Surgical Science
Vol. 3 No. 4 (2012) , Article ID: 18650 , 6 pages DOI:10.4236/ss.2012.34038
Patterns of Antiplatelet and Anticoagulant Agents Use in Urological Inpatients and Their Perception of Adverse Reactions
1st Department of Urology, Athens Medical School, Athens, Greece
Email: Tasmih10@gmail.com
Received January 17, 2012; revised March 6, 2012; accepted March 17, 2012
Keywords: Antithrombotics; Adverse Reactions; Urologic Patients
ABSTRACT
Purpose: To evaluate the rate of any type of anticoagulant drug use in urological inpatients and patients awareness of their effect on coagulation. Material and Methods: This observational study was conducted prospectively in a cohort of 193 consecutive urological inpatients who were asked to state the medications they were taking and following that, were specifically asked whether they were taking aspirin or other antiplatelet/anticoagulant agents. In case they did so, they were further asked why they were taking them, whether they knew their effect on coagulation and who had informed them on the matter. Results: Forty-seven patients received some kind of antithrombotic treatment. Twenty-nine per cent of aspirin users had to be specifically prompted in order to state its use, in comparison to 35.7% and 25% of other antiplatelets and warfarin users, respectively. Half of patients receiving warfarin were not aware of its effect on coagulation in comparison to 32.3% and 21.4% of those taking aspirin and other antiplatelets, respectively. Conclusion: Urologists should be aware of the high use of such agents by their patients and that not all patients are aware of their effect on coagulation, while some, even fail to report their use and have to be specifically prompted.
1. Introduction
The vast majority of urological inpatients is composed of middle-age and elderly individuals in whom, the use of long-term antithrombotic therapy is becoming increasingly common for primary or secondary prevention of coronary heart disease. This is probably the result of public health messages ensued from previous clinical trials that have influenced both clinical practice and the public behavior [1,2]. In a U.S. population-based cohort study, the overall prevalence of aspirin use among 45- to 64-year-olds was 23% with only 8% of them reporting it as a prescribed medication [1]. In Europe, aspirin use for primary prevention might be lower than in the U.S. as shown in a population-based study in Switzerland [3]. In Denmark, 21% of patients undergoing transurethral prostatectomy (TUR-P) during the years 1992-1994 were using aspirin and/or nonsteroidal anti-inflammatory drugs (NSAIDs) [4], while in Ireland, 60% of patients with a median age of 64 yrs (55 - 74 yrs) admitted on acute medical call were receiving aspirin, 13% warfarin, and 10% NSAIDs [5]. Aspirin use has recently greatly increased even in two low cardiovascular risk groups in whom its benefits are not certain: in those with cardiac risk factors only (e.g. hypertension) [6], and in healthy patients who purchase aspirin over-the-counter [7].
Even though most related studies have low statistical power, they clearly show that aspirin increases the frequency of bleeding complications in the perioperative period by approximately 50% [8]. Aspirin increases the bleeding time, a routine measure of platelet dysfunction, by a factor of 1.7 [9], while, when it is combined with clopidogrel it may increase by 3-fold [10]. However, a 1.7-fold increase in bleeding time is an abnormal value for only 15% - 25% of patients [11] who may have an exaggerated response to aspirin [12]. Moreover, bleeding time has not proven its usefulness as a predictor of the risk of hemorrhage associated with surgical procedures [13]. On the contrary, with discontinuation of low-dose aspirin, hazardous events such as stroke, myocardial infarction or even cardiovascular death may occur [8]. Many urologists prefer to cease them prior to even minor interventions, while others believe that the cardiovascular benefits outweigh the urological risks [14-16]. In Greece it is generally recommeneded to stop warfarin 3 - 5 days before surgery and clopidogrel 7 days before surgery; there is no clear recommendation as far as the cessation of aspirin or NSAIDs is concerned.
A considerable proportion of patients are unaware of both their illness and treatment [17], and risk perception may be different in patients and health professionals [18]. Health professionals rank anticoagulants and anti-inflammatory drug as carrying the highest risk, while aspirin was ranked sixth in a list of 13 categories [19]. The perceived risk of hemorrhage is reported as high among warfarin users, and quite low among aspirin and other antiplatelet users [5]. We evaluated the rate of any type of anticoagulant drug use in urological inpatients and patients’ awareness of their effect on coagulation in relation to probable confounding factors such as age, education background and the length of their use.
2. Materials and Methods
This observational study was conducted prospectively in a cohort of consecutive urological inpatients. Besides gender, age, education level, and reason for hospitalization, patients were asked to state the medications they were taking and following that, they were specifically asked whether they were taking aspirin or other antiplatelet/anticoagulant agents or NSAIDs using a standardized questionnaire, while the residents asking the questions were blinded to what therapy the patient was under. In case they did not include these drugs in their initial report and recalled their use only after been specifically asked for, their answer was recorded as “prompted”. Patients receiving aspirin or other antiplatelet/anticoagulants or NSAIDs were further asked about treatment duration, why they were taking them, whether they knew their effect on coagulation, and who had informed them on the matter.
Chi-square test was used to examine the relationship between education level and either the need of prompting in order to state the use of or the knowledge of the effect of the various antiplatelet/anticoagulant drugs and NSAIDs on coagulation. The impact of age and the time length of using the specific drugs on the parameters mentioned before was estimated by comparing the corresponding mean values of dichotomized (yes or no) groups using student’s t-test.
3. Results
Our study cohort was comprised of 193 consecutive urological inpatients (81.3% men). Their age ranged from 18 to 91 years (mean, 64.9 ± 13.8 yrs). Thirty-one patients (16.1%; age: 58 - 83, 71.2 ± 6.4; 96.8% men) were taking aspirin for 1 to 132 months (mean, 41.5 ± 43.5 months), 14 (7.3%; age: 60 - 82, 72.1 ± 6.9; 100% men) were taking clopidogrel for 4 to 120 months (mean, 27.6 ± 28.9 months), 8 (4.1%; age: 66 - 83, 75.1 ± 5.9; 87.5% men) were using warfarin for 3 to 348 months (mean, 65.6 ± 115.9 months) and 6 (3.1%; age: 39 - 86, 70.5 ± 16.4; 50% men) were using NSAIDs for 1 to 136 months (mean, 11.3 ± 14.9 months); there were no patients under dual antiplatelet treatment (Table 1). Twenty-nine per cent of aspirin users had to be specifically prompted in order to state its use, in comparison to 35.7%, 25% and 0% of clopidogrel, warfarin, and NSAID users, respectively, and in any case it was independent of their age, education level and the duration of its use (Tables 2 & 3, Figure 1). Surprisingly, 50% of patients receiving warfarin were not aware of its effect on coagulation in comparison to 32.3%, 21.4% and 20% of those taking aspirin, clopidogrel, and NSAIDs, respectively (Table 2). Again, patients’ awareness of this effect was independent of their age, education level and the time length they were using it (Table 4, Figure 2).
4. Discussion
The aim of this study was firstly to evaluate the rate of any type of antiplatelet/anticoagulant drug use in urological inpatients and secondly to assess patients’ awareness
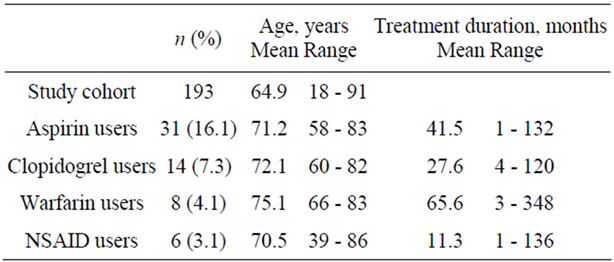
Table 1. Characteristics of the study population.

Table 2. Patients receiving any type of drug affecting coagulation who had to be specifically asked for using it and their knowledge of its side effect on coagulation.
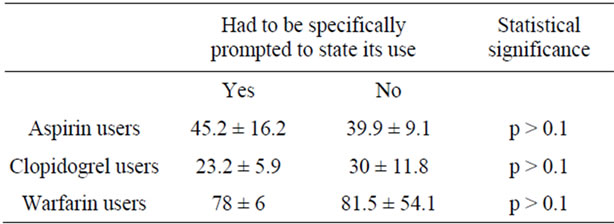
Table 3. Duration of drug use (months; mean ± SE) and need of prompting to state the use of drugs.
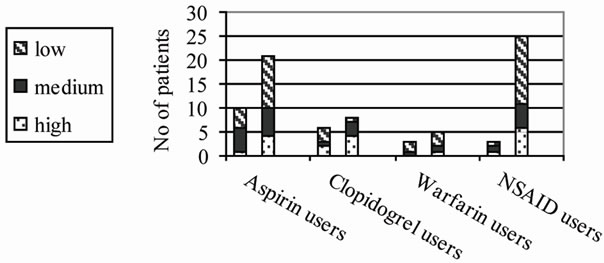
Figure 1. Education level distribution and need of prompting to state the use of drugs (left column in each category): no correlation, p > 0.1.

Table 4. Duration of drug use (months; mean ± SE) and knowledge of drug effect on coagulation.

Figure 2. Education level distribution and knowledge of drug effect on coagulation (left column in each category): no correlation, p > 0.1.
of their effect on coagulation in relation to probable confounding factors such as age, education background and the treatment duration. Knowledge of their use is important in surgical everyday practice, since many urologists prefer to cease them prior to even minor elective interventions in anticipation of difficulties in surgical haemostasis [14-16], while regional anesthesia, which is frequently the method of choice for many urological procedures, could be complicated [20]. Aspirin and NSAIDs result in irreversible and reversible inhibition of platelet cyclooxygenase, respectively, that impairs the thromboxane-dependent platelet aggregation. Clopidogrel is a novel platelet aggregation inhibitor that acts on the adenosine diphosphate receptor and thus inhibiting the ADP-induced platelet aggregation [10]. However, routine screening of coagulation profiles may not unveil platelet dysfunction [11] that can be revealed only by performing specific tests such as the platelet function analyzer (PFA- 100) test [21]. Warfarin inhibits the hepatic synthesis of vitamin K-dependent clotting factors (II, VII, IX, and X) [22]. Most reports on the frequency of bleeding complications in the perioperative period in aspirin users, albeit of low statistical power, show an increase by approximately 50%. In a recent study it was shown that oral anticoagulation had a significant and independent impact on TURP outcome in terms of bleeding complications [23]. However, with the exception of intra-cranial and transurethral prostate surgery, the bleeding complications were no more severe in patients taking aspirin. In contrast, several studies report that, with discontinuation of low-dose aspirin, hazardous events such as stroke, myocardial infarction or even cardiovascular death may occur [8]. A considerable proportion (38%) of British urologists do not ask their patients to stop aspirin prior to TUR-P; 90% of them believe that the cardiovascular benefits of aspirin outweigh the urological risks while 58% of them believe that it does increases blood loss [14]. As far as urological guidelines are concerned, the only ones dealing with the matter are those of the German Urological Society where it is stated that aspirin is not a priori a contraindication for surgery [24].
As expected, the vast majority of our study group were of middle age or older, mainly men, that represent the part of the population in which the use of long-term antithrombotic therapy for primary or secondary prevention of coronary heart disease is, or should be, quite common. A large study has shown that 50% of men undergoing prostate surgery have concurrent hypertension and 20% have ischemic heart disease [25]; some of them might be expected to be under treatment with aspirin or another antithrombotic. Almost one-third of our patients were under some type of antithrombotic treatment (i.e. aspirin, clopidogrel or warfarin) or NSAIDs. Population-based studies have shown that as far as aspirin is concerned, the prevalence of its use is about 23% in U.S. and somehow lower in Europe [1,3]. In Ireland, 60% and 13% of patients admitted on acute medical call were receiving aspirin and warfarin, respectively [5]. There are no other reports on the prevalence of its use in urological patients with the exception of that from Denmark, where 21% of patients undergoing transurethral prostatectomy (TUR-P) during the years 1992-1994 were using aspirin and/or NSAIDs [4].
Clinical trials showing long-term benefits for antiplatelet users have probably influenced both clinical practice and the public behavior [1,2] that in turn has resulted in an increase in aspirin use even in low cardiovascular risk groups [6,7].
A considerable proportion of our patients did not state using aspirin (29%), clopidogrel (35.7%) or warfarin (25%) when their medical history was taken and had to be specifically prompted to do so. Previous studies investigating patient ability to recall their medications correctly are limited and their results vary significantly. Two studies conducted in the emergency department setting showed that 48% and 42.8% of the patients, respectively, could recall all of their medications [26,27]. In the general practice setting the corresponding data vary significantly from 10.9% [28] to 85% [29]. Fifty per cent [30] and 31% [31] of hospital patients interviewed immediately after consultation made errors in recalling their prescription drugs. These poor outcomes have been attributed to low education level [28], while others implicate increasing age, lower household income and multiple drug use [29]. In our study, prompt recalling of taking antiplatelet/anticoagulants was independent of patient age, education level and treatment duration; we cannot provide data in relation to household income and the total number of drugs used. However, increasing age was independent of correct recalling in some studies [26,30] or even significantly correlated to it [28]. As far as education level is concerned, the relationship found by others [28] could be attributed to the quite high proportion (15%) of patients with no formal education; no such patients were present in our group.
Even though one could not expect all patients to be fully aware of their medications, lack of knowledge of specific probable side effects (such as that on coagulation) could lead to serious adverse events. Patients on oral anticoagulants have been found to have significant knowledge gaps [32], a fact that, in a particular study, was related to age and treatment duration [33]. Data from the same study showed that illiteracy was the main reason for not reading the information booklet on warfarin. In our study, 50% of patients receiving warfarin were not aware of its effect on coagulation in comparison to 32.3%, 21.4% and 20% of those taking aspirin, clopidogrel, and NSAIDs, respectively. Again, patients’ awareness of this effect was independent of their age, education level and the time length they were using it, indicating a significant gap in patient education that has to be further analyzed and finally, to be properly confronted. A limitation of our study is that it does not provide data on whether these patients were ever informed on the matter by the prescribing physician or misunderstood the risk associated with their use. Although patients’ understanding of risk is (or rather should be) an essential component of shared decision making [34], risk perception may be different in patients and health professionals [18]. As far as anticoagulants and anti-inflammatory drugs are concerned, health professionals rank them as carrying the highest risk, while ranking aspirin sixth in a list of 13 categories [19]. The perceived risk of hemorrhage is reported as high among warfarin users, and quite low among aspirin and other antiplatelet users [5].
Despite differences in urologists’ attitude towards cessation or not of antiplatelet/anticoagulants prior to diagnostic or therapeutic interventions, we should be aware of the high use of such agents by urological patients. In absence of multidisciplinary guidelines, urologists should be at least aware that not all patients are aware of their effect on coagulation, while some even fail to report their use and have to be specifically prompted. Other health care providers prescribing these agents should also inform their patients more thoroughly on possible adverse drug reactions, especially in relation to possible surgical interventions.
REFERENCES
- E. Shahar, A. R. Folsom, F. J. Romm, K. M. Bisgard, P. A. Metcalf, L. Crum, P. G. McGovern, R. G. Hutchinson, and G. Heiss for the ARIC Study Investigators, “Patterns of Aspirin Use in Middle-Aged Adults: The Atherosclerosis Risk in Communities (ARIC) Study,” American Heart Journal, Vol. 131, No. 5, 1996, pp. 915-922. doi:10.1016/S0002-8703(96)90173-8
- N. L. Smith, B. M. Psaty, C. D. Furberg, et al., “Temporal Trends in the Use of Anticoagulants among Older Adults with Atrial Fibrillation,” Archives of Internal Medicine, Vol. 159, No. 14, 1999, pp. 1574-1578. doi:10.1001/archinte.159.14.1574
- N. Rodondi, J. Cornuz, P. Marques-Vidal, et al., “Aspirin Use for the Primary Prevention of Coronary Heart Disease: A Population-Based Study in Switzerland,” Preventive Medicine, Vol. 46, No. 2, 2008, pp. 137-144. doi:10.1016/j.ypmed.2007.08.006
- F. S. Wierod, N. J. Frandsen, J. D. Jacobsen, et al., “Risk of Haemorrhage from Transurethral Prostatectomy in Acetylosalicylic Acid and NSAID-Treated Patients,” Scandinavian Journal of Urology and Nephrology, Vol. 32, No. 2, 1998, pp. 120-122. doi:10.1080/003655998750014495
- G. Cullen, E. Kelly and F. E. Murray, “Patients’ Knowledge of Adverse Reactions to Current Medications,” British Journal of Clinical Pharmacology, Vol. 62, No. 2, 2006, pp. 232-236. doi:10.1111/j.1365-2125.2006.02642.x
- Collaborative Group of the Primary Prevention Project, “Low Dose Aspirin and Vitamin E in People at Cardiovascular Risk: A Randomized Trial in General Practice,” Lancet, Vol. 357, No. 9250, 2001, pp. 89-95. doi:10.1016/S0140-6736(00)03539-X
- E. V. Hersh, P. A. Moore and G. L. Ross, “Over-theCounter Analgesics and Antipyretics: A Critical Assessment,” Clinical Therapeutics, Vol. 22, No. 5, 2000, pp. 500-548. doi:10.1016/S0149-2918(00)80043-0
- W. Burger, J.-M. Chemnitius, G. D. Kneissl and G. Rucker, “Low-Dose Aspirin for Secondary Cardiovascular Prevention—Cardiovascular Risks after Its Perioperative Withdrawal versus Bleeding Risks with Its Continuation—Review and Meta-Analysis,” Journal of Internal Medicine, Vol. 257, No. 5, 2005, pp. 399-414. doi:10.1111/j.1365-2796.2005.01477.x
- S. K. Samra, R. L. Harrison, D. E. Bee and V. Valero, “A Study of Aspirin Induced Changes in Bleeding Time, Platelet Aggregation, and Sonoclot Coagulation Analysis in Humans,” Annals of Clinical & Laboratory Science, Vol. 21, No. 5, 1991, pp. 315-327.
- D. A. Payne, P. D. Hayes, C. I, Jones, P. Belham, A. R. Naylor and S. Bjorkman, “Combined Therapy with Clopidogrel and Aspirin Significantly Increases the Bleeding Time through a Synergistic Antiplatelet Action,” Journal of Vascular Surgery, Vol. 35, No. 6, 2002, pp. 1204-1209. doi:10.1067/mva.2002.122027
- J. R. Sonksen, K. L. Kong and R. Holder, “Magnitude and Time Course of Impaired Primary Haemostasis after Stopping Chronic Low and Medium Dose Aspirin in Healthy Volunteers,” British Journal of Anaesthesia, Vol. 82, No. 3, 1999, pp. 360-365.
- L. D. Fiore, M. T. Brophy, A. Lopez, P. Janson and D. Deykin, “The Bleeding Time Response to Aspirin. Identifying the Hyperresponder,” American Journal of Clinical Pathology, Vol. 94, No. 3, 1990, pp. 292-296.
- P. Peterson, T. E. Hayes, C. F. Arkin, et al., “The Preoperative Bleeding Time Test Lacks Clinical Benefit: College of American Pathologists’ and American Society of Clinical Pathologists’ Position Article,” Archives of Surgery, Vol. 133, No. 2, 1998, pp. 134-139. doi:10.1001/archsurg.133.2.134
- M. K. Enver, I. Hoh and F. I. Chinegwundoh, “The Management of Aspirin in Transurethral Prostatectomy: Current Practice in the UK,” Annals of the Royal College of Surgeons of England, Vol. 88, No. 3, 2006, pp. 280-283. doi:10.1308/003588406X95084
- J. Masood, A. Hafeez, J. Calleary and J. M. Barua, “Aspirin Use and Transrectal Ultrasonography-Guided Prostate Biopsy: A National Survey,” British Journal of Urology International, Vol. 99, No. 5, 2007, pp. 965-966. doi:10.1111/j.1464-410X.2006.06671.x
- S. E. J. Connor and J. P. Wingate, “Management of Patients Treated with Aspirin or Warfarin and Evaluation of Haemostasis Prior to Prostatic Biopsy: A Survey of Current Practice amongst Radiologists and Urologists,” Clinical Radiology, Vol. 54, No. 9, 1999, pp. 598-603. doi:10.1016/S0009-9260(99)90022-3
- M. Radhamanohar, M. Than and S. Rizvi, “Assessment of Patients’ Knowledge about Their Illness and Treatment,” The British Journal of Clinical Practice, Vol. 47, No. 1, 1993, pp. 23-25.
- J. K. Aronson, “Risk Perception in Drug Therapy,” British Journal of Clinical Pharmacology, Vol. 62, No. 2, 2006, pp. 135-137. doi:10.1111/j.1365-2125.2006.02739_1.x
- V. Bongard, S. Menard-Tache, H. Bagheri, et al., “Perception of the Risk of Adverse Drug Reactions: Differences between Health Professionals and Non Health Professionals,” British Journal of Clinical Pharmacology, Vol. 54, No. 4, 2002, pp. 433-436. doi:10.1046/j.1365-2125.2002.01674.x
- S. Mak and P. Amoroso, “Stop Those Antiplatelet Drugs before Surgery!” British Journal of Urology International, Vol. 91, No. 7, 2003, pp. 593-594. doi:10.1046/j.1464-410X.2003.04209.x
- R. Karger, N. Donner-Banzhoff, H. H. Muller, V. Kretschmer and M. Hunink, “Diagnostic Performance of the Platelet Function Analyzer (PFA-100) for the Detection of Disorders of Primary Haemostasis in Patients with a Bleeding History—A Systematic Review and Meta-Analysis,” Platelets, Vol. 18, No. 4, 2007, pp. 249-260. doi:10.1080/09537100601100366
- J. Hirsh, J. E. Dalen, D. R. Anderson, et al., “Oral Anticoagulants Mechanism of Action, Clinical Effectiveness, and Optimal Therapeutic Range,” Chest, Vol. 119, Suppl. 1, 2001, pp. 8-21. doi:10.1378/chest.119.1_suppl.8S
- A. Descazeaud, et al., “Impact of Oral Anticoagulationon on Morbidity of Transurethral Resection of the Prostate,” World Journal of Urology, Vol. 29, No. 2, 2011, pp. 211-216. doi:10.1007/s00345-010-0561-3
- V. Kretschmer, “Perioperative Risk of Haemorrhage in Urological Operations Caused by Administration of Aspirin,” Urologe A, Vol. 37, No. 6, 1998, p. 675.
- J. H. Wasson, D. J. Reda, R. C. Bruskewitz, J. Elinson, A. M. Keller and W. G. Henderson, “A Comparison of Transurethral Surgery with Watchful Waiting for Moderate Symptoms of Benign Prostatic Hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate,” The New England Journal of Medicine, Vol. 332, No. 2, 1995, pp. 75-79. doi:10.1056/NEJM199501123320202
- G. M. Vike, A. Marino, J. Iskander and T. C. Chan, “Emergency Department Patient Knowledge of Medications,” The Journal of Emergency Medicine, Vol. 19, No. 4, 2000, pp. 327-330. doi:10.1016/S0736-4679(00)00257-2
- M. K. Chung and J. M. Bartfield, “Knowledge of Prescription Medications among Elderly Emergency Department Patients,” Annals of Emergency Medicine, Vol. 39, No. 6, 2002, pp. 605-608. doi:10.1067/mem.2002.122853
- A. Akici, S. Kalaca, M. U. Ugurlu, H. Z. Toklu, E. Iskender and S. Oktay, “Patient Knowledge about Drugs Prescribed at Primary Healthcare Facilities,” Pharmacoepidemiology and Drug Safety, Vol. 13, No. 12, 2004, pp. 871-876. doi:10.1002/pds.1020
- C. Jaye, J. Hope and I. R. Martin, “What Do General Practice Patients Know about Their Prescription Medications?” New Zealand Medical Journal, Vol. 115, No. 1162, 2002, p. 183.
- D. S. Brody, “An Analysis of Patient Recall of Their Therapeutic Regimens,” Journal of Chronic Diseases, Vol. 33, No. 1, 1980, pp. 57-63. doi:10.1016/0021-9681(80)90086-7
- T. Silva, E. P. Schenkel and S. S. Mengue, “Information Level about Drugs Prescribed to Ambulatory Patients in a University Hospital,” Cadernos de Saúde Pública, Vol. 16, No. 2, 2000, pp. 449-455. doi:10.1590/S0102-311X2000000200015
- S. Jank, T. Bertsche, W. Herzog and W. E. Haefeli, “Patient Knowledge on Oral Anticoagulants: Results of a Questionnaire Survey in Germany and Comparison with the Literature,” International Journal of Clinical Pharmacology and Therapeutics, Vol. 46, No. 6, 2008, pp. 280-288.
- E. O. Y. L. Tang, C. S. M. Lai, K. K. C. Lee, R. S. M. Wong, G. Cheng and T. Y. K. Chan, “Relation between Patients’ Warfarin Knowledge and Anticoagulation Control,” The Annals of Pharmacotherapy, Vol. 37, No. 1, 2003, pp. 34-39. doi:10.1345/aph.1A198
- H. Thornton, “Patients’ Understanding of Risk,” British Medical Journal, Vol. 327, 2003, pp. 693-694. doi:10.1136/bmj.327.7417.693

