Paper Menu >>
Journal Menu >>
 Advances in Chemical Engi neering and Science , 2011, 1, 61 -64 doi:10.4236/aces.2011.12010 Published Online April 2011 (http://www.SciRP.org/journal/aces) Copyright © 2011 SciRes. ACES Prospect of Natural Gas Utilization in China Wei Huang1, Hongjun You2 1School of En vironment and Biological Engineering, Liao Ning Shihu a University, Fushun, China 2Southern Alberta In stitute of Technology Polytechnic, Calgary, Canada E-mail: youhongjun@hotmail.com Received February 15 , 20 11; revised Marc h 21 , 20 1 1; accepted March 28, 2011 Abstract This paper introduces the source and utility circumstance of natural gas in China. The utility circumstance of natural gas is discussed in detail, such as, natural gas as feedstock is produced to acetylene; natural gas is used to cut metal; natural gas is applied to generate ethylene; natural gas instead of heavy oil is fired to pro- duce chromium trioxide. In so doing, the energy demand of China will be better met with least damage to the environment. According to the full utility of natural gas sources, their good economic and social benefits are obtained. Keywords: Natural Gas, Utilization, China, Environment, Energy Demand 1. Introduction With the decrease of petroleum energy, it is very impor- tant to utilize Chin ese traditional en ergy such as co al and natural gas and improve natural gas as high value pro- duction. It has a good economic benefit and society ben- efit [1]. Chinese economic and social development is limited due to the shortage of natural resource. The out- put of Chinese petroleum basically keeps at between 1.8 * 108 t per year and 2.5 * 108 t per year [2]. However, Chinese usable petroleum source are reserved at 32.7 * 108 t per year. Chinese petroleum exploration only has 16 years. And 52% petroleum comes from overseas. So Chinese government uses the different policy to decrease the power loss and increase production efficiency and decrease tax for those companies that try to keep the na- ture source and develop the new source such as wind re- source, water resource and natural gas source, and so on. At present, utilization of natural gas in China is low efficiency and proceeds in a way that quickly degrades the environment and caused serious pollution. The local residents almost depend on natural gas for cooking and space heating. Most of natural gas is directly burnt as fuel for cooking. Due to using outdated stoves, the burn- ing efficiency is very low, utilizing less than 30% of electricity energy. In conclusion, the overuse of traditional energy (natu- ral gas) as fuel in partial area damages the fragile envi- ronment. There exists an urgent need to utilize natural gas in a rational way, which can be done through using new method to improve production value. This will not only improve energy efficiency, but also reduce pollution and damage the ecological environment. In this paper, the five main methods are introduced. Natural gas as feeds- tock is produced to acetylene, ethylene and chromium trioxide, respectivel y; natural gas is used to cut metal and produced to chromium trioxide inst ead of he avy oi l . 2. Natural Gas Utilization in China 2.1. Natural Gas Produced to Acetylene Zhang Xiangfu [3] introduced that natural gas as feeds- tock is produced to acetylene with the plasma method. The three methods for acetylene production were com- pared from the view point of technology and economics. The plasma method for acetylene production has more advantages than the partial oxidation and calcium carbide method. It was effectively used to save energy and de- crease production capital and protect environment. Figure 1 shows the relationship between temperature and free energy of hydrocarbons. When temperature is between 900 K and 1200 K, free energies o f hydrocarbon compounds are more than 0 and increase with the in- crease of temperature. But free energy of acetylene has high value at low temperature and decreases with the in- crease of temperature. When temperature is more than 1800 K, free energy of acetylene is less than that of oth- ers. This proves that acetylene is firstly obtained than others. 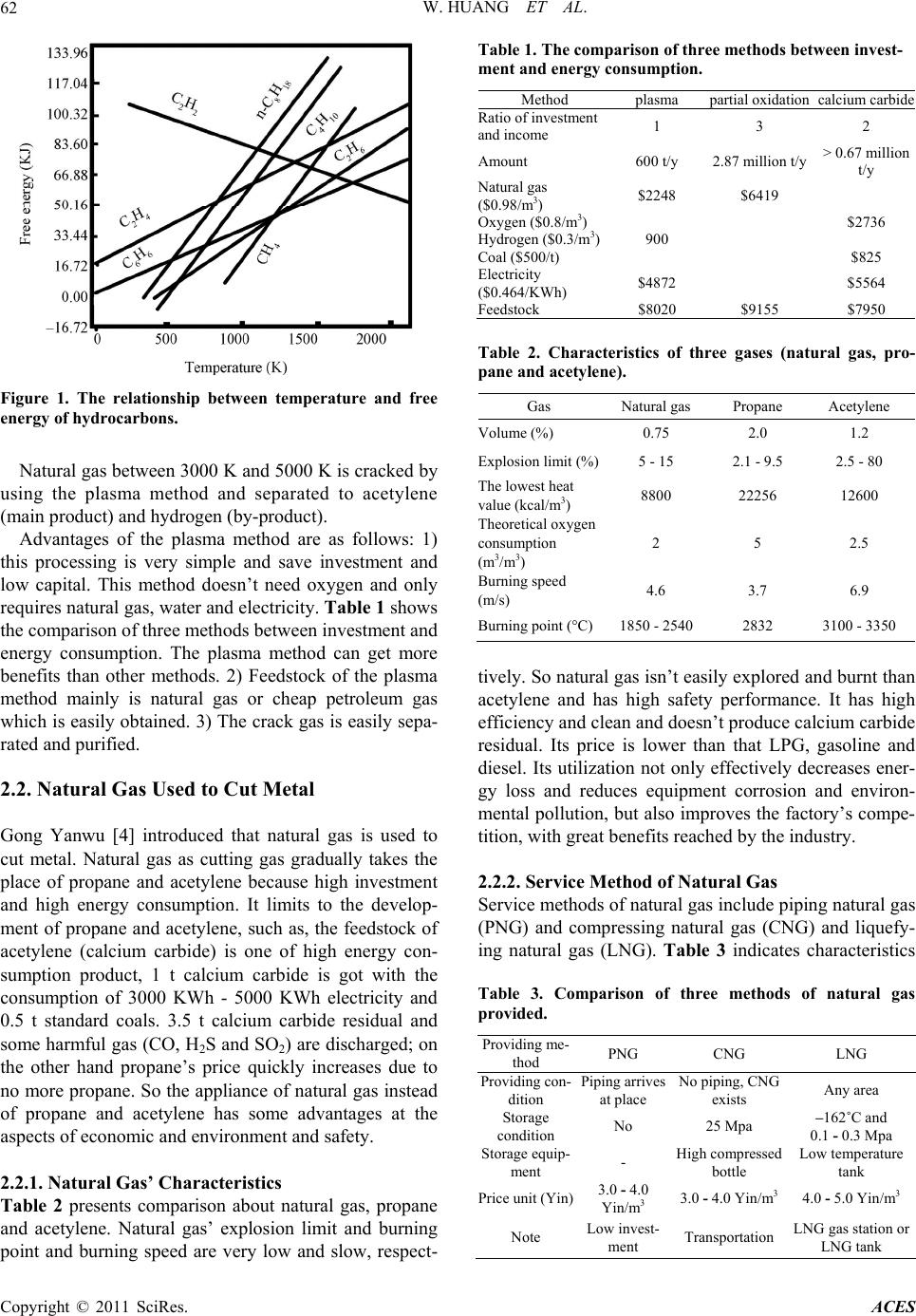 W. HUANG ET AL. Copyright © 2011 SciRes. ACES 62 Figure 1. The relationship between temperature and free energy of hydr ocarbons. Natural gas betw een 3000 K and 5000 K is cracked by using the plasma method and separated to acetylene (main product) and hydrogen (by-product). Advantages of the plasma method are as follows: 1) this processing is very simple and save investment and low capital. This method doesn’t need oxygen and only requires natural gas, water and electricity. Table 1 shows the comparison of three methods between investment and energy consumption. The plasma method can get more benefits than other methods. 2) Feedstock of the plasma method mainly is natural gas or cheap petroleum gas which is easily obtained . 3) The crack gas is easily sepa- rated and purified. 2.2. Natural Gas Used to Cut Metal Gong Yanwu [4] introduced that natural gas is used to cut metal. Natural gas as cutting gas gradually takes the place of propane and acetylene because high investment and high energy consumption. It limits to the develop- ment of propane and acetylene, such as, the feedstock of acetylene (calcium carbide) is one of high energy con- sumption product, 1 t calcium carbide is got with the consumption of 3000 KWh - 5000 KWh electricity and 0.5 t standard coals. 3.5 t calcium carbide residual and some harmful gas (CO, H2S and SO2) are discharged; on the other hand propane’s price quickly increases due to no more propane. So the appliance of natural gas instead of propane and acetylene has some advantages at the aspects of economic and environment and safety. 2.2.1. Natural Gas’ Charac teristics Table 2 presents comparison about natural gas, propane and acetylene. Natural gas’ explosion limit and burning point and burning speed are very low and slow, respect- Table 1. The comparison of three methods between invest- ment and energy consumption. Method plasma partial oxidation calcium carbide Ratio of investment and income 1 3 2 Amount 600 t/y 2.87 million t/y > 0.67 million t/y N atural gas ($0.98/m3) $2248 $6419 Oxygen ($0.8/m3) $2736 Hydrogen ($0.3/ m3)900 Coal ($500/t) $825 Electricity ($0.464/KWh) $4872 $5564 Feedstock $8020 $9155 $7950 Table 2. Characteristics of three gases (natural gas, pro- pane and acetylene) . Gas Natural gas Propane Acetylene Volume (%) 0.75 2.0 1.2 Explosion limit (%)5 - 15 2.1 - 9.5 2.5 - 80 The lowest heat value (kcal/m3) 8800 22256 12600 Theoretical oxygen consumption (m3/m3) 2 5 2.5 Burning speed (m/s) 4.6 3.7 6.9 Burning point (° C)1850 - 2540 2832 3100 - 3350 tively. So natural gas isn’t easily exp lored and burnt than acetylene and has high safety performance. It has high efficiency and clean and doesn’t produce calcium carbide residual. Its price is lower than that LPG, gasoline and diesel. Its utilization not only effectively decreases ener- gy loss and reduces equipment corrosion and environ- mental pollution, but also improves the factory’s compe- tition, with great benefits reached by the industry. 2.2.2. Service Metho d of Natural Gas Service methods of natural gas include piping natural gas (PNG) and compressing natural gas (CNG) and liquefy- ing natural gas (LNG). Table 3 indicates characteristics Table 3. Comparison of three methods of natural gas provided. Providing me- thod PNG CNG LNG Providing con- dition Piping arrives at place No piping, CNG exists Any area Storage condition No 25 Mpa –162˚C and 0.1 - 0.3Mpa Storage equip- ment - High compressed b o t tle Low temperature tan k Price unit (Yin)3.0 - 4.0 Yin/m3 3.0 - 4.0 Yin/m3 4.0 - 5.0 Yin/m3 Note Low invest- ment Transportation LNG gas station or LNG tank  W. HUANG ET AL. Copyright © 2011 SciRes. ACES 63 of three service methods. LNG has high price due to complicated processing and higher investment. So its sale prices are more than that of PNG and CNG. But LNG can be compressed into bottle and is good for per- sonal requirement. 1 kg acetylene and 1 kg natural gas’ prices are 16 Chinese Yin and 12 Chinese Yin, respectively. Using natural gas as fuel instead of acetylene can save 4000 Yin per day and decrease oxygen consumption. It can reduce capital investments about 2 millions Chinese Yin per year. 2.3. Natural Gas Used to Cut Metal Tang Hongqing [5] introduced how to synthesize ethy- lene with natural gas. Three methods, which include three steps, two steps and one step, were discussed in detail. Three steps method, two steps method and one step method mean natural gas-synthetic gas-methanol -ethylene, natural gas-methanol-ethylene and natural gas-eth-ylene, respectively. 0.5 t per year unit is established by Norsk Hydro Company with three steps method in 1995 . This unit was continually operated at 90 days. The experimental results showed that unit was very stable and catalyst perfor- mance was well and high yield of ethylene and propylene were obtained. Table 4 shows product yield of MTO (Methanol- To-Olefins) unit (500 t per year ethylene). 2370 kt me- thanol coming from 20 billions natural gases was re- quired to produce to 500 t per year ethylene. At the same time 345 kt propylene and 100 kt butylenes were ob- tained. Table 5 presents evaluation of naph tha an d natural gas as feedstock to produce ethylene. The investment of us- ing natural gas method is more than 43.6% that of using naphtha method, while the operating cost of using natural gas method is only 20% that of using naphtha method. Natural gas is oxidized to methanol and then produced to ethylene with MTO method. This is two steps method. It is seldom used because it is very difficult to control the first step (oxidation reaction) and a lot of by-produc ts are Table 4. Product yield of MTO (Methanol-To-Olefins) unit (500 t/y ethylene). Name Quantity, kt Yield, % Ethylene 500 48.0 Propylene 345 33.0 Butylenes 100 9.6 C5+ 25 2.4 H2, C1, C2, C3 paraffin 37 3.5 CO2 5 0.5 Coke 30 3.0 Water 1328 0.0 Total 2370 100 Table 5. Evaluation of naphtha and natural gas as feedstock to produce ethylene. naphtha natural gas Investment, billion Yin 5.5 7.9 Operating cost, billion Yin 549 247 By-product recycle –447 –379 Net feedstock capital 102 –132 Interest, % 25.9 29.9 got. At the same time the separation processing is very complicated and has high requirement for reactor’s ma- terial. Natural gas is hydrogenated to produce ethylene. A lot of researcher studied on this method (OCM). And the good results were obtained. Table 6 shows the different catalysts having an effect on OCM method. Reaction temperature and conversion of ethylene are above 870°C and 36%, respectively. 2.4. Natural Gas Instead of Heavy Oil Fired to Produce Chromium Trioxide Song Ying [6] introduced that natural gas took the place of heavy oil as fuel to produce chromium trioxide. Dis- advantages of heavy oil was used as fuel, such as, chro- mium trioxide processing is no continuing operation, heavy oil can’t be fully burnt at the beginning and is discharged black flue and pollute environment; heavy oil’s viscosity and freezing point are high. The heating systems are required due to storing and transporting and burning heavy oil; the quality of chromium trioxide has relationship with melting point; chromium trioxide is cracked with the increase of reaction temperature. The composition of natural gas was shown in Table 7. Advantages of heavy oil was used as fuel, such as, unit is very simple and easily operated; no more black flue is discharged and environment is protected; heat efficiency is improved in Table 8, heat efficiency increases 45% Table 6. An effect of the different catalys t s on OCM method . Catalyst Temperature (˚C) CH4/O2 Conversion, % Li-Mn-Ti 1073 2.5 43.9 Li-Mn 1023 1.7 47.3 Li-Na-Mn 1073 3.0 38.3 Na-W-Mn/SiO21073 2.6 38.1 Li-Na-Sn 1073 2.0 41.2 Li-Mn-Ti 1073 2.5 37.3 SnO2 953 1.5 44.9 Li-Mg 998 1.7 47.3 Li-Mg-Nd 1003 2.0 57.1 Li-Re 873 - 1073 41.3 Li-Mg-Mn 973 44.35 Na-Mg-B 1013 36.4  W. HUANG ET AL. Copyright © 2011 SciRes. ACES 64 Table 7. Composition of natural gas, v%. Composition Volume, v% CH4 95.86 C2H6 1.54 C3H8 0.17 C4H10 0.62 C5H12 0.06 C6H14 0.03 N2 1.13 CO2 1.19 Table 8. Comparing consumption of natural gas and heavy oil. Project Natural gas Heavy oil Consumption 106.9 Nm3 150 kg Total input heat energy, kJ 380.2 * 104 627.4 * 104 O2 consumption, Nm3 212.410 334.138 CO2, Nm3 110.748 242.4 H2O, Nm3 214.516 205.2 N2, Nm3 800.253 1294.8 SO2, Nm3 0 0.158 while amount of flue is decreased to 35.41%; operating cost can be decreased, its equipment is very cheap and a lot of electricity and water and steam are saved. Economic benefits are listed as follows: 1) Amount of chromium trioxide is 7000 t per year, prices of heavy oil and natural gas are 1500/t and 1.6/m3, respectively, consumption of heavy oil and natural gas are 0.15 t/tCrO3 and 106.9 Nm3/tCrO3, respectively, Costs of heavy oil per year 1500 * 0.15 * 7000 = 1.575 mil- lions dollars Costs of natural gas per year 106.9 * 1.6 * 7000 = 1.197 millions dollars Saving cost = 1.575 mil- lions dollars – 1.197 millions dollars = 0.378 millions dollars. 2) The equipment of water circulation for heavy oil is easily fragile and about 20 parts needs to be replaced. The price of one part is 2000 Chinese Yin. Factory can save 40000 Chinese Yin per year. The energy consumption of the natural gas system is reduced, such as, cooling water, steam and electricity. 3. Conclusions In a word, using natural gas as high value production is promising option for energy reserve in China. Benefits of natural gas are listed as follow: 1) Displace the traditional energy (coal) and protect the local fragile environment, substitute for fossil fuel consumption transported from at least 1000 km and avoid pollutions emissions, such as SO2, NO, Total Sus- pended Particles and CO2. 2) Make good benefits for public and factory in the south and east of China cost-effectively. 3) Facilitate the local sustainable development and in- crease the local income and job opp ortunity. 4. References [1] Z. Y. Ma, X. Y. Meng, H. W. Wang, W. Zhou and H. Y. Jiang, “Progress in Research of Oxalate Synthesis by Gas Phase Catalytic Coupling of CO,” Petrochemical, Vol. 38, No. 3, 2009, pp. 456-461. [2] Z. L. Li, “Economic Introduction for Methanol to Gener- ate Lower Carbon Olefin,” Chemical Catalysis and Me- thanol Technology, Vol. 6, No. 1, 2007, pp. 16-23. [3] X. F. Zhang and D. Q. Zheng, “The Plasma Method for Acetylene Production from Natural Gas,” Natural Gas Industry, Vol. 23, No. 4, 1998, pp. 39-43. [4] Y. W. Gong, F. H. Liu and Y. K. Lai, “Technology and Appliance of Natural Gas Cutting Metal,” Natural Gas Industry, Vol. 28, No. 8, 2003, pp. 117-119. [5] H. Q. Tang, Z. W. Yuan and D. J. Jiang, “Study on and Develop Natural Gas Produced to Ethylene Processing,” Chemical Engineering Design, Vol. 9, No. 1, 1999, pp. 5- 8. [6] Y. Song, W. Ji and X. Li, “Replacing Heavy Oil with Natural Gas to Produce Chromium Trioxide,” Shangdong Chemical Industry, Vol. 30, No. 4, 2001, pp. 37-38. |

