Perforation of the Nasal Septum and Nasal Ulcers29
Centre for PCR study and specific Leishmania culture.
Pending the outcome of specific cultures, empirical
therapy was replaced with liposomal amphotericin B 3
mg/kg/day (six weekly doses with a received dose of 900
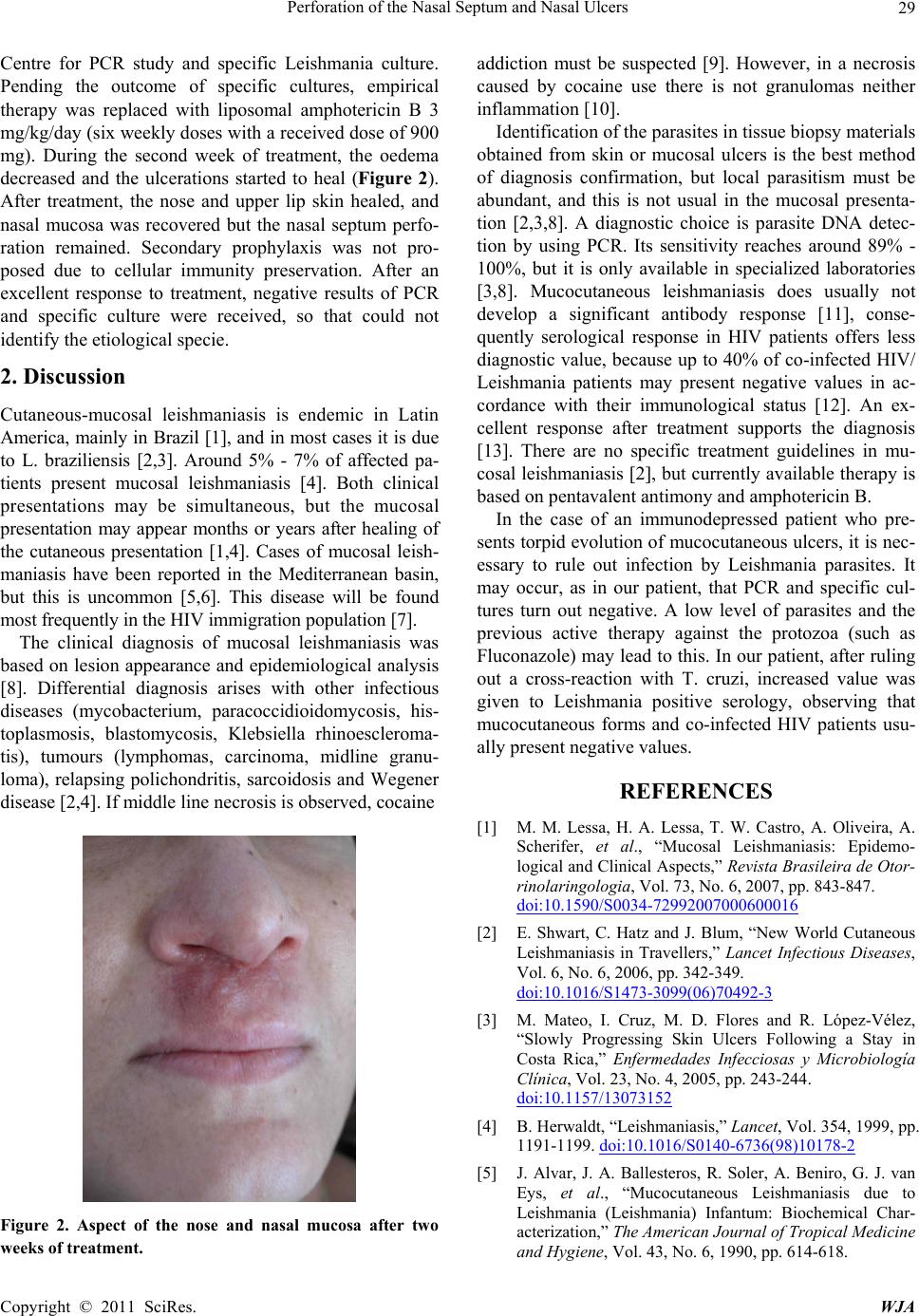
mg). During the second week of treatment, the oedema
decreased and the ulcerations started to heal (Figure 2).
After treatment, the nose and upper lip skin healed, and
nasal mucosa was recovered but the nasal septum perfo-
ration remained. Secondary prophylaxis was not pro-
posed due to cellular immunity preservation. After an
excellent response to treatment, negative results of PCR
and specific culture were received, so that could not
identify the etiolog ical specie.
2. Discussion
Cutaneous-mucosal leishmaniasis is endemic in Latin
America, mainly in Brazil [1], and in most cases it is due
to L. braziliensis [2,3]. Around 5% - 7% of affected pa-
tients present mucosal leishmaniasis [4]. Both clinical
presentations may be simultaneous, but the mucosal
presentation may appear months or years after healing of
the cutaneous presentation [1,4]. Cases of mucosal leish-
maniasis have been reported in the Mediterranean basin,
but this is uncommon [5,6]. This disease will be found
most frequentl y i n t he HIV immigrati on po pul at i on [7] .
The clinical diagnosis of mucosal leishmaniasis was
based on lesion appearance and epidemiological analysis
[8]. Differential diagnosis arises with other infectious
diseases (mycobacterium, paracoccidioidomycosis, his-
toplasmosis, blastomycosis, Klebsiella rhinoescleroma-
tis), tumours (lymphomas, carcinoma, midline granu-
loma), relapsing polichondritis, sarcoidosis and Wegener
disease [2,4]. If middle line necrosis is observed, cocaine
Figure 2. Aspect of the nose and nasal mucosa after two
weeks of treatment.
addiction must be suspected [9]. However, in a necrosis
caused by cocaine use there is not granulomas neither
inflammation [10].
Identification of the parasites in tissue biopsy materials
obtained from skin or mucosal ulcers is the best method
of diagnosis confirmation, but local parasitism must be
abundant, and this is not usual in the mucosal presenta-
tion [2,3,8]. A diagnostic choice is parasite DNA detec-
tion by using PCR. Its sensitivity reaches around 89% -
100%, but it is only available in specialized laboratories
[3,8]. Mucocutaneous leishmaniasis does usually not
develop a significant antibody response [11], conse-
quently serological response in HIV patients offers less
diagnostic value, because up to 40% of co-infected HIV/
Leishmania patients may present negative values in ac-
cordance with their immunological status [12]. An ex-
cellent response after treatment supports the diagnosis
[13]. There are no specific treatment guidelines in mu-
cosal leishmaniasis [2], but currently availab le therapy is
based on pentavalent antimony and amphotericin B.
In the case of an immunodepressed patient who pre-
sents torpid evolution of mucocutaneous ulcers, it is nec-
essary to rule out infection by Leishmania parasites. It
may occur, as in our patient, that PCR and specific cul-
tures turn out negative. A low level of parasites and the
previous active therapy against the protozoa (such as
Fluconazole) may lead to this. In our patient, after ruling
out a cross-reaction with T. cruzi, increased value was
given to Leishmania positive serology, observing that
mucocutaneous forms and co-infected HIV patients usu-
ally present negative values.
REFERENCES
[1] M. M. Lessa, H. A. Lessa, T. W. Castro, A. Oliveira, A.
Scherifer, et al., “Mucosal Leishmaniasis: Epidemo-
logical and Clinical Aspects,” Revista Brasileira de Otor-
rinolaringologia, Vol. 73, No. 6, 2007, pp. 843-847.
doi:10.1590/S0034-72992007000600016
[2] E. Shwart, C. Hatz and J. Blum, “New World Cutaneous
Leishmaniasis in Travellers,” Lancet Infectious Diseases,
Vol. 6, No. 6, 2006, pp. 342-349.
doi:10.1016/S1473-3099(06)70492-3
[3] M. Mateo, I. Cruz, M. D. Flores and R. López-Vélez,
“Slowly Progressing Skin Ulcers Following a Stay in
Costa Rica,” Enfermedades Infecciosas y Microbiología
Clínica, Vol. 23, No. 4, 2005, pp. 243-244.
doi:10.1157/13073152
[4] B. Herwaldt, “Leishmaniasis,” Lancet, Vol. 354, 1999, pp.
1191-1199. doi:10.1016/S0140-6736(98)10178-2
[5] J. Alvar, J. A. Ballesteros, R. Soler, A. Beniro, G. J. van
Eys, et al., “Mucocutaneous Leishmaniasis due to
Leishmania (Leishmania) Infantum: Biochemical Char-
acterization,” The American Journal of Tropical Medicine
and Hygiene, Vol. 43, No. 6, 1990, pp. 614-618.
Copyright © 2011 SciRes. WJA