World Journal of Vaccines
Vol. 2 No. 2 (2012) , Article ID: 19314 , 5 pages DOI:10.4236/wjv.2012.22012
Difference of the Pain during the DPT (Diphtheria-Pertussis-Tetanus) Vaccination*
![]()
1Pediatrics, Sempos Tokyo Takanawa Hospital, Tokyo, Japan; 2Department of Pediatrics, School of Medicine, Showa University, Tokyo, Japan.
Email: #yuitsuji@ybb.ne.jp
Received March 25th, 2012; revised April 22nd, 2012; accepted May 10th, 2012
Keywords: Diphtheria-pertussis-tetanus vaccine; Immunization pain; thimerosal; phenoxyethanol; questionnaire
ABSTRACT
Background: The objective of this study is to determine whether analyze of the infant’s pain associated with diphtheria-pertussis-tetanus (DPT) immunization is useful for vaccination in children. It is not known whether the immunization pain can be prevented with the adequate choice of DPT vaccines among several manufacturers in Japan. Further, it is not clear whether the difference of the reaction during vaccination between gender and age. Design: Three manufacturer’s Japanese DPT vaccines were used in this study. The parents assessed their infant’s pain on a modified visual analogue scale (MVAS), the start of the crying and total crying time during the immunization. Results: The A manufacturer’s DPT vaccine was significantly lower on the proportion of crying, the duration of crying and MVAS score than the other two manufacturer’s DPT vaccines. The proportion of crying, the duration of crying and MVAS score was lower in the boy than in the girl. On the other hand, it had not found the difference of their reactions with age. Conclusions: Our studies have found that the adequate choice of DPT vaccine decreased vaccination pain. The studies also indicate that some tendencies with vaccinations were shown in children. To consider these tendencies was useful in performing less painful vaccination.
1. Introduction
DPT vaccine is a most common vaccine in Japan. The current schedule as recommended by the Japan Pediatric Society calls for four times of DPT vaccine injections from 3 months to 19 months. Recently in Japan, Haemophilus influenzae type b vaccine was authorized in 2008 and pneumococcal vaccine was also authorized in 2010. The introductions of new vaccines are also planned in future in Japan. These resulted in increasing the opportunities of vaccination. There are not a few children would cry before vaccination, nor want to go to medical institution. We must make allowance for the possible measures that might prevent a guardian from taking a child to medical facilities for vaccination. Vaccination is one of the important medical acts to a pediatrician and to occupy the large percentage of medical practices in the pediatric section. From the point of these views, we have to consider the decreasing vaccination pain. We think that it is useful to evaluate various pain-reducing methods and to try the method of the pain reducing during infant’s immunization. Reducing the pain of childhood vaccination an evidence-based clinical practice guideline has been issued from Canada in 2010 [1]. Then we started our study in DPT vaccination which is most frequency vaccine in Japan for the purpose of contributing to the alleviation of pain during vaccination. For the simply verification, the object of this study was only the single DPT vaccination. And for the objectivity, this study was performed by a questionnaire which was obtained from parent, not from our medical stuff. We wanted to determine if this varies with manufacturers, genders, age and times of DPT vaccination.
2. Methods
The study was conducted from October, 2008 through September, 2011.
2.1. DPT Vaccine
The setting of this study was single-center at pediatric outpatient clinic, Sempos-Tokyo-Takanawa Hospital in Minato-ku, Tokyo, Japan. The vaccination doctor limited to three pediatricians for the reducing the difference by the maneuver.
2.1.1. Comparison among Three Manufacturers of DPT Vaccine
DPT vaccine (Adsorbed Diphtheria-Purified PertussisTetanus Combined Vaccine): We used three types of DPT vaccines from different manufacturers in Japan (A: Takeda, B: Kaketsuken-Astellas, C: Kitasato-DaiichiSankyo).
These three vaccines are almost same effective component without additions. A-manufacturer vaccine contains phenoxyethanol without thimerosal. B-manufacturer vaccine contains thimerosal without phenoxyethanl. C-manufacturer vaccine has not contains phenoxyethanl nor thimerosal. These differences are described in (Table 1).
65 children were enrolled on A-manufacturer vaccine, 76 children were enrolled on B-manufacturer vaccine and 44 children were enrolled on C-manufacturer vaccine. Total 185 children were enrolled on this study.
2.1.2. Comparison among Times, Genders and Ages
On this comparison, we only used A-makers vaccine. We enrolled 235 (116: boys, 119: girls) children. Genders were divided into boy and girl. Ages were divided into seven groups: 1) 3 months: 18 children, 2) 4 - 6 months: 158 children, 3) 7 - 9 months: 27 children, 4) 10 - 12 months: 0 child, 5) 13 - 18 months: 11 children, 6) 19 - 24 months: 21 children, 7) over 25 months: 5 children. Times of DPT vaccination were divided into first to fourth.
2.2. Participants
Healthy infants 3 to 24 months of age undergoing routine immunization. We excluded infants with acute febrile illness, chronic medical conditions, and allergy to any of the vaccine components. We did not use of the systemic analgesia (e.g., ibuprofen, acetoaminophen) and sucrose before the vaccination. Participants were enrolled from October, 2008 through September, 2011.
Main outcome measures: All results were obtained from questionnaire which was signed by the guardian who attended the infant being vaccinated. Pediatricians,
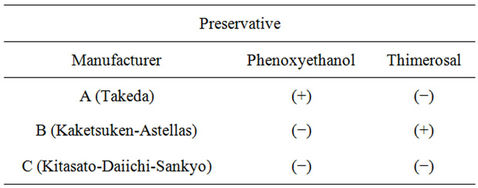
Table 1. The differences of preservative among three manufacturers on phenoxyethanol and thimerosal.
nurses and other medical staffs had not participated in the reply of this questionnaire. The questionnaire was presented after the immunization by the guardian. This questionnaire was given in (Figure 1). On this questionnaire, the primary outcome was infant pain during vaccine injection as assessed by modified visual analog scale (MVAS) which was made out for this purpose. Crying (yes/no, duration and when started) was also fill out. There were three items of the questions; 1) whether crying or not and when started if crying, 2) duration of crying, 3) facial pain response. We regulated the rank of start and duration of crying (Figure 1). Guardian rated pain using our MVAS, where the left anchor denoted “no cry and no change of facial expression” and right anchor denoted “crying hard and worst possible pain”. Informed, signed consent was obtained from guardian. This observation began with the entry into the consulting room and ended the leaving the room or when stopped crying.
The immunization procedure was standardized. Infants were held during vaccination by the guardian. During injection, the needle was inserted subcutaneously at about 45 with steady pressure in the anterolateral aspect of the left or right upper arm. The vaccine material was rapidly injected within 1 to 2 seconds followed by rapid withdrawal of the needle. All of the infants were immunized using a 26-gauge, 22 mm needle. We excluded infants with acute febrile illness, chronic medical conditions, and allergy to any of the vaccine components. This study was non-intervention and non-aggression, but we got the approval of the Ethical Review Board including the outside well-informed person in our hospital, because it was a clinical study to handle personal information. We selected the statistical significance level was P < 0.05. The official approval used t-test and One-way Factorial ANOVA test. All analyses were conducted using a commercially available software program (Stat View, version 5.0; Abacus Concepts Inc, California, USA).
3. Results
We found that the analysis among manufacturers showed
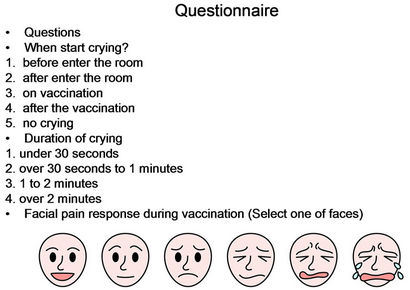
Figure 1. Questionnaire to guardian.
the crying proportion, duration and facial pain response score were significantly lower when using of the Amanufacturer vaccine than other manufacturers (Table 2). In addition, the analysis between genders showed that the crying proportion, duration and facial pain response score were significantly lower in boys than in girls (Table 3). Furthermore, the analysis between ages and times of DPT vaccination showed that the crying proportion, duration and facial pain response score were not significantly differences (Tables 4 and 5).
4. Discussion
This study investigated the adequate choice of DPT vaccine decreased vaccination pain in Japan. Jackson et al. reported that routine vaccination results in significant levels of distress in children and no evidence on clinical benefit of using with acetaminophen or ibuprofen for prevention of local reactions [2]. Infant pain response during routine subcutaneous vaccine injection was affected by the order of administration of the vaccine. In
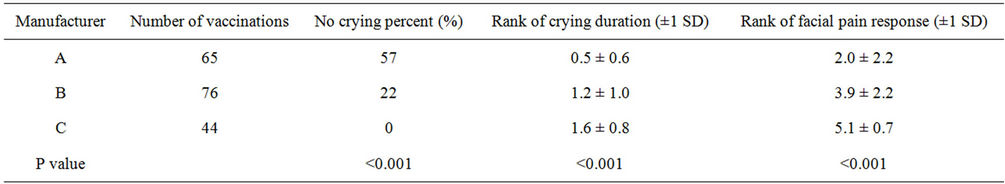
Table 2. The differences of the state of crying and facial pain response among three manufacturers.
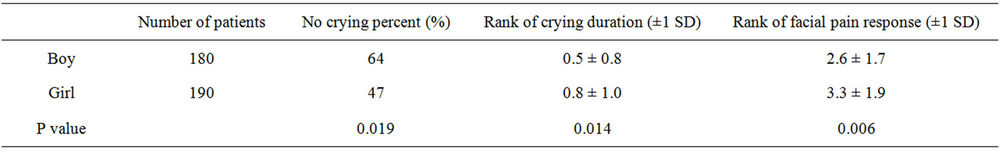
Table 3. The differences of the state of crying and facial pain response between boy and girl.
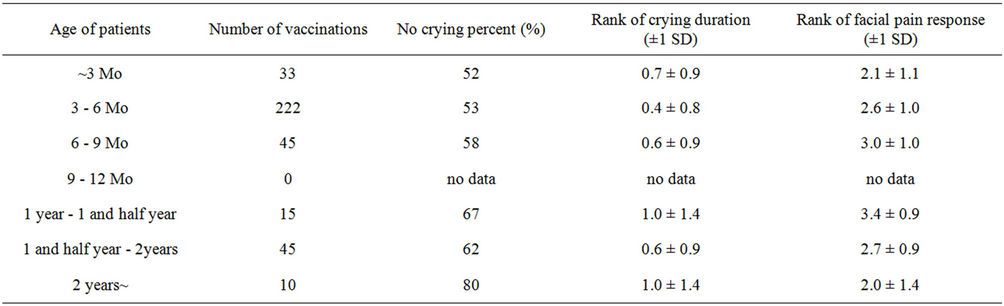
Table 4. The differences of the state of crying and facial pain response among age of patients.
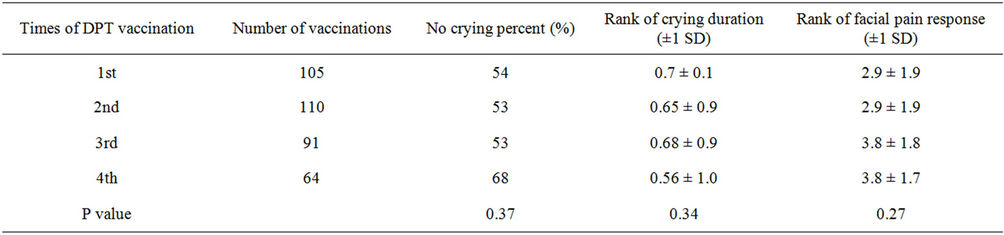
Table 5. The differences of the state of crying and facial pain response among times of DPT vaccination.
this study of children receiving their fourth consecutive DPT vaccination, we found a significant reduction in pain among children assigned to A-manufacturer’s DPT vaccine compared with those assigned to other manufacturers’ DPT vaccines. The three types of DPT vaccines used in this study have differences on additions (Table 1). The reason for the pain differences is presumed to be related to the physicochemical properties of the vaccine, especially additions in the vaccine. Terada et al. reported that the difference of pain between with thimerosal or phenoxyethanol [3]. In view of the reducing vaccinial pain, our data suggest that the least painful vaccine should be administered between several types of DPT vaccines in Japan. Minimizing the pain of vaccine injection experienced by infants and children is currently receiving considerable attention by clinicians. Woodin et al. described that continued education and reassurance of parents and physicians is needed to address concerns about children becoming “pincushions” from immunizations [4]. Ipp et al. reported that the order of vaccine reduced the pain of vaccine [5]. Cohen et al. described that distraction was more economical than eutectic mixture of local anesthetics [6]. Jacobson et al. reported that a significant proportion of children suffer substantial pain and distress from vaccination [7]. Piira et al. reported that parental behavior in the treatment room has a key role in influencing how infants respond to painful procedures [8]. In a recent study of pediatricians in the United States, parental vaccine refusal is commonly happened. Therefore, reductions in vaccination pain have the potential to improve compliance with the vaccination schedule. Further, it could prevent the prevalence of vaccine-preventable infections. Varying the order of vaccine administration to reduce pain is a strategy that is simple and effective, very low cost, and easily incorporated into clinical practice. In considering methods of reducing pain with vaccination, vaccine manufacturers have to play a more integral role in attempting to produce vaccine formulations that are less painful. We also conclude that boys were more patience than girls on this study. The cause of this result is unclear but we have to take care of girls more than boys during vaccination. Humphrey et al. found no association of gender with venipuncture distress [9]. Our study was based on the questionnaire from guardian. Similar papers which deal with vaccination pain were performed by the nurse [6-8]. Our aim of this study was assessment from patients and their guardian who had no interest in manufacturers completely. But usually, guardian was not self-possession at the vaccination of their infant. Therefore, the answers may not be accurate. On this point, more research is needed. Further, we think that more study is necessary to determine why becoming the difference of pain among DPT vaccines.
On the basis of our results, on less pain vaccination, selection of DPT vaccine among manufacturers in Japan and the adequate care for girls are important for children during vaccination.
5. Acknowledgements
The authors appreciate the constructive comments of Yoshifusa Abe M.D.
REFERENCES
- A. Taddio, A. Appleton, R. Bortolussi, et al., “Reducing the pain of Childhood Vaccination: An Evidence-Based Clinical Practice Guideline (Summary),” Canadian Medical Association Journal, vol. 182, no. 18, 2010, pp. 1989- 1995. doi:10.1503/cmaj.092048
- L. A. Jackson, M. Dunstan, P. Starkovich, J. Dunn, O. Yu, J. C. Nelson, T. Rees and A. Zavitkovsky, “Prophylaxis with acetaminophen or ibuprofen for prevention of local reactions to the Fifth Diphtheria-Tetanus Toxoids-Acellular Pertussis Vaccination: A Randomized, Controlled Trial,” Pediatrics, Vol. 117, No. 3, 2006, pp. 620-625. doi:10.1542/peds.2005-1217
- K. Terada, M. Inoue, T. Yamaguchi, et al., “Difference of response in an Inoculation Site and pain between vaccines with thimerosal or phenoxyethanol,” Infection and Immunity in childhood, Vol. 22, No. 2, 2010, pp. 145- 150.
- K. A. Woodin, L. E. Rodewald, S. G. Humiston, M. S. Carges, S. J. Schffer and P. G. Szilagyi, “Are children Becoming Pincushions from immunizations?” Archives of Pediatrics & Adolescent Medicine, Vol. 149, No. 8, 1995, pp. 845-849. doi:10.1001/archpedi.1995.02170210019003
- M. Ipp, P. C. Parkin, N. Lear, M. Goldbach and A. Taddio, “Order of vaccine injection and Onse,” Archives of Pediatrics & Adolescent Medicine, Vol. 163, No. 5, 2009, pp. 469-472. doi:10.1001/archpediatrics.2009.35
- L. L. Cohen, R. L. Blount, R. J. Cohen, E. R. Schaen and J. F. Zaff, “Comparative study of distraction versus topical anesthesia for Pediatric Pain Management during immunizations,” Health Psychology, Vol. 18, No. 6, 1999, pp. 591-598. doi:10.1037/0278-6133.18.6.591
- R. M. Jacobson, A. Swan, A. Adegbenro, S. L. Ludington, P. C. Wollan and G. A. Poland, “Making vaccines more Acceptable-Methods to prevent and minimaize pain and other common adverse events associated with vaccines,” Vaccine, Vol. 19, No. 17-19, 2001, pp. 2418-2427. doi:10.1016/S0264-410X(00)00466-7
- T. Piira, G. D. Champion, T. Bustos, N. Donnelly and K. Lui, “Factors associated with infant pain following an immunization injection,” Early Human Development, Vol. 83, No. 5, 2007, pp. 319-326. doi:10.1016/j.earlhumdev.2006.06.007
- G. B. Humphrey, C. M. Boon, G. F. van Linden van den Heuvell and H. B. van de Wiel, “The occurrence of High Levels of Acute Behavioral Distress in children and adolescents undergoing routine venipunctures,” Pediatrics, Vol. 90, No. 1, 1992, pp. 87-91.
Abbreviation
DPT vaccine—adsorbed diphtheria-purified pertussistetanus combined vaccine,
NOTES
*Financial disclosure: No financial relationships relevant to this article to disclose.
Conflict of Interest statement: No conflict of interest.
#Corresponding author.

