 Journal of Crystallization Process and Technology, 2011, 1, 1-7 doi:10.4236/jcpt.2011.11001 Published Online April 2011 (http://www.SciRP.org/journal/jcpt) Copyright © 2011 SciRes. JCPT 1 A Facile Route to Phosphanylborohydrides: Synthesis, Crystal Structure and Spectroscopic Properties of 1,2-Bis(Diphenylphosphinoborane)Ethane Leyla Tatar Yildirim1, Mehdi Masjedi2, Saim Özkar2 1Department of Engineering Physics, Hacettepe University, Ankara, Turkey; 2Department of Chemistry, Middle East Technical University, Ankara, Turkey E-mail:1tatar@hacettepe.edu.tr, 2mehdimasjedi@yahoo.com, 3sozkar@metu.edu.tr Received February 22nd, 2011; revised March 16th, 2011; accepted March 17th, 2011. ABSTRACT A novel and simple synthetic way using NaBH4 in the mixture of H2O-THF was applied to prepare 1,2-bis(diphenyl- phosphinoborane)ethane, dppe(BH3)2, in high yield and purity. The phosphanylborohydride compound dppe(BH3)2 was isolated in the form of colorless crystals and characterized by single crystal X-ray diffractio n, 1H, 13C, 31P and 11B NMR spectroscopy. Prismatic colorless crystals of dppe(BH3)2 were obtained in monoclinic crystal system and space group P21 with two asymmetric units in the unit cell. Lattice parameters were: a = 11.657(2), b = 17.237(2), c = 12.764(2) Å, = 98.735(14)˚, 2535.0 (7) Å3. Keyword s: Crystal Structure, Synthesis, Phosphinoborane, Sodium Boro h ydride, Phosphanylborohydride, X-Ray Diffraction 1. Introduction A recent study [1] has reported the catalytic activity of ruthenium (III) acetylacetonate in the presence of differ- ent phosphorus compounds such as 1,2-bis(diphenyl- phosphino)ethane, dppe, in the hydrolysis of sodium borohydride. At the end of catalytic reaction, in addition to the unreacted dppe, unexpectedly we isolated a new species which contains two BH3 molecules coordinated to dppe. Obviously, in this catalytic reaction, NaBH4 acts not only as a substrate to generate hydrogen, but also as a BH3 supplier in forming phosphanylborohydrides such as 1,2-bis(diphenylphosphinoborane)ethane, dppe(BH3)2. In literature, phosphanylborohydrides have been prepared by using the mixture of sodium borohydride and iodine in monoglyme [2] or using the other borane sources: dppe(BH3)2 by complexation of dppe with BH3·S(CH3)2 [3], rac/meso-[HP(BH3)(Ph)CH2]2 from the reaction of BH3.thf [4] or reaction of phosphine oxides with dibo- rane [5], from the reaction of trialkylphosphines with bromoboranes or bromochloroboranes [6]. In addition following phosphanylborohydrides have been reported: tertiary mono and diphosphine-borane complexes [7-9], cyclic phosphine-boranes [10], phosphine-carborane clusters [11], phosphinyl-borane radicals [12] and phosphine alkylene boranes [13]. It is noteworthy that the phosphanylborohydride [P(BH3)Ph2]- forms dative bonds of higher p character and establish more stable σ adducts towards the acceptor orbital of the Lewis acid in com- parison with its neutral counterpart P(CH3)Ph2 [14]. A similar phenomenon was observed in the study of chal- cogenated phosphanylborohydrides K[EP(BH3)R2] (E: O, S, Se, Te; R: Ph, t-Bu) with a certain degree of E=P mul- tiple bond character [15]. Borane complexes of phosphorus compounds, a very common oxidation free relay for cata- lytic ligands (phosphines, phosphites or phosphinites) can be easily deprotected by treatment with polymer- supported piperazine, N-methylpiperazine [16] or pyrrol derivatives [3]. Phosphanylborohydrides supported by amines such as polypyrroles, are very useful for homogeneous cataly- sis due to more efficient recovery and purification [3]. Despite the known examples given above, the chemistry of phosphanylborohydrides is still largely undeveloped [17-22]. Herein we report a new and simple synthetic way using NaBH4 in an homogeneous aqueous-organic solution to yield  A Facile Route to Phosphanylborohydrides: Synthesis, Crystal Structure and Spectroscopic 2 Properties of 1,2-Bis(Diphenylphosphinoborane)Ethane characterization by single crys- 1H, 13C, 31P and 11B NMR spec- tal ith ace- ious rinsing with distilled water 0˚C in oven for a few hours. P NMR. Posi- tiv hydrogen liberated during hy- , 129.87, 131.85, 137.59. P {H} NMR R (CD2Cl2, ppm): BH3) and phenyl rin drogen’s of the comp 1,2-bis(diphenylphosphinoborane)ethane, dppe(BH3)2, and its tal X-ray diffraction, troscopy. 2. Experimen 2.1. Materials Sodium borohydride, NaBH4 (98%) and 1,2-bis(di- phenylphosphino)ethane, dppe, were purchased from Aldrich. Tetrahydrofuran, THF and dichloromethane, CH2Cl2 were purchased from Merck. All glassware and Teflon-coated magnetic stir bars were cleaned w tone, followed by cop before drying at 15 2.2. Equipment All reactions involving air sensitive compounds were performed under argon or nitrogen atmospheres. 1H, 13C, 31P and 11B NMR spectra were recorded on Bruker Avance DPX 400 MHz spectrometer (400.1 MHz for 1H; 100.6 MHz for 13C; 161.3 MHz for 31P; 128.2 MHz for 11B). TMS was used as internal reference for 1H and 13C NMR chemical shifts. BF3·(C2H5)2O was used as external reference for 11B NMR chemical shifts. H3PO4 (85% in glass capillary) was used as reference for 31 e ion mass spectrometry data was obtained on a Bruker Micro TOF-LC/ESI/Ms system. The experimental setup consists of a 75 mL jacketed reaction flask containing a Teflon-coated stir bar placed on a magnetic stirrer (Heidolph MR-301) which can be thermostated to 25.0˚C by circulating water through its jacket from a constant temperature bath (RL6 LAUDA water bath). Note that drolysis of sodium borohydride was released from the flask through a bubbler. 2.3. Synthesis of 1,2-Bis(Diphenylphosphinobo- rane)Ethane, DPPE(BH3)2 For the preparation of 1,2-bis(diphenylphosphinoborane) ethane, dppe(BH3)2, 140 mg (0.35 mmol) of 1,2-bis(di- phenylphospino)ethane, dppe, was dissolved in 10 mL of THF by vigorous stirring. Then, the solution was trans- ferred into a 75 mL jacketed reaction flask containing 30 mg (0.79 mmol) NaBH4 dissolved in 40 mL water and thermostated at 25.0˚C. The reaction was started by turning on the magnetic stirrer (Heidolph MR-301) at 1000 rpm under inert atmosphere (argon or nitrogen). After 3 h stirring, the mixture was extracted with di- chloromethane and the combined organic extracts were cooled in order to precipitate out traces of sodium boro- hydride or metaborate remaining in organic extracts. Then, the solution was dried over magnesium sulfate, filtered and evaporated in vacuum giving 144 mg of pure dppe(BH3)2 complex (96% yield). Colorless crystals of dppe(BH3)2 were obtained by crystallization from the hexane-dichloromethane solution at 0˚C after one week, which were separated by filtration. [Ph2P(BH3)CH2]2: 1H NMR (CD2Cl2, ppm): δ 1.99 (t, 6H, JP-H = 4.8 Hz, 2BH3), 2.15 (br d, 2H, JP-H = 6.4 Hz, CH2), 2.38 (br d, JP-H = 2.8 Hz, 2H, CH2), 7.38 (m, 12H, H-m,p), 7.54(m, 4H, H-o), 7.61 (m, 4H, H-o). 13C {1H} NMR (CD2Cl2, ppm): δ 22.93, 127.8131 1 (CD2Cl2, ppm): δ –12.5. 11B {1H} NM δ –40.06. 3. Crystal Structure Analysis X-ray diffraction measurements were performed with MoKα radiation on an Enraf-Nonius CAD4 diffractome- ter [23] equipped with a graphite monochromator. Inten- sity data were collected by /2 scan mode. The cell parameters were determined from a least-squares refine- ment of 18 centered reflections in the range of 10.12˚ 18.03˚. Cell refinement was carried out using CAD-4 EXPRESS. Data reduction was carried out using XCAD4 [24]. The structures were solved by Patterson methods and refined using the program SHELX [25]. A full-matrix least-squares refinement on F2 was done. For all non-hydrogen atoms anisotropic displacement pa- rameters were refined. Borane(g hy- ound were placed geometrically and a riding model was used with ()1.5() iso eq UH UC and ()1.2() iso eq UH UC , respectively. Methylene hydro- gen’s were taken from a difference Fourier map and re- fined. Single crystal X-ray diffraction analysis of the colorless crystal shows the crystallization in monoclinic system with space group P21 and two asymmetric units with a formula of C26H30B2P2 and four molecules per unit cell. Table 1 shows the crystal data and crystal refine- ment of dppe(BH3)2. The atomic coordinates and iso- tropic displacement parameters are listed in Table 2. Selected bond lengths and angles are given in Table 3. ORTEP [26] drawing of the dppe(BH3)2 complex with the atomic numbering scheme is given in Figure 1. The unit cell of the structure as shown in Figure 2. The con- formations of molecules and molecular packing geome- try were analyzed using PLATON [27]. The structure includes several pi-ring interactions between two asym- ng interaction geome-metric moieties. Details of the pi-ri try are given in Table 4. 4. Results and Discussion When an aqueous solution of sodium borohydride is dded to a solution of 1,2-bis(diphenylphospino)ethane, a Copyright © 2011 SciRes. JCPT 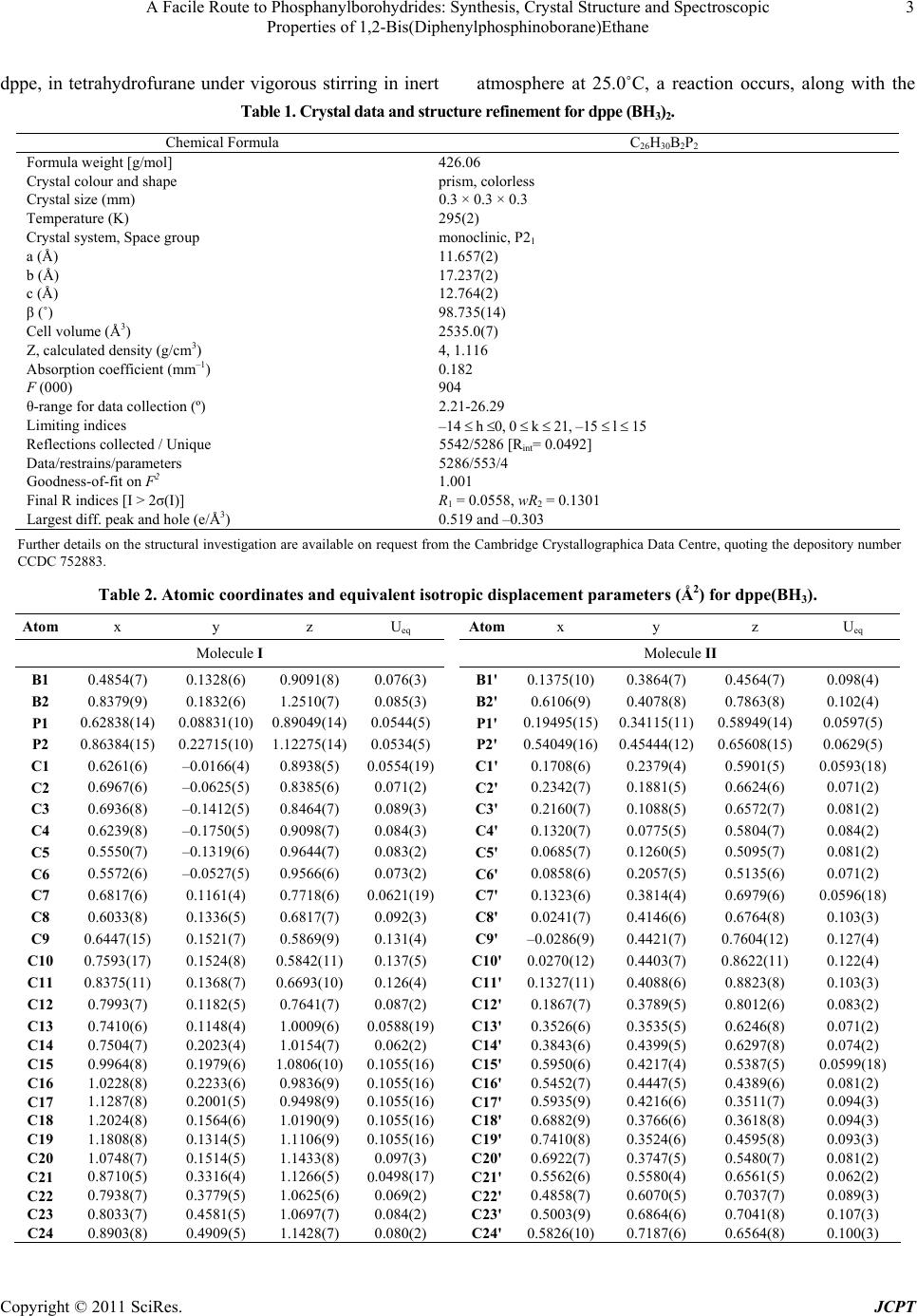 A Facile Route to Phosphanylborohydrides: Synthesis, Crystal Structure and Spectroscopic Properties of 1,2-Bis(Diphenylphosphinoborane)Ethane Copyright © 2011 SciRes. JCPT 3 pe, in tetrahydrofurane undeaction occurs, along with the . Crystal data and structure refinement for dppe emical Formula C26H30B2P2 dpr vigorous stirring in inert atmosphere at 25.0˚C, a re Table 1(BH3)2. Ch Formula weight [g/mol] 426.06 Crystal colour and shape system, Space group , P21 6 l 15 que int= 0.0492] 0.1301 prism, colorless Crystal size (mm) erature (K) 0.3 × 0.3 × 0.3 Temp l 295(2) Crysta monoclinic a (Å) 11.657(2) b (Å)17.237(2) c (Å) 12.764(2) 4)β (˚) 3 98.735(1 (7)Cell volume (Å) lated density (g/cm3) 2535.0 11Z, calcu 4, 1. Absorption coefficient (mm–1) 0.182 F (000) 904 θ-range for data collection (º) 2.21-26.29 k 21, –15 Limiting indices Uni –14 h 0, 0 286 [RReflections collected / 5542/5 Data/restrains/parameters 25286/553/4 Goodness-of-fit on F Final R indices [I > 2σ(I)] 1.001 R = 0.0558, wR = 1 2 Largest diff. peak and hole (e/Å3) 0.519 and –0.303 Further details on the structural investigation are available on request from the Cambridge Crystallographica Data Centre, quoting the depository number CCDC 75288 Table 2. Atomic coordinates and equivalotropplacemt paramet (Å2) for dppe(BH3). Atom x z Ueq Atom x z Ueq 3. ent isic diseners y y Molecule I Molecule II B1 0.4854(7) 0.1328(6) 0.9091(8) 0.076(3) B1' 0.1375(10)0.3864(7) 0.4564(7) 0.098(4) B2 0.8379(9) 0.1832(6) 1.2510(7) 0.085(3) B2' 0.6106(9) 0.4078(8) 0.7863(8) 0.102(4) P1 0.62838(14) 0.08831(10) 0.89049(14) 0.0544(5)P1' 0.19495(15)0.34115(11) 0.58949(14) 0.0597(5) P2 0 1 00 0 00 00 0 0 .86384(15) 0.22715(10) .12275(14) 0.0534(5)P2' .54049(16).45444(12).65608(15)0.0629(5) C1 0.6261(6) –0.0166(4) 0.8938(5) .0554(19)C1' 0.1708(6) 0.2379(4) 0.5901(5) .0593(18) C2 0.6967(6) –0.0625(5) 0.8385(6) 0.071(2) C2' 0.2342(7) 0.1881(5) 0.6624(6) 0.071(2) C3 0.6936(8) –0.1412(5) 0.8464(7) 0.089(3) C3' 0.2160(7) 0.1088(5) 0.6572(7) 0.081(2) C4 0.6239(8) –0.1750(5) 0.9098(7) 0.084(3) C4' 0.1320(7) 0.0775(5) 0.5804(7) 0.084(2) C5 0.5550(7) –0.1319(6) 0.9644(7) 0.083(2) C5' 0.0685(7) 0.1260(5) 0.5095(7) 0.081(2) C6 0.5572(6) –0.0527(5) 0.9566(6) 0.073(2) C6' 0.0858(6) 0.2057(5) 0.5135(6) 0.071(2) C7 0.6817(6) 0.1161(4) 0.7718(6) .0621(19)C7' 0.1323(6) 0.3814(4) 0.6979(6) .0596(18) C8 0.6033(8) 0.1336(5) 0.6817(7) 0.092(3) C8' 0.0241(7) 0.4146(6) 0.6764(8) 0.103(3) C9 0.6447(15) 0.1521(7) 0.5869(9) 0.131(4) C9' –0.0286(9)0.4421(7) 0.7604(12) 0.127(4) C10 0.7593(17) 0.1524(8) 0.5842(11) 0.137(5) C10' 0.0270(12)0.4403(7) 0.8622(11) 0.122(4) C11 0.8375(11) 0.1368(7) 0.6693(10) 0.126(4) C11' 0.1327(11)0.4088(6) 0.8823(8) 0.103(3) C12 0.7993(7) 0.1182(5) 0.7641(7) 0.087(2) C12' 0.1867(7) 0.3789(5) 0.8012(6) 0.083(2) C13 0.7410(6) 0.1148(4) 1.0009(6) 0.0588(19)C13' 0.3526(6) 0.3535(5) 0.6246(8) 0.071(2) C14 0.7504(7) 0.2023(4) 1.0154(7) 0.062(2) C14' 0.3843(6) 0.4399(5) 0.6297(8) 0.074(2) C15 0.9964(8) 0.1979(6) 1.0806(10) 0.1055(16)C15' 0.5950(6) 0.4217(4) 0.5387(5) .0599(18) C16 1.0228(8) 0.2233(6) 0.9836(9) 0.1055(16)C16' 0.5452(7) 0.4447(5) 0.4389(6) 0.081(2) C17 1.1287(8) 0.2001(5) 0.9498(9) 0.1055(16) 0 C17' 0.5935(9) 0.4216(6) 0.3511(7) 0.094(3) C18 1.2024(8) 0.1564(6) 1.0190(9) .1055(16)C18' 0.6882(9) 0.3766(6) 0.3618(8) 0.094(3) C19 1.1808(8) 0.1314(5) 1.1106(9) 0.1055(16)C19' 0.7410(8) 0.3524(6) 0.4595(8) 0.093(3) C20 1.0748(7) 0.1514(5) 1.1433(8) 0.097(3) C20' 0.6922(7) 0.3747(5) 0.5480(7) 0.081(2) C21 0.8710(5) 0.3316(4) 1.1266(5) .0498(17)C21' 0.5562(6) 0.5580(4) 0.6561(5) 0.062(2) C22 C23 0.7938(7) 0.8033(7) 0.3779(5) 0.4581(5) 1.0625(6) 1.0697(7) 0.069(2) 0.084(2) C22' C23' 0.4858(7) 0.5003(9) 0.6070(5) 0.6864(6) 0.7037(7) 0.7041(8) 0.089(3) 0.107(3) C24 0.8903(8) 0.4909(5) 1.1428(7) 0.080(2) C24' 0.5826(10)0.7187(6) 0.6564(8) 0.100(3)  A Facile Route to Phosphanylborohydrides: Synthesis, Crystal Structure and Spectroscopic 4 Properties of 1,2-Bis(Diphenylphosphinoborane)Ethane C25 0.9654( 0.118(4) 7) 0.4449(5) 1.2062(6) 0.081(2) C25' 0.6512(10)0.6726(7) 0.6109(9) C26 0.9546(6) 0.3663(4)6) 0.069(2) C26' 0.6405(8) 0.6082(7) 0.092(3) 1.1979(0.5929(5) Tabcted bond length [Å] and angles˚] for tmetric units oH3)2. Molecule I Molecule II le 3. Sele [wo asymf dppe(B B1 – P1 1.882(8) B1' – P1' 1.896(9) B2 – P2 1.870(9) B2' – P2' 1.914(10) P1 – C1 1.809(7) P1' – C1' 1.802(8) P1 – C7 1.788(7) P1' – C7' 1.799(7) P1 – C13 1.832(7) P1' – C13' 1.837(8) P2 – C14 P2 – C15 1.805(7) 1.784(9) P2' – C14' 2' – C15' 1.818(8) 1.805(7) P P2 – C21 1.803(7) P2' – C21' 1.794(8) C13 – C14 1.521(9) C13' – C14' 1.533(11) B1 – P1 – C1 112.8(4) B1' – P1' – C1' 112.1(4) B1 – P1 – C7 115.3(4) B1'– P1' – C7' 113.6(5) B1 – P1 – C13 110.2(4) B1' – P1' – C13' ' 112.0(5) B2 – P2 – C14111.8(5) B2' – P2' – C14112.7(5) B2 – P2 – C15 114.3(5) B2' – P2' – C15' 115.6(5) B2 – P2 – C21 113.2(4) B2' – P2' – C21' 112.8(5) C1 – P1 – C7 107.2(3) C1' – P1' – C7' 107.2(3) C1 – P1 – C13 104.0(3) C1' – P1' – C13' 105.3(4) C7 – P1 – C13 106.5(3) C7' – P1' – C13' 106.0(4) C14 – P2 – C15 105.8(5) C14' – P2' – C15' 105.8(4) C14 – P2 – C21 C15 – P2 – C21 106.5(3) 104.6(4) C14' – P2' – C21' C15' – P2' – C21' 103.7(4) 105.2(3) P1 – C13 – C14 111.8(5) P1' – C13' – C14' 110.4(5) P2 – C14 – C13 111.0(5) P2' – C14' – C13' 111.7(5) Ttural parameters oing interaceometry (e title compound. CDg able 4. Strucf pi-rtion gÅ,˚) for th D H Cg DH gH DCg HC C9 H9Cg4' 0.93 2.81 3.685(14) 158 C11H11Cg1i 0.93 2.79 3.634(12) 151 C25 H25Cg1ii 0.93 2.91 3.662(12) 138 Symmetry codes [i: x, y, 1 + z and ii: 1 – x, ½ + y,1 – z] Copyright © 2011 SciRes. JCPT 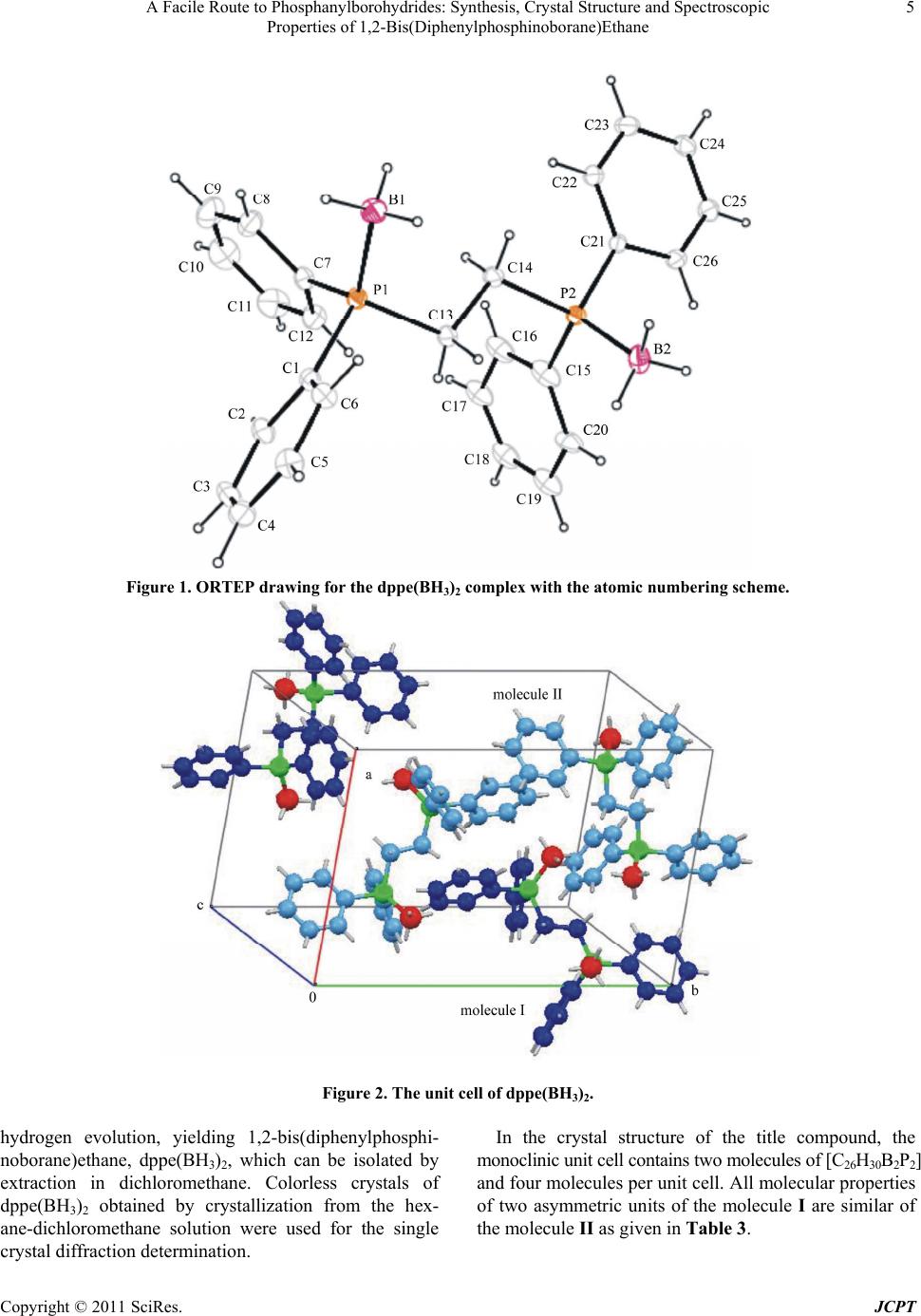 A Facile Route to Phosphanylborohydrides: Synthesis, Crystal Structure and Spectroscopic Properties of 1,2-Bis(Diphenylphosphinoborane)Ethane Copyright © 2011 SciRes. JCPT 5 Figure 1. ORTEP drawing for the dppe(BH3)2 complex with the atomic numbe ring scheme. ll of dppe(BH3)2. In the crystal structure of the title compound, the monoclinic unit cell contains two molecules of [C26H30B2P2] and four molecules per unit cell. All molecular properties of two asymmetric units of the molecule I are similar of the molecule II as given in Table 3. Figure 2. The un hydrogen evolution, yielding 1,2-bis(diphenylphosphi- noborane)ethane, dppe(BH3)2, which can be isolated by extraction in dichloromethane. Colorless crystals of dppe(BH3)2 obtained by cr it ce ystallization from the hex- ane-dichloromethane solution were used for the single crystal diffraction determination.  A Facile Route to Phosphanylborohydrides: Synthesis, Crystal Structure and Spectroscopic 6 Properties of 1,2-Bis(Diphenylphosphinoborane)Ethane angles around phosphorus atoms are nyl rings in coordi Cg1-Cg2 = 76.4(3) Å, Cg3- le I and Cg5-Cg6 = 71.2(3) on NMR data of 1,2-bis(dipheny phosphinoborane are also in good agreement with tcture. It is note- 1 ns of phenyl rings ylene groups, or- ore shielded than corre- )CH ] complex facile BH3 source loro- ontaining nearly by the Turkish Academy of esting Catalytic tonate in the Pres- 2 The average bond 112.9˚, 113.1˚, 105.8˚, and 105.5˚ for B-P-C, B'-P'-C', C-P-C and C'-P'-C', respectively. Study of the interac- tions between P atoms and contact atoms in coordination sphere with average distances (P-B: 1.876 Å for the molecule I, 1.905 Å for II and P-C 1.804 Å for I, 1.809 Å for II) reveals that the P atoms are surrounded by four atoms (one boron and three carbon atoms) in nearly ideal tetrahedral geometry. Both molecules in an asymmetric unit have a similar three-dimensional conformation. Dihedral angles be- tween the least square planes of phe- Sc nation sphere of P atoms are Cg4 = 88.2(3) Å for molecu Å, Cg7-Cg8 = 71.5(3) Å for molecule II. Figure 2 shows the unit cell of dppe(BH3)2. There is no classic hydrogen bond in the structure but the compound includes several pi-ring interactions be- tween two asymmetric moieties. Rings are composed of atoms Cg1 = C1/C6, Cg2 = C7/C12, Cg3 = C15/C20, Cg4 = C21/C26, Cg1' = C1'/C6', Cg2' = C7'/C12', Cg3' = C15'/C20', Cg4' = C21'/C26'. Details of the pi-ring inter- action geometry are given in Table 4. The solutil- en )ethane, dppe(BH3)2, he single crystal stru worthy that the H NMR spectrum gives two doublets at 2.15 and 2.38 ppm for the prochiral methylene groups [28] while the 13C NMR spectrum exhibits only one signal at 22.93 ppm for the methylene carbons. Moreover, two separated multiplets at 7.54 and 7.61 ppm are observed for ortho-hydroge and similar to hydrogens of meth tho-hydrogens of phenyl rings are not identical due to their different positions with respect to borane moie- ties. 31P NMR spectrum shows a peak at –12.5 ppm which is about 10 ppm m sponding peak of meso-[HP(BH3)(Ph)CH2]2 complex [4], indicating that dppe(BH3)2 complex containing more phenyl rings has more electron-rich phosphorus atoms. However, 11B NMR gives a peak at –40.06 ppm for borane groups comparable to the value of –41.6 ppm reported for meso-[HP(BH3)(Ph 22 [ 4]. MS does not show the molecular ion peak ex- pected at m/z = 425. Instead, it shows peaks at m/z = 429 or 431 due to oxidation of dppe(BH3)2 during the sampling/ionization whereby BH3 groups are replaced by the oxo groups. 5. Conclusions In conclusion, sodium borohydride is a in the synthesis of phosphanylborohydride compounds such as 1,2-bis(diphenylphosphinoborane)ethane, dppe (BH3)2, in high yield. Sodium borohydride and metabo- rate are easily separated by extraction with dich methane, providing an easy separation for the preparation of phosphanylborohydrides with high purity. The adduct dppe(BH3)2 crystallizes in monoclinic system with space group P21 and two asymmetric units c ideal tetrahedral phosphorus atoms. 6. Acknowledgements Partial support of this work iences and the Scientific and Technological Research Council of Turkey (TUBITAK, Project No.: 105M357) is gratefully acknowledged. L.T. Yildirim thanks Hacettepe University Scientific Research Unit (grant No. 04 A602004) for financial support. MM thanks TUBITAK for awarding a PhD fellowship. We thank Mr. Bunyamin Cosut from Gebze Institute of Technology for performing mass analysis. REFERENCES [1] M. Masjedi, T. Demiralp and S. Ozkar, “T Activity of Ruthenium (III) Acetylace ce of Trialkylphosphite or Trialkylphosphine in Hy- drogen Generation from the Hydrolysis of Sodium Boro- hydride,” Journal of Molecular Catalysis A: Chemical, Vol. 310, No. 1-2, 2009, pp. 59-63. doi:10.1016/j.molcata.2009.05.02 chkewitsch, “A New Synthe-[2] K. C. Nainan and G. E. Rys sis of AMINE- and Phosphine-Boranes,” Inorganic Chemistry, Vol. 8, No. 12, 1969, pp. 2671-2674. doi:10.1021/ic50082a027 [3] O. Stephan, N. Riegel and S. Juge, “Po Diphosphine Borane Groups: A lypyrrole Bearing New Way to Prepare Polymer-Supported Phosphine Ligands. Application to Palladium Catalysed Reactions,” Journal of Electroana- lytical Chemistry, Vol. 421, No. 1-2, 1997, pp. 5-8. doi:10.1016/S0022-0728(96)01027-3 [4] F. Dornhaus, M. Bolte, H-W “The First Bidentate Phospha . Lerner and M. Wagner, nylborohydrides: Synthesis, Structure and Reactivity Towards [CpFe(CO)2I],” Journal of Organometallic Chemistry, Vol. 692, No. 14, 2007, pp. 2949-2955. doi:10.1016/j.jorganchem.2007.03.006 [5] R. A. Geanangel, “The Reaction of Amine and Phosphine Oxides with Diborane,” Journal of Inorganic and Nu- clear Chemistry, Vol. 36, No. 6, 1974, pp. 1397-1398. doi:10.1016/0022-1902(74)80085-0 [6] G. Jugie, J. P. Laussac and J. P. Laurent, “Addition Compounds of Trialkylphosphines (R3P) with Bromobo- ranes or Bromochloroboranes,” Bulletin de la Societe Chimique de France, Vol. 7, 1970, pp. 2542-2544. [7] S. Juge, M. Stephan, J. A. Laffitte and J. P. Genet, “Effi- cient Asymmetric Synthesis of Optically Pure Tertiary Mono and Diphosphine Ligands,” Tetrahedron Letters, Copyright © 2011 SciRes. JCPT  A Facile Route to Phosphanylborohydrides: Synthesis, Crystal Structure and Spectroscopic Properties of 1,2-Bis(Diphenylphosphinoborane)Ethane Copyright © 2011 SciRes. JCPT 7 63-1 Vol. 31, No. 44, 1990, pp. 6357-6360. doi:10.1016/S0040-4039(00)970 , J. P. Genet, J. Uziel and S. Juge, [8] S. Juge, M. Stephan, R. Merdes, J. P. Genet and S. Halut- Desportes, “Stereochemistry of the P-C Bond Formation in an Oxazaphospholidine Borane Complex,” ChemIn- form, 1993, pp. 531-533. [9] B. E. Kaloun, R. Merdes “Asymmetric Synthesis of (S,S)-(+)-1,1'-bis-(Methyl- phenyl-Phosphino) Ferrocene,” Journal of Organometal- lic Chemistry, Vol. 529, No. 1-2, 1997, pp. 455-463. doi:10.1016/S0022-328X(96)06690-9 [10] H. Schmidbaur, M. Si Structure of Cyclic Pho gl and A. Schier, “Synthesis and sphine-Boranes,” Journal of Or- ganometallic Chemistry, Vol. 529, No. 1-2, 1997, pp. 323-327. doi:10.1016/S0022-328X(96)06616-8 [11] R. Nunez, C. Vinas, F. Teixidor, R. Sillanpaa and R. Kivekas, “Phosphine-Boranes Incorporating the Carbo- rane Cluster,” Journal of Organometallic Chemistry, Vol. 657, No. 1-2, 2002, pp. 224-231. doi:10.1016/S0022-328X(02)01326-8 [12] J. A. Baban and B. P. Roberts, “Homolytic Reactions of Ligated Boranes. Part 3. Electron Spin Resonance Studies of Radicals Derived from Dialkyl-Amine Boranes,” Journal of the Chemical Society, Perkin Transactions 2, Vol. 10, 1986, pp. 1607-1611. doi:10.1039/p29860001607 [13] H. Schmidbaur, G. Mueller and G. Blaschke, “Darstel- lung und Eigenschaften Einfacher und Stark Sterisch Ge- hinderter Phosphanalkylenborane,” Chemische Berichte, Vol. 113, No. 4, 1980, pp. 1480-1486. doi:10.1002/cber.19801130427 [14] F. Dornhaus, M. Bolte, H.-W. Lerner and M. Wagner, “Phosphanylborohydrides: First Assessment of the Rela- tive Lewis Basicities of [BH3PPh2]-, CH3PPh2 and HPPh2,” European Journal of Inorganic Chemistry, Vol. 2006, No. 9, 2006, pp. 1777-1785. doi:10.1002/ejic.200501126 [15] F. Dornhaus, M. Bolte, H-W. Lerner and M. Wagner, “A Comparative Study of Chalcogenated Phosphanylboro- hydrides [EPR2BH3]- (R = Ph, tBu) and Triorganophos- phane Chalcogenides EPPh2CH3 (E = O, S, Se, Te),” European Journal of Inorganic Chemistry, 2006, pp. 5138-5147. doi:10.1002/ejic.200600753 [16] S. Sayalero and M. A. Pericas, “Work-up-Free Deprotec- tion of Borane Complexes of Phosphines, Phosphites and Phosphinites with Polymer-Supported Amines,” Synlett, Vol. 16, 2006, pp. 2585-2588. [17] W. Angerer, W. S. Scheldrick and W. Malisch, “Uber- gangsmetallsubstituierte Phosphane, Arsane und Stibane, XLVI, Einige Reaktionen des Ferrio-Phosphanes C5Me5(CO)2 Fe-PPh2 und Molekulstruktur Seines Boran-Addukts,” Chemische Berichte, Vol. 118, 1985, pp. 1261-1266. doi:10.1002/cber.19851180339 [18] D. A. Hoic, W. M. Davis and G. C. Fu, “Diphenylphosphi- doboratabenzene: An Anionic Analogue of Triphenyl- phosphine,” Journal of the American Chemical Society, Vol. 118, 1996, pp. 8176-8177. doi:10.1021/ja9615740 [19] A.-C. Gaumont, M. B. Hursthouse, S. J. Coles and J. M. Brown, “Isolation of the Reactive Intermediate in Palla- dium-Catalysed Coupling of Secondary Phosphine-Boranes with Aryl Halides,” Chemical Communications, Vol. 1, 1999, pp. 63-64. doi:10.1039/a807830k [20] H. Dorn, C. A. Jaska, R. A. Singh, A. J. Lough and I. Manners, “Synthesis and Novel Reactivity of Platinum Phosphine-Borane Complexes trans-[PtH(PPhR.BH3) (PEt3)2] (R = H, Ph),” Chemical Communications, Vol. 12, 2000, pp. 1041-1042. doi:10.1039/b002615h [21] G. Muller and J. Brand, “Mono(Borane)Phosphides as Ligands to Lithium and Aluminum,” Organometallics, Vol. 22, 2003, pp. 1463-1467. doi:10.1021/om0209430 [22] C. A. Jaska, A. J. Lough and I. Manners, “Linear Hybrid Aminoborane/Phosphinoborane Chains: Synthesis, Pro- ton-Hydrid Interactions and Thermolysis Behavior,” In- organic Chemistry, Vol. 43, No. 3, 2004, pp. 1090-1099. [23] Enraf-Nonius, “CAD-4 EXPRESS,” Enraf-Nonius, Delft, 1994. [24] K. Harms and S. Wocadlo, “XCAD4,” University of Marburg, Marburg, 1995. [25] G. M. Sheldrick, “A Short History of SHELX,” Acta Crystallographica, Vol. A64, 2008, pp. 112-122. [26] L. J. Farrugia, “XRDIFF: Simulation of X-Ray Diffrac- tion Patterns,” Journal of Applied Crystallography, Vol. 30, No. 5-1, 1997, pp. 565-566. [27] A. L. Spek, “Single-Crystal Structure Validation with the Program PLATON,” Journal of Applied Crystallography, Vol. 36, No. 1, 2003, pp. 7-13. doi:10.1107/S0021889802022112 [28] R. K. Harris, “Comments on N. M. R. Spectra of the XnAA’Xn’ Type,” Canadian Journal of Chemistry, Vol. 42, No. 10, 1964, pp. 2275-2281. doi:10.1139/v64-334
|