showed that, depending on the type of plants and the chemical structure decumbenones A, B and C (Figure 1) have different effect on the growth of seedling roots of buckwheat, spring wheat, barley and maize (Figure 2).
Thus, decumbenone A showed stimulatory effect on the growth of seedling roots of spring wheat at concentrations of 10–6 (114%), 10–14 (114%), 10–17 (111%) M. The reduction of activity to the level of control and lower was observed at concentrations of 10–9 (90%) and 10–15 (103%) M. Also decumbenone A showed stimulatory effect on the growth of seedling roots of maize at concentrations of 10–8 (127%), 10–10 (120%), 10–13 (115%) M. Decumbenone A showed small stimulatory effect (108%) on the growth of seedling roots of spring barley at a concentration of 10–12 M. This compound was inactive with respect of seedling roots of buckwheat.
Decumbenone B showed small stimulatory effect on the growth of seedling roots of buckwheat, spring wheat and spring barley. Its stimulatory effect on the growth of seedling roots of buckwheat appeared at concentrations of 10–6 (111%), 10–12 (109%), 10–17 (109%) M. The reduction of activity of decumbenone B to the level of control was observed at concentrations of 10–9 (104%) and 10–13 (101%) M. Weak stimulating action of decumbenone B was observed on the growth of seedling roots of wheat at concentrations of 10–11 (109%), 10–13 (111%), 10–18 (111%) M. Decrease activity of decumbenone B to the level of control was observed at concentrations of 10–10 (101%), 10–12 (102%), 10–16 (102%) M. Decumbe-
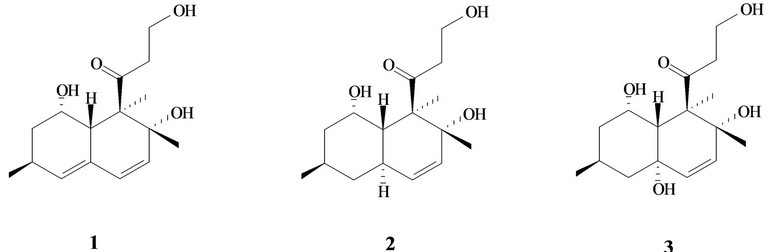
Figure 1. Structure of decumbenones A (1), B (2) and C (3) from the marine-derived strain of the fungus Aspergillus sulphureus.
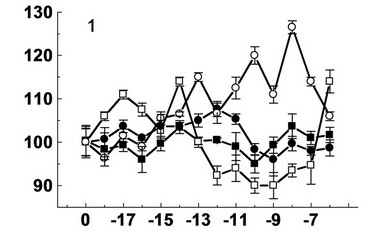
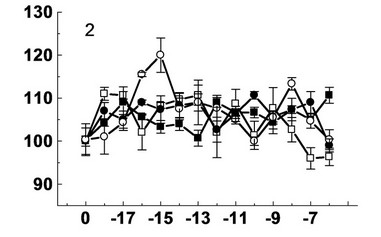
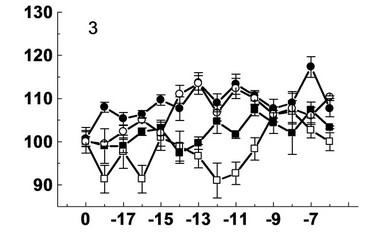
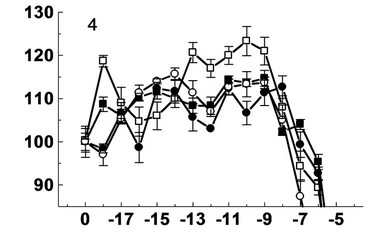
Figure 2. Effect decumbenones A (1), B (2), C (3) from marine fungus Aspergillus sulphureus and kinetin (4) on the growth of seedling roots of buckwheat (- ■ -), spring wheat (- □ -), spring barley (- ● -) and corn (- ○ -). On the X-axis—lg molar concentration of biologically active substances; on Y-axis—length of roots, % of control.
none B showed a small stimulatory effect on the growth of seedling roots of wheat at concentrations of 10–7 (109%), 10–10 (111%), 10–13 (110) and 10–16 (109%) M. The reduction of activity of decumbenone B to the level of control was observed at a concentration of 10–12 (103%) М. Decumbenone B acted differently on the growth of seedling roots of maize. Its stimulatory effect was observed at two concentrations 10–8 (113%) и 10–15 (120%) М. Decrease of activity of decumbenone B to the level of control was observed at a concentration 10–10 М.
Deсumbenone C showed the greatest stimulatory effect on the growth of seedling roots of spring barley at concentrations of 10–7 (117%), 10–11 (113%), 10–13 (114%) M and on the growth of seedling roots of corn—10–6 (110%), 10–11 (112%), 10–13 (114%) M. This compound had a low stimulatory effect on the growth of seedling roots of spring wheat at concentrations of 10–8 (107%), 10–9 (106%) M and on the growth of seedling roots of buckwheat—10–7 (107%), 10–10 (107%) M.
The positive control was kinetin, which exhibits both stimulatory and inhibitory activity on the growth of seedling roots of the test cultures. It showed a stimulatory effect on the growth of seedling roots of buckwheat at concentrations of 10–9 - 10–11 и 10–15 M. Kinetin had a stimulatory effect on the growth of seedling roots of spring wheat at concentrations of 10–9 - 10–13 and 10–18 M, and at a concentration of 10–16 M stimulatory effect came nearer to the control. Kinetin had a stimulatory effect on the growth of seedling roots of spring barley at concentrations of 10–8 - 10–9, 10–11, 10–14 - 10–15 M, and at a con-
centration of 10–12 M stimulatory effect came nearer to the control. Kinetin had a stimulatory effect on the growth of seedling roots of maize at concentrations of 10–9 (113%) and 10–14 (117%) M and at a concentration of 10–12 (107%) M stimulatory effect came nearer to the control.
Using natural plant growth regulators in agricultural practices found a number of difficulties associated with the high cost of their production. In this connection it is necessary to search for natural compound which in small and very small concentrations can increase plant growth and improve qualities of production of agricultural crops. Some secondary metabolites of marine fungi in ultra-low concentrations act as stimulators of the initial stages of crop [7-9].
The study of action of ultra-low doses of regulators of growth of plants is perspective, because it can be basis for new ways of application of biologically active substances in plant growing.
4. CONCLUSION
The effect of deсumbenones A, B and C from marine fungus Aspergillus sulphureus on the growth of seedling roots of agricultural plants was studied. It was shown, that the most effective stimulators on the growth of seedling roots of buckwheat was decumbenone B, of spring wheat—decumbenone A, of spring barley—decumbenone C, of maize—decumbenones A, B, C. These metabolites exerted a stimulating effect in ultra-low concentrations. Decumbenones A, B and C can be recommended for study in the field conditions as growth promoters buckwheat, spring wheat, barley and maize.
5. ACKNOWLEDGEMENTS
The study was supported by the program grant from the Russian Foundation for Basic Research (REBR N 12-03-31406), by the Program of the Presidium RAS “Molecular and Cell Biology” and the President of the Russian Federation Program for support of the Leading Scientigic schools Grant N 546.2012.4.
REFERENCES
- Bhadury, P., Mohammad, B.T. and Wright, P.C. (2006) The current status of natural products from marine fungi and their potential as anti-infective agents. Journal of Industrial Microbiology and Biotechnology, 33, 325-337. doi:10.1007/s10295-005-0070-3
- Debbab, A., Aly, A.H., Lin, W.H. and Proksch, P. (2010) Bioactive compounds from marine bacteria and fungi. Microbial Biotechnology, 3, 544-563. doi:10.1111/j.1751-7915.2010.00179.x
- Greve, H., Mohamed, I.E., Pontius, A., Kehraus, S., Gross, H. and König, G.M. (2010) Fungal metabolites: Structural diversity as incentive for anticancer drug development. Phytochemistry Reviews, 9, 537-545. doi:10.1007/s11101-010-9198-5
- Proksch, P., Putz, A., Ortlepp, S., Kjer, J. and Bayer, M. (2010) Bioactive natural products from marine sponges and fungal endophytes. Phytochemistry Reviews, 9, 475- 489. doi:10.1007/s11101-010-9178-9
- Rateb, M.E. and Ebel, R. (2011) Secondary metabolites of fungi from marine habitats. Natural Product Reports, 28, 290-344. doi:10.1039/c0np00061b
- Anisimov, M.M., Chaikina, E.L., Afiyatullov, Sh. and Kuznetsova, T.A. (2010) Influence of diterpene glycosides from a sea mushroom acremonium striatisporum on growth of roots of corn sprouts (Zea mays L.). Agrochemistry, 5, 34-38.
- Afiyatullov, Sh., Chaikina, E.L., Kraskovskaya, N.A. and Anisimov, M.M. (2012) Effect of alkaloids from the marine-derived strain of the fungus Aspergillus fumigatus Fresen. Оn the root growth of corn (Zea mays L.) seedlings. Agrochemistry, 7, 39-42.
- Anisimov, M.M., Chaikina, E.L., Afiyatullov, Sh. and Klykov, A.G. (2012) Influence alkaloids from the marine-derived strain of the fungus Aspergillus fumigatus Fresen. On the growth of seedling roots of buckwheat (Fagopyrum esculentum Moench). International Journal of Research and Reviews in Applied Sciences, 13, 326-329.
- Afiyatullov, Sh., Zhuravleva, O.I., Chaikina, E.L. and Anisimov, M.M. (2012) A new spirotryprostatin from the marine isolate of the fungus Aspergillus fumigatus. Chemistry of Natural Compounds, 48, 95-98. doi:10.1007/s10600-012-0166-8
- Ciegler, A. (1972) Bioproduction of ochratoxin A and penicillic acid by members of Aspergillus-ochraceus group. Canadian Journal of Microbiology, 18, 631-636. doi:10.1139/m72-100
- Fujii, Y., Asahara, M., Ichinoe, M. and Nакajima, H. (2002) Fungal melanin inhibitor and related compounds from Penicillium decumbens. Phytochemistry, 60, 703-708.
- Zhuravleva, O.I., Afiyatullov, Sh., Vishchuk, O.S., Denisenko, V.A., Slinkina, N.N., Smetanina, O.F. and Decumbenone, C., (2012) A new cytotoxic decaline derivative from the marine fungus Aspergillus sulphureus KMM 4640. Archives of Pharmacal Research, 35, 1771- 1776. doi:10.1007/s12272-012-1007-9

