Journal of Materials Science and Chemical Engineering
Vol.03 No.06(2015), Article ID:57078,7 pages
10.4236/msce.2015.36007
Determination of Molecular Mass of Strong Acids by Differential Temperature Model (DTM) Using H3PO4 and HBF4 for Classical Demonstration
I. A. Akpan
Thermodynamics and Electrochemical Laboratory, Department of Chemistry, University of Uyo, Uyo, Nigeria
Email: iaakpanchem2007@yahoo.com


Received February 2015

ABSTRACT
A new chemical hypothesis based on the differential temperature model (DTM) for estimation of molecular masses of some strong acids (H2SO4, HNO3 and HCl) in solutions have previously been propounded and tested theoretically and analytically by the author. The results were published in the Bulletin of Pure and Applied Sciences?Chemistry in 2012. The changes in temperature following various dilutions of the acids were found to be proportional to their molecular properties. The new chemical hypothesis and model is hereby tested on H3PO4 and HBF4 and their exact molecular masses have been evaluated analytically and theoretically. The validity of the hypothesis and the model is hereby presented for chemical proof and adoption to theory by chemists.
Keywords:
Differential Temperature, Molecular Mass, Strong Acids

1. Introduction
Strong acids are strong electrolytes with high ionization potential in solution.
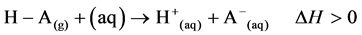 (1)
(1)
The reaction in the above chemical equation is an endothermic process. A lattice energy is absorbed by the reacting system and thus, is responsible for the ionisation of the acidic species in solution. Proton theory of acids developed by Bronsted and Bjerrum in Denmark and Lowry in England in 1923 defines acids as proton-trans- ferring species [1]. The proton discharged during ionisation process has a great tendency to be solvated being an empty orbital. It then implies that, a proton does not exist in a free state in solution but in the solvated form. Water molecule as a solvent, interacts with proton, forming a solvated ion known as an oxonium ion. Solvation reaction is an exothermic process, accompanied by evolution of hydration or solvation energy in the form of heat.
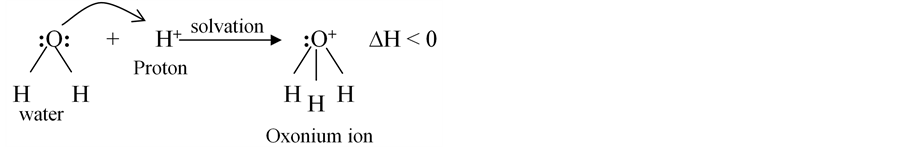 (2)
(2)
The extent of ionisation or dissociation of acids depends upon the intrinsic acidic strength and upon the degree of affinity of the solvent for proton (protophilicity). Hence all acids which ionise completely in solution at finite dilution are called strong acids.
2. Methods of Determination of Molecular Mass
Hitherto, several methods abound for the determination molecular mass. Physical methods include measurements via colligative properties in ideal solution [2], diffusion rates, etc. Analytical methods make use of titrimetry which depends on the stoichiometric relationship between the amount of the standard solution and that of the analyte (solute). The volume-mass relationship of the analyte is then used in the determination of molecular mass [3].
Instrumental methods make use of electronic instrument such as mass spectrometry, X-ray diffraction analysis, viscometer etc. [4] [5].
Apart from the high financial costs associated with procurement of most apparatus used for the determination of molecular properties in physical chemistry laboratories spectrophotometric equipment such as mass spectrophotometer and X-ray diffraction machines often break down and remain obsolete due to electric current fluctuations which characterise our economy.
The author’s interest is to study the thermochemical potentials in solutions of chemical substances and to develop hypothesis in order to devise alternative methods of determining their molecular properties without recourse to sophisticated instruments.
Attention is first focused on strong acids and bases because of their wide applications in chemical studies. Earlier research efforts in our electrochemical laboratory had led to the discovery of thermal constants of strong acids at various dilutions.
From the thermal constant discovered we had proposed an hypothesis and developed a valid mathematical model for the calculation of molecular mass of strong acids and tests their validity in HCl, HNO3 and H2SO4 [6].
The present work is yet a further application of the mathematical model for the determination of molecular masses of H3PO4 and HBF4.
2.1. The Propounded Hypothesis and Its Mathematical Implication
“At constant temperature and pressure, the differential temperature 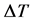 of the dilution of equal volumes of strong acids in a fixed volume of water is directly proportional to the product of the sum of the relative masses of the ionised species and the square of the basicity of the acid”.
of the dilution of equal volumes of strong acids in a fixed volume of water is directly proportional to the product of the sum of the relative masses of the ionised species and the square of the basicity of the acid”.
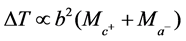 (3)
(3)
where  and
and 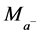 are the relative masses of the cation and anion species respectively and b, the basicity of the acid.
are the relative masses of the cation and anion species respectively and b, the basicity of the acid.
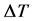 is the change in temperature between the maximum temperature attained after dilution and the initial temperature of the solvent (water) before dilution.
is the change in temperature between the maximum temperature attained after dilution and the initial temperature of the solvent (water) before dilution.
If a constant is introduced into the proportion in 1 above, we obtain
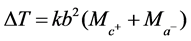 (4)
(4)
k is the thermal constant of strong acids at equivalent dilution at constant pressure and temperature
From Equation (2)
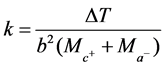 (5)
(5)
Method of evaluation of k for HBF4 and H3PO4 can apply to other strong acids.
2.2. Experimental
The solvent de-ionsied water and the solutes H3PO4 and HBF4 were purchased from BDH Limited. Both solutes were used as purchased without further purification. The reaction was carried out in a well-insulated vessel, known as the colorimeter as described elsewhere [7]. Being perfectly insulated, it could effectively measure the heat energy transferred during the reaction.
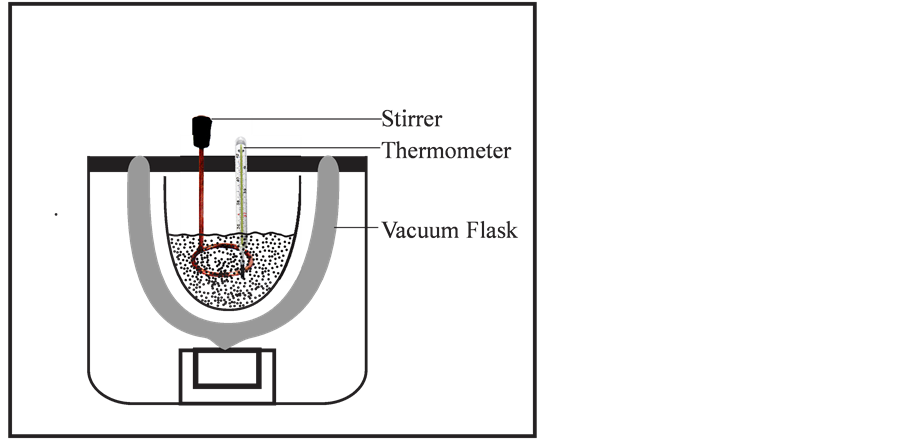
Dewar flask was used as calorimeter as shown in Figure 1, because it has a large heat capacity. The inner surface of the vessel was silvered and a space between the inner and outer wall was evacuated in order to minimise exchange of heat energy with the surrounding. A cork stopper was fitted at the top of the mouth and it contained a thermometer. The heat was measured in calories and converted to Joules. The gram-calorie is the amount of heat required to raise the temperature of 1 g of water through 1˚C. The amount of heat evolved in the process was measured as, mass of the system multiplied by rise in temperature, multiplied by specific heat of the system. Thermal constants and other thermochemical properties were evaluated and recorded.
3. Result and Discussion
3.1. Results
The results of the experiments are presented in Tables 1-4. Relevant plots are presented in Figure 1 and Figure 2.
3.2. Discussion
3.2.1. Thermal Mechanism of Fluoroboric Acid (HBF4)
The thermochemical process resulting from the ionisation of Fluoroboric Acid following the absorption of lattice energy attracts solvation of the proton with the evolution of heat energy according to the mechanism.
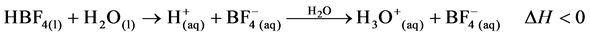 (6)
(6)
The thermal constant following the ionisation process is calculated as follows
Mass of solvated cation = 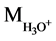 = 19, Mass of anion =
= 19, Mass of anion = 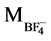 = 87
= 87
(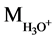 +
+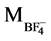 ) = (19 + 87) = 106
) = (19 + 87) = 106
Figure 1. Dewar flask for thermochemical measurements.
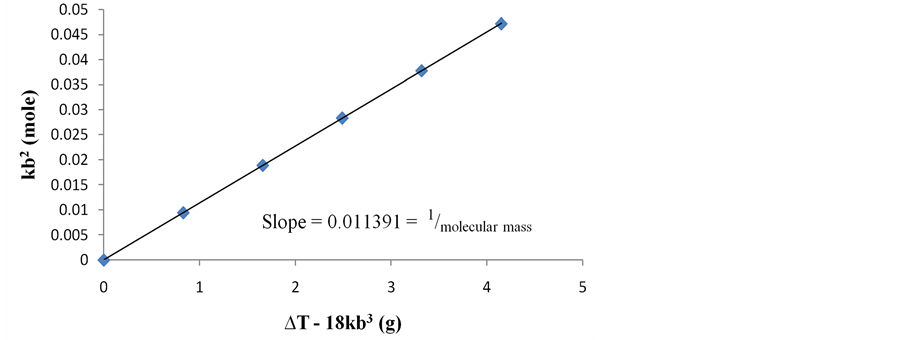
Figure 2. Variation of basic constant kb2(mole) versus thermohydrobasic constant ∆T ? 18 kb3 (g) for the determination of the molecular mass of Fluoroboric Acid (HBF4).
Table 1. Thermal constants k determined for Fluoroboric acid (HBF4 at various concentrations at constant temperature and pressure (25˚C and 1 atm).
Table 2. Values of basic constants (kb2) hydrobasic constants (18kb2) and thermohydrobasic constant ( T ? 18kb3) for Fluoroboric acid at various concentrations at constant temperature and pressure.
For HBF4, k = 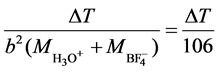
where b represents the basicity of 
From thermal constant, the value of basic constant kb2 and thermohydrobasic constant 
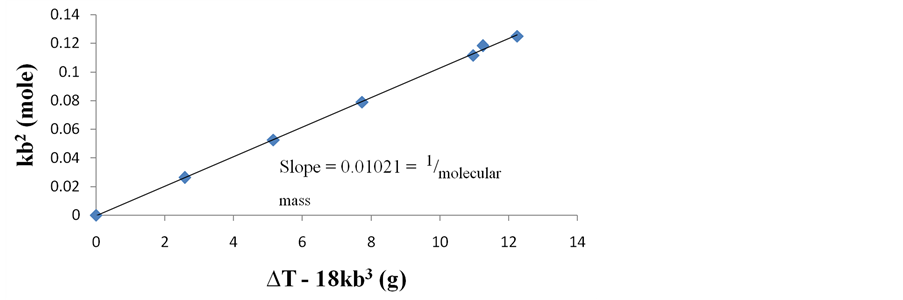
Figure 3. Variation of basic constant kb2 (mole) versus thermohydrobasic constant ∆T ? 18kb3 (g) for the determination of the molecular mass of Phosphoric Acid (H3PO4).
Table 3. Thermal constants for phosphoric acid (H3PO4) at various dilution at constant temperature and pressure (28˚C and 1atm).
Table 4. Values of basic constants (kb2), hydrobasic constants (18kb2) and thermohydrobasic constant ( T ? 18kb3) for phosphoric acid, at various dilution at constant temperature and pressure (28˚C and 1atm).
3.2.2. Thermal Mechanism of Phosphoric Acid H3PO4
The ionisation of H3PO4 catalysed by adsorption of lattice energy is followed by solvation which is an exothermic process, according to the mechanism.
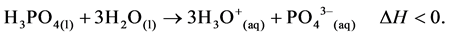
The thermal constant following the ionisation process is evaluated as follows:
Mass of solvated cation 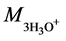
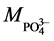
(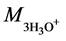
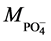
For H3PO4, k = 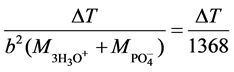
where b = 3 because the acid is tribasic.
Other related constants are evaluated from thermal constant as previously explained [8].
3.2.3. Determination of Molecular Mass by Differential Temperature Model (DTM)
The Differential Temperature 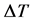
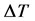
The plot of kb2 (mole) versus 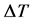
Similar plot for H3PO4 reveals a straight line from the origin with slope = 0.0102 which is the reciprocal of the molecular mass, such that the experimental molecular mass of 98.003 is obtained for H3PO4
4. Conclusion
The actual molecular masses of HBF4 and H3PO4 are 87.81 g∙mol−1 and 97.99 g∙mol−1 respectively. The result shows that both the propounded hypothesis and the Differential Temperature Model are valid for the determination of the molecular masses of strong acids.
5. Recommendation
The correspondent author, Dr. I. A. Akpan has propounded a new chemical hypothesis for chemical challenge. He has discovered a thermochemical model for the determination of the relative molecular masses of strong acids. The discovery has added to the list of physical methods available for the determination of molecular properties of substances. Additional chapter has been opened for numerous chemical calculations of molecular properties of strong acids in solution such as thermal constant, basic constant, hydrobasic constant and thermohydrobasic constants. The author expects that the relevant society of chemistry will subject this hypothesis and discovery to test to approve the hypothesis for chemical theory such that a new law credited to the author may come to nobel.
Acknowledgements
The author is grateful to his 1998 final year project students and 2013 third year students for their assistance in taking measurements. Financial assistance received from my wife is specially acknowledged. Useful discussions and advice received from my mentors, Prof. A. I. Onuchukwu (Professor of Physical and Industrial Chemistry), Prof. A. C. I. Anusiem (Professor of Physical Chemistry) and Prof. A. A. Ayuk (Professor of Theoretical and Quantum Chemistry) are also acknowledged.
Cite this paper
I. A. Akpan, (2015) Determination of Molecular Mass of Strong Acids by Differential Temperature Model (DTM) Using H3PO4 and HBF4 for Classical Demonstration. Journal of Materials Science and Chemical Engineering,03,41-47. doi: 10.4236/msce.2015.36007
References
- 1. Ebbing, D.D. and Gammon, S.D. (1999) General Chemistry. 6th Edition, Houghton Miffin Company; Boston, New York, 105, 320, 485.
- 2. Sharma, K.K. and Sharma, L.K. (1999) A Textbook of Physical Chemistry. 4th Rev. Edition, Vikas Publishing House, PVT Ltd. India, 160, 165-167.
- 3. Mendham, J., Denney, R.C. and Thomas, M.J.K. (2000) Vogel’s Textbook of Quantitative Chemical Analysis. 6th Edition, Person Education Limited, Edinburgh Gate, Harlow, Essex, 34-35
- 4. Mahan, B.H. (1980) University Chemistry. 3rd Edition, Addison-Wesley Publishing Company, Inc., Philippines, 248- 449.
- 5. Berry, S.R., Rice, S.A. and Ross, J. (2000) Physical Chemistry. 2nd Edition, Oxford Uni-versity Press, Oxford, New York, 35-38.
- 6. Akpan, I.A. (2012) Thermochemical Model for the Determination of the Relative Molecular Mass of Strong Acids from Heat of Solution. Bulletin of Pure and Applied Sciences, Vol. 31C - Chemistry (No.1), 93-100.
- 7. Sharma, K.K. and Sharma, D.S. (1982) An Introduction to Practical Chemistry. 1st Edition, Vikas Publishing House PVT Ltd. India, 254-255.
- 8. Akpan, I.A. (2012) Test for Ionization and Formation of Oxonium in Acid Solutions. Bulletin of Pure and Applied Sciences. Vol. 31c - Chemistry (No. 1), 11-14.