 International Journal of Analytical Mass Spectrometry and Chromatography, 2013, 1, 95-102 Published Online December 2013 (http://www.scirp.org/journal/ijamsc) http://dx.doi.org/10.4236/ijamsc.2013.12012 Open Access IJAMSC A Simple and Fast Separation Method of Fe Employing Extraction Resin for Isotope Ra tio Determi nation by Multicollector ICP-MS Akio Makishima The Pheasant Memorial Laboratory for Geochemistry and Cosmochemistry (PML), Institute for Study of the Earth’s Interior, Okayama University, Misasa, Tottori, Japan Email: max@misasa.okayama-u.ac.jp Received October 17, 2013; revised November 13, 2013; accepted December 16, 2013 Copyright © 2013 Akio Makishima. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT A new, simple and fast separation method for Fe using an extraction chromatographic resin, Aliquat 336 (commercially available as TEVA resin) has been developed. A one milliliter column containing 0.33 mL TEVA resin on 0.67 mL CG-71C was used. Iron was adsorbed with 6 mol·L−1 HCl + H2O2 on TEVA resin, and recovered with 2 mol·L−1 HNO3. The recovery yield and total blank were 93.5 ± 6.5% and 6 ng, respectively. The separation method is simple, and takes <2 hours. For evaluation of the Fe separation, Fe isotope ratios were measured by a double-spike method employing multicollector inductively coupled plasma source mass spectrometry (MC-ICP-MS) with repeatability of 0.06‰ (SD) for the standard solution and ~0.05‰ for the silicate samples. Therefore, the column chemistry developed in this study is a viable option for Fe isotope ratio measurement by MC-ICP-MS. Keywords: Fe Separation; Fe Isotope Ratio; Multicollector Inductively Coupled Plasma Source Mass Spectrometry 1. Introduction Trioctylmethylammonium chloride (Aliquat 336) works as an anion exchanger, and is used in extraction chroma- tography (commercially sold as TEVA resin) [1]. Yang and Pin [2] and Grahek and Macefat [3] have tried to use the TEVA resin for Fe separation. The eluent volumes of 60 mL for 1 mL of the TEVA resin and 120 mL for 3 g (equates ~2.7 mL) TEVA resin were used, respectively. However, these volumes are too mamy to handle; large space is required and evaporation takes a long time. Re- cently, Makishima and Nakamura [4] successfully puri- fied Zn using a one milliliter column composed of 0.33 mL TEVA resin on 0.67 mL CG-71C resin. They used the advantage of acid resistance of the TEVA resin [5], namely, HNO3 was used in the final step to recover Zn that was strongly adsorbed on the resin. Based on this novel finding, it was noticed that the anionic character of the TEVA resin could be applied to separation of Fe. This study applies the TEVA resin for purification of Fe for isotope ratio measurements using multicollector (MC)-ICP-MS for the first time. An advantage of the new TEVA resin column chemis- try is that the whole column chemistry (from sample loading to Fe collection) finishes <2 hours. Anion ex- change resins, AG 1X8 or AG MP-1 employing Fe(III) chloro complex are commonly used [6,7]. In a one milli- liter of AG 1X8 column case, for example, the total volume of 55 mL [7], was used from washing the column to collection of Fe. Using an AG MP-1 column, total elution volume for Fe was reduced to 22 mL [6]. For evaluation of column chemistry and mass spec- trometry developed in this study, three USGS (the US Geological Survey) standard silicate reference materials, seven GSJ (the Geological Survey of Japan) standard silicate reference materials and three carbonaceous chon- drites of Orgueil, Murchison and Allende were used as test samples. Then Fe isotope ratios were measured using a double spike method [8-11] at high mass resolution by MC-ICP-MS to show the validity of the method. 2. Experimental 2.1. Reagents All experiments were performed in clean rooms and clean benches with HEPA (High Efficiency Particulate  A. MAKISHIMA 96 Air) filter in the Pheasant Memorial Laboratory (PML) [12]. Water and HF were purified as described elsewhere [12]. Hydrochloric acid was distilled by a Teflon-two- bottle distiller [12]. TAMAPURE-AA-100 grade per- chloric acid (Tama Chemicals Co., Ltd., Japan), electric industry (EL) grade HNO3 and ultrapure hydrogen per- oxide (Kanto Chemical Co. Inc., Japan) were used with- out purification. Six mol·L−1HCl with 0.05 % (v/v) H2O2 was prepared just before the column chemistry. For the column calibration, two multi-element standard solutions (Specpure, Nos. 42885 and 44270, Alfa Aesar, USA) were used. CAUTION: HF, HCl, HNO3, HClO4 and H2O2 are highly corrosive and toxic. Inhalation and contact with skin and eyes should be avoided. They should be handled with protective glasses and gloves. Iron standard metal, IRMM-014 was dissolved, and finally diluted into 1 μg·mL−1 with 0.5 mol·L−1 HNO3 and used as the isotopic standard for MC-ICP-MS. TE- VA extraction resin (100 - 150 μm, Eichrom Technolo- gies, Inc., USA) and Amberchrom CG-71C (Rohm and Haas, Co., USA) were soaked and stored in water. TEVA resin and CG-71C were not reused. 2.2. Iron Double Spike A 57Fe-58Fe double spike [10,11] was chosen. Iron iso- topes of 57Fe and 58Fe, with enrichments of 86.06 and 73.51%, respectively, were purchased from Oak Ridge National Laboratory (USA). Each spike was dissolved and diluted with HNO3, then mixed and used as a Fe double spike. Ideal isotopic abundances of the double spike were 47.65 and 52.35% for 57Fe and 58Fe, respec- tively [10], and the best sample: spike mole ratio is 55:45 [10]. Therefore, 1 μg sample Fe was mixed with 0.8 μg double-spike. To keep this ratio, the Fe concentration in the sample solution was determined before the spike ad- dition by high-resolution ICP-MS [13]. 2.3. Silicate Samples Three USGS silicate reference materials, BHVO-1 (ba- salt), AGV-1 (andesite) and PCC-1 (peridotite) and seven GSJ silicate reference materials, JB-1, JB-2, JB-3 (ba- salts), JA-1, JA-2, JA-3 (andesites) and JP-1 (peridotite) were used as test samples. Powder of bulk carbonaceous chondrites, Orgueil (CI1), Murchison (CM2) and Allende (CV3) were also employed. 2.4. TEVA Resin Column and Silicate Sample Solution The TEVA resin column was prepared by packing 0.33 mL of TEVA resin on 0.67 mL of CG-71C in a 1 mL polypropylene column (5 cm × 5 mm in diameter, Muro- machi Chemicals Inc., Japan) [4]. The CG-71C resin was used for absorbing organic materials and controlling the elution rate. Silicate powder samples were digested by a usual sample digestion method in our laboratory [13]. In short, samples were digested with HF-HClO4, dried to decom- pose fluorides with HClO4 again [14], evaporated with HCl, and finally dissolved with 0.5 mol·L−1 HNO3. The final dilution was typically ~250 times (20 mg silicate samples into 5 mL). 2.5. Iron Separation by the TEVA Resin Column A Fe separation procedure using the TEVA resin column is summarized in Table 1. All H2O2 concentration in Table 1 is 0.05 % (v/v) H2O2. The resin bed was washed twice with 2 mol·L−1 HNO3, subsequently washed once with 6 mol·L−1HCl, and conditioned. The sample solu- tion containing 1 μg of Fe was added with the solution containing 0.8 μg Fe double spike and 0.6 mL of 6 mol· L −1 HCl, and dried. Then the sample was dissolved with 0.1 mL of 6 mol·L−1 HCl with H2O2, loaded on the resin bed and left for 5 min (see Results and Discussion). The major elements were washed away by addition of 3.2 mL of 6 mol·L−1 HCl with H2O2. Then Fe was col- lected with 6.4 mL of 2 mol·L−1 HNO3. The Fe fraction was dried at 80˚C for 10 hours with one drop of HClO4 to decompose organic materials and small resin particles. To evaporate HClO4 completely, the sample was finally heated at 195˚C for 6 hours. Then the purified Fe was dissolved with 1 mL of 0.5 mol·L−1 HNO3, which is ready for MC-ICP-MS measurement. Although the flow rate of the TEVA column is 0.3 mL· mi n −1, which is rather high, breakthrough of Fe does not occur. As the total elution volume including washing of the column is 32.1 mL, the column chemistry can be finished within two hours including washing and condi- tioning the resin bed. This is one of the largest advan- tages of the TEVA resin column chemistry developed in this study. In a case of 1 mL of AG 1X8, the flow rate of a column is ~0.2 mL·min−1, and the total elution volume is ~55 mL [7]. Thus it takes more than 5 hours to collect Fe. In addition in other studies, one pass of anion ex- Table 1. Chemical procedure for Fe purification using TE- VA resin column. 2 mol·L−1 HNO3 6.4 mL water 1.6 mL 2 mol·L−1 HNO3 6.4 mL water 1.6 mL Washing 6 mol·L−1 HCl 3.2 mL Conditioning 6 mol·L−1 HCl + H2O23.2 mL Loading the sample (leave 5 min) 6 mol·L−1 HCl + H2O20.1 mL Removing major elements 6 mol·L−1 HCl + H2O23.2 mL Collecting Fe 2 mol·L−1 HNO3 6.4 mL Open Access IJAMSC 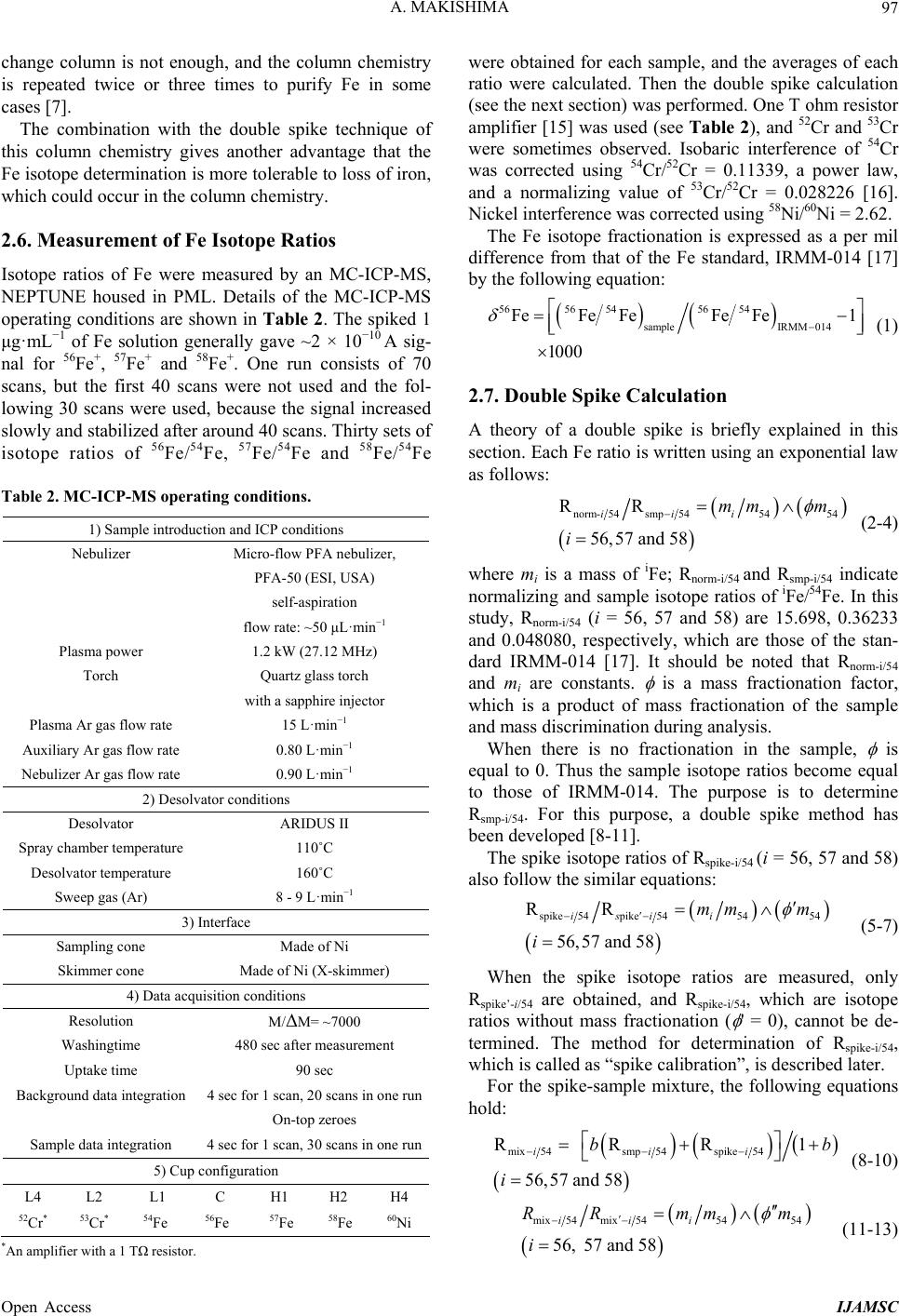 A. MAKISHIMA 97 change column is not enough, and the column chemistry is repeated twice or three times to purify Fe in some cases [7]. The combination with the double spike technique of this column chemistry gives another advantage that the Fe isotope determination is more tolerable to loss of iron, which could occur in the column chemistry. 2.6. Measurement of Fe Isotope Ratios Isotope ratios of Fe were measured by an MC-ICP-MS, NEPTUNE housed in PML. Details of the MC-ICP-MS operating conditions are shown in Table 2 . The spiked 1 μg·mL−1 of Fe solution generally gave ~2 × 10−10 A sig- nal for 56Fe+, 57Fe+ and 58Fe+. One run consists of 70 scans, but the first 40 scans were not used and the fol- lowing 30 scans were used, because the signal increased slowly and stabilized after around 40 scans. Thirty sets of isotope ratios of 56Fe/54Fe, 57Fe/54Fe and 58Fe/54Fe Table 2. MC-ICP-MS operating conditions. 1) Sample introduction and ICP conditions Nebulizer Micro-flow PFA nebulizer, PFA-50 (ESI, USA) self-aspiration flow rate: ~50 μL·min−1 Plasma power 1.2 kW (27.12 MHz) Torch Quartz glass torch with a sapphire injector Plasma Ar gas flow rate 15 L·min−1 Auxiliary Ar gas flow rate 0.80 L·min−1 Nebulizer Ar gas flow rate 0.90 L·min−1 2) Desolvator conditions Desolvator ARIDUS II Spray chamber temperature 110˚C Desolvator temperature 160˚C Sweep gas (Ar) 8 - 9 L·min−1 3) Interface Sampling cone Made of Ni Skimmer cone Made of Ni (X-skimmer) 4) Data acquisition conditions Resolution M/ΔM= ~7000 Washingtime 480 sec after measurement Uptake time 90 sec Background data integration 4 sec for 1 scan, 20 scans in one run On-top zeroes Sample data integration 4 sec for 1 scan, 30 scans in one run 5) Cup configuration L4 L2 L1 C H1 H2 H4 52Cr* 53Cr* 54Fe 56Fe 57Fe 58Fe 60Ni *An amplifier with a 1 TΩ resistor. were obtained for each sample, and the averages of each ratio were calculated. Then the double spike calculation (see the next section) was performed. One T ohm resistor amplifier [15] was used (see Table 2), and 52Cr and 53Cr were sometimes observed. Isobaric interference of 54Cr was corrected using 54Cr/52Cr = 0.11339, a power law, and a normalizing value of 53Cr/52Cr = 0.028226 [16]. Nickel interference was corrected using 58Ni/60Ni = 2.62. The Fe isotope fractionation is expressed as a per mil difference from that of the Fe standard, IRMM-014 [17] by the following equation: 5656 5456 54 sampleIRMM 014 FeFe FeFe Fe1 1000 (1) 2.7. Double Spike Calculation A theory of a double spike is briefly explained in this section. Each Fe ratio is written using an exponential law as follows: norm- 54smp545454 RR 56,57 and 58 iii mm m i (2-4) where mi is a mass of iFe; Rnorm-i/54 and Rsmp-i/54 indicate normalizing and sample isotope ratios of iFe/54Fe. In this study, Rnorm-i/54 (i = 56, 57 and 58) are 15.698, 0.36233 and 0.048080, respectively, which are those of the stan- dard IRMM-014 [17]. It should be noted that Rnorm-i/54 and mi are constants. is a mass fractionation factor, which is a product of mass fractionation of the sample and mass discrimination during analysis. When there is no fractionation in the sample, is equal to 0. Thus the sample isotope ratios become equal to those of IRMM-014. The purpose is to determine Rsmp-i/54. For this purpose, a double spike method has been developed [8-11]. The spike isotope ratios of Rspike-i/54 (i = 56, 57 and 58) also follow the similar equations: spike 54pike545454 RR 56,57 and 58 isi i mm m i (5-7) When the spike isotope ratios are measured, only Rspike’-i/54 are obtained, and Rspike-i/54, which are isotope ratios without mass fractionation ( ' = 0), cannot be de- termined. The method for determination of Rspike-i/54, which is called as “spike calibration”, is described later. For the spike-sample mixture, the following equations hold: mixsmp spik5454 54e 56, R RR 57 and 58 1 iii bb i (8-10) mix 54mix545454 56, 57and58 iii RRmm m i (11-13) Open Access IJAMSC  A. MAKISHIMA 98 where b is mixing mole ratio of the sample to the spike. The three isotope ratios of the spike-sample mixtures (Rmix'-i/54; i = 56, 57 and 58) are measured. As there are nine variables (Rsmp-i/54, Rmix-i/54, b, and ''; i = 56, 57 and 58) and nine equations (Equations (2-4); (8-13)), there should be solutions for these variables. In this study, a calculation [9] to solve these equations was followed, in which exponential approximation is used. Finally, a fractionation degree of the unknown sample, δ56Fe (Equation (1)) can be determined. The spike calibration method is as follows. First, the pure spike is measured. In this study, the averages of the pure spike isotope ratios, Rspike'-i/54 (i = 56, 57 and 58) are obtained to be 25.927, 52.110 and 58.973, respec- tively. Then, mixtures of the spike and the standard, IRMM-014 were prepared and measured. Then, the spike isotope ratio of Rspike-56/54 and ' are determined to mini- mize the difference between the 56Fe/54Fe ratio of IRMM-014 and the average of the 56Fe/54Fe ratio ob- tained from the double spikes calculation using Micro- soft-Excel “Solver”. In the actual sample measurement, the spike-sample mixture is bracketed by the spike-standard mixture. Then from all the spike-standard isotope ratios, the average of Rspike-56/54 and the optimum ' in one session are obtained. Then the 56Fe/54Fe ratios of the sample and the standard before and after the sample measurement are calculated. Finally, δ56Fe of each sample is determined. In this cal- culation, the typical error of 56Fe/54Fe of the standard solution was 0.06‰ (SD). 3. Results and Discussion 3.1. Kinetic Effects in Adsorption of Fe For the TEVA resin, kinetic effects in adsorption of Fe are not negligible [5]. Therefore, the kinetic effects in the adsorption of Fe were examined. The Fe standard solu- tions were loaded on the TEVA column with adsorption time of 5, 10, 25 and 55 min. Then Fe was collected, and the yields were measured. Analytical results are shown in Figure 1. The recovery of Fe was ~100% after 5 min. However, the recovery yields of >5 min seem a bit lower than 100%. Therefore, the optimal adsorption reaction time is determined as 5 min. When the adsorption reaction time becomes longer than 5 min, some Fe ion cannot be desorbed from the resin by 6.4 mL of 2 mol·L−1 HNO3. 3.2. Elution Curves of Fe in the TEVA Column An elution curve of Fe of the TEVA column is shown in Figure 2. In the figure, only Mg and Fe are plotted, how- ever, other major elements in silicate samples such as Na, Al, P, Ca, Cr, Mn and Ni behave similarly to Mg. As shown in Figure 2, almost 100 % of major elements in 0 20 40 60 80 100 0 102030405060 Fe Recovery yi eld (%) Time min Figure 1. The sample adsorption time (min) after sample loading vs. the recovery yield (%). Error bars are the quan- titative analytical error of ~7%. The dotted horizontal line shows 100% yield. 0.1 1 10 100 Mg Fe 02468 Fraction (%) 10 LFe fracti on Eluent mL Wash Figure 2. Elution curves of Fe and Mg for the TEVA col- umn. Mg represents behaviors of Na, Al, P, K, Ca, Cr, Mn, Fe, Co and Ni. The horizontal axis shows total eluent vol- ume (mL). The vertical axis indicates fraction (%) recov- ered from each eluent fraction (%) compared to the added amounts on the column. The vertical axis is in the logarith- mic scale. The horizontal arrows at the top show the eluents for wash and Fe fraction, respectively. “L” indicates the sample loading solution. silicate samples added in the column are washed away in the first 1.6 mL of 6 mol·L−1HCl with H2O2. The total yield of Fe using actual silicate samples was 93.5 ± 6.5 % (SD). This result means that there could be a small loss of Fe in this study. Such loss of Fe could cause iso- topic fractionation [18]. However, such fractionation can be corrected by the double spike method employed in this study. Zinc, Ga, Nb, Mo, Ta, W and U were contained in the Fe fraction with yields of 60% - 90%. However, in usual silicate samples, amounts of these elements compared to 1 μg Fe are <ng levels, therefore, effects of these impuri- ties are inconsequential. Furthermore, total yields of Nb, Ta and W from the sample solution should be lower than Open Access IJAMSC  A. MAKISHIMA Open Access IJAMSC 99 those of the starting solution, because co-precipitation with Ti oxides of Nb, Ta and W should occur during sample evaporation using HClO4 [19]. The total blanks including sample digestion were 6 ng (n = 6). As δ56Fe range of all natural samples is <±3‰ [7], δ56Fe of blank can be assumed to be <±3‰. Since Fe in the sample is 1 μg, the maximum shift of δ56Fe by the blank should be <±0.02‰. As the repeatability of the standard solution was found to be 0.06‰, the blank ef- fects are less than one thirds of the repeatability of the standard solution. Thus the blank effect can be neglected. 3.3. Evaluation of Accuracy in Fe Isotope Ratio Measurement It is difficult to evaluate the accuracy in stable isotope mass spectrometry for less popular elements such as Fe, because the standard materials with accurate isotope ra- tios are limited. In this study, to examine the accuracy with variable isotope ratios and matrix elements, the synthesized samples were made by mixing of two sam- ples with different isotope ratios and matrix element compositions. Then the isotope ratios of the mixture were compared with the calculated isotope ratios. Such mixing tests were done in studies of Zn and Tl isotopes to evalu- ate the accuracy of the method [4,20]. For one sample, one of the JB-2 solutions of δ56Fe = −0.27 ± 0.03 (see the next section) was used. For the other sample, one of ferruginous bodies-digested solu- tions [21] of δ56Fe = −3.16 ± 0.09 (n = 4) (private com- munication, Makishima and Nakamura, Okayama Uni- versity, Dec. 2012) was used. This solution was prepared by ashing the ferruginous bodies separated from human lung and dissolving with nitric acid. This solution has a very low δ56Fe value, and is mostly composed of Fe [21]. The JB-2 and ferruginous bodies-digested solutions were taken and mixed to contain 1 μg of Fe. Three types of the mixtures with different matrix compositions were made. For each type of the mixtures, four or five samples were made. Each sample was added with 0.4 mL 6 mol·L−1 HCl and the Fe double spike, dried and passed through the TEVA column. Then Fe was collected and its isotope ratio was determined by MC-ICP-MS. Analytical results of the δ56Fe value of each mixture are shown in Table 3. The calculated values are also shown in Table 3 . The error in calculation for each mix- ture was based on concentration error of ~5% of two starting solutions, and no other errors are taken into ac- count. From Table 3, the observed values are consistent with the calculated values within 2SD ranges, although the three samples had different major element composi- tions. Therefore, it is suggested that the double spike method for Fe isotope measurements using MC-ICP-MS in this study gives accurate isotope ratios of Fe. 3.4. Fe Isotope Ratio Measurements in Silicate Reference Materials The TEVA column chemistry developed in this study was applied to analysis of the silicate reference materials, and analytical results are presented in Table 4, together with the reported values [11,22-26]. To make the com- parison between δ56Fe of this study and those of refer- ences easier, they are plotted in Figure 3. The δ56Fe value of BHVO-1 in this study of 0.18‰ is a little higher than those of references [22-24,26]. The δ56Fe values of AGV-1 and PCC-1 of this study are −0.10 and 0.00‰, respectively, which are consistent with those of previous studies [21,25,26] when errors are taken into account. Repeatability for silicate reference materials (Table 4) is 0.03‰ - 0.06‰, and the average is 0.05‰. This 0.05‰ should be considered as repeatability of actual silicate analysis by MC-ICP-MS in this study. As dis- cussed previously, the blank effects are ±0.02‰, how- ever, this can be negligible to 0.05‰, which is consid- ered to be repeatability of this method. This value is similar to that of the pure standard solution (0.06‰). The repeatability in the Fe standard solution is com- pared with those of references here. The SD values of replicate analyses of the standard solution are 0.013, 0.10, 0.010, 0.050 and 0.041‰ in Chicago [22], Woods Hole [23], Hannover [24], Madison [25], and Frankfurt [26] groups, respectively. Therefore the repeatability of the MC-ICP-MS methods of these leading groups which use the bracketing method is similar or a bit better than that of this study. However, all groups use AG 1X8, so they need repetitive column chemistry in many cases, because Fe cannot be purified enough by the single column chemistry to be used in the mass spectrometry. However, other groups [11] which use AG MP-1 can purify Fe by a single column chemistry. Recently, Millet et al. [11] achieved “ultra-precise” Fe measurements, using the same pair of the double spike, 57Fe-58Fe. They constantly achieved the repeatability of Table 3. Analytical results of mixing experiments. Calculated Observed δ56Fe SD (‰) δ56Fe SD (‰) n Mixture#1 −1.12 0.08 −1.36 0.04 4 Mixture#2 −1.90 0.13 −2.00 0.19 4 Mixture#3 −2.50 0.17 −2.64 0.05 5  A. MAKISHIMA 100 Table 4. δ56Fe values of USGS and GSJ silicate reference materials and carbonaceous chondrites. Sample Average SD n References δ56Fe (‰) (Error is SD) BHVO-1 0.18 0.03 3 0.105 ± 0.008 [22], 0.110 ± 0.060 [23], 0.109 ± 0.024 [24], 0.117 ± 0.015 [26] AGV-1 −0.10 0.06 3 0.04 ± 0.01 [24] PCC-1 0.00 0.02 3 0.025 ± 0.012 [22], −0.06 ± 0.06 [25], 0.043 ± 0.013 [26] JB-1 −0.14 0.05 4 JB-2 −0.27 0.03 4 0.073 ± 0.014 [11] JB-3 −0.35 0.08 3 JA-1 −0.13 0.06 4 0.060 ± 0.010 [11] JA-2 −0.02 0.03 4 JA-3 0.09 0.06 4 JP-1 −0.54 0.06 4 Orgueil −0.05 0.06 3 −0.015 ± 0.074 [26], 0.38 [27], 0.04 ± 0.06 [28] Murchison −0.10 0.12 3 −0.12 ± 0.06 [28] Allende −0.03 0.03 3 −0.007 ± 0.012 [22], −0.04 [27], −0.06 ± 0.01 [28] -0.4 -0.3 -0.2 -0.1 0 0.1 0.2 0.3 0.4 -0.4-0.3-0.2-0.100.1 0.2 0.3 0.4 Orgue il Murchison Al len de BH VO-1 AGV - 1 PCC- 1 56Fe of references 56Fe of this study Figure 3. Iron isotope ratios (δ56Fe) of this study vs. those of references. The error bars show one standard deviation of repeatability (SD, ‰). The vertical and horizontal dotted lines show δ56Fe = 0 of this study and references, respec- tively. The dotted slope line indicates slope = 1, which means that there is no difference in δ56Fe between this study and references. 0.01‰ using double-spike-MC-ICP-MS. The largest difference of this study from their study is 1) enrichment of the double spike and 2) larger usage of the sample. The 56Fe/54Fe, 57Fe/54Fe and 58Fe/54Fe ratios of the spike in this study are 24.5521, 53.0182 and 48.0436, while those of their spike are 2031, 67300 and 6812, respec- tively. This means that the denominator isotope, 54Fe of this study is far more abundant than that or Millet et al. [11], resulting in lower precision. In addition, Millet et al. [11] used a 100 μL·min−1 nebulizer and 2 μg·mL−1 solu- tion, totally 4 times larger amounts of Fe are used than that in this study. 3.5. Application to Measurements of δ56Fe in Carbonaceous Chondrites The δ56Fe values of carbonaceous chondrites, Orgueil, Murchison and Allende were measured by the method developed in this study. The δ56Fe value of Orgueil of this study agrees well with those of Weyer et al. [26] and Kehm et al. [28], but that of Zhu et al. [27] seems a bit higher. The δ56Fe values of Allende of this study also agrees well with those of previous studies [22,27,28]. However, numbers of analyses of carbonaceous chon- drites in literatures are limited, and carbonaceous chon- drites could be heterogeneous, thus further studies are required. Interference ratios of 54Cr/54Fe and 58Ni/58Fe in these carbonaceous chondrite analyses after the column chem- istry were <1.6 × 10−3 and <1.4 × 10−4, respectively. Large Cr and Ni corrections were needed in the TEVA column chemistry developed in this study, however, the analytical results suggest that the single-pass TEVA column is sufficient even in analyses of Cr rich samples such as peridotites (~3000 μg·g−1) or chondrites (~4000 μg·g−1). 4. Conclusions Using an extraction resin, TEVA, new column chemistry for separating Fe has been developed for Fe isotope ratio determination by MC-ICP-MS. Iron was purified by 6 mol· L −1 HCl + H2O2, and major elements were separated. Fe was finally recovered with 2 mol·L−1 HNO3. The re- covery yields and total blanks were 93.5% ± 6.5% (SD) and 6 ng, respectively. For evaluation of the separation method, Fe isotope ra- tios were measured by a double spike method using MC-ICP-MS, respectively. Repeatability obtained from actual analyses of USGS standard reference materials of Open Access IJAMSC  A. MAKISHIMA 101 BHVO-1, AGV-1, PCC-1, and GSJ standard silicate ma- terials of JB-1, JB-2, JB-3, JA-1, JA-2 and JA-3 were 0.05‰ (SD). Fe isotope ratios of carbonaceous chon- drites of Orgueil, Murchison and Allende were also de- termined by the column chemistries developed in this study. 5. Acknowledgements The author thanks to E. Nakamura for suggestions and supports at various aspects of this study. The author is also grateful to K. Tanaka for doing column chemistry; and T. Moriguti, C. Sakaguchi and all members of PML for maintaining the clean laboratory. The author is also grateful to K. Okabe for donating ferruginous protein bodies. REFERENCES [1] E. P. Horwitz, M. L. Dietz, R. Chiarizia, H. Diamond, S. L. III Maxwell and M. R. Nelson, “Separation and Precon- centration of Actinides by Extraction Chromatography Using a Supported Liquid Anion-Exchanger—Applica- tion to the Characterization of High-Level Nuclear Waste Solutions,” Analytica Chimica Acta, Vol. 310, No. 1, 1995, pp. 63-78. http://dx.doi.org/10.1016/0003-2670(95)00144-O [2] X.-J. Yang and C. Pin, “Separation of Hafnium and Zir- conium from Ti and Fe-Rich Geological Materials by Ex- traction Chromatography,” Analytical Chemistry, Vol. 71, No. 9, 1999, pp. 1706-1711. http://dx.doi.org/10.1021/ac980833w [3] Z. Grahek and M. R. Macefat, “Isolation of Iron and Strontium from Liquid Samples Anddetermination of Fe-55 and Sr-89, Sr-90 in Liquid Radioactive Waste,” Analytica Chimica Acta, Vol. 511, No. 2, 2004, p. 339. http://dx.doi.org/10.1016/j.aca.2004.01.049 [4] A. Makishima and E. Nakamura, “Low-Blank Chemistry for Zn Stable Isotope Ratio Determination Using Extrac- tion Chromatographic Resin and Double Spike-Multiple Collector-ICP-MS,” Journal of Analytical Atomic Spec- trometry, Vol. 28, 2013, pp. 127-133. http://dx.doi.org/10.1039/c2ja30271c [5] A. Makishima, M. Nakanishi and E. Nakamura, “A Group Separation Method of Ruthenium, Palladium, Rhe- nium, Osmium, Iridium and Platinumusing Their Bromo Complexes and an Anion Exchange Resin,” Analytical Chemistry, Vol. 73, No. 21, 2001, pp. 5240-5248. http://dx.doi.org/10.1021/ac010615u [6] D. M. Borrok, R. B. Wanty, W. I. Ridley, R. Wolf, P. J. Lamothe and M. Adams, “Separation of Copper, Iron, and Zinc from Complex Aqueous Solutions for Isotopic Meas- urement,” Chemical Geology, Vol. 242, No. 3-4, 2007, pp. 400-414. http://dx.doi.org/10.1016/j.chemgeo.2007.04.004 [7] N. Dauphas and O. Rouxel, “Mass Spectrometry and Natural Variations of Iron Isotopes,” Mass Spectrometry Reviews, Vol. 25, No. 4, 2006, pp. 515-550. http://dx.doi.org/10.1002/mas.20078 [8] M. H. Dodson, “A Theoretical Study of the Use of Inter- nal Standards for Precise Isotopic Analysis by the Surface Ionization Technique: Part I—General First-Order Alge- braic Solutions,” Journal of Scientific Instruments, Vol. 40, 1963, pp. 289-295. http://dx.doi.org/10.1088/0950-7671/40/6/307 [9] C. M. Johnson and B. L. Beard, “Correction of Instru- mentally Produced Mass Fractionation during Isotopic Analysis of Fe by Thermal Ionization Mass Spectrome- try,” International Journal of Mass Spectrometry, Vol. 193, No. 1, 1999, pp. 87-99. http://dx.doi.org/10.1016/S1387-3806(99)00158-X [10] J. F. Rudge, B. C. Reynolds and B. Bourdon, “The Dou- ble Spike Toolbox,” Chemical Geology, Vol. 265, No. 3-4, 2009, pp. 420-431. http://dx.doi.org/10.1016/j.chemgeo.2009.05.010 [11] M.-A. Millet, J. A. Baker and C. E. Payne, “Ultra-Precise Stable Fe Isotope Measurement by High Resolution Mul- tiple-Collector Inductively Coupled Plasma Mass Spec- trometry with a 57Fe-58Fe Double Spike,” Chemical Ge- ology, Vol. 304-305, 2012, pp. 18-25. http://dx.doi.org/10.1016/j.chemgeo.2012.01.021 [12] E. Nakamura, A. Makishima, T. Moriguti, K. Kobayashi, C. Sakaguchi, T. Yokoyama, R. Tanaka, T. Kuritani and H. Takei, “Comprehensive Geochemical Analyses of Small Amounts (100 mg) of Extraterrestrial Samples for the Analytical Competition Related to the Sample-Return Mission, MUSES-C,” The Institute of Space and Astro- nautical Science Report, SP No. 16, 2003, pp. 49-101. [13] A. Makishima and E. Nakamura, “Determination of Ma- jor, Minor and Trace Elements in Silicate Samples by ICP-QMS and ICP-SFMS Applying Isotope Dilution-In- ternal Standardization (ID-IS) and Multi-Stage Internal Standardization,” Geostandards and Geoanalytical Re- search, Vol. 30, No. 3, 2006, pp. 245-271. http://dx.doi.org/10.1111/j.1751-908X.2006.tb01066.x [14] T. Yokoyama, A. Makishima and E. Nakamura, “Evalua- tion of the Coprecipitation of Incompatible Trace Ele- ments with Fluoride during Silicate Rock Dissolution by Acid Digestion,” Chemical Geology, Vol. 157, No. 3, 1999, pp. 175-187. http://dx.doi.org/10.1016/S0009-2541(98)00206-X [15] A. Makishima and E. Nakamura, “Precise Isotopic De- termination of Hf and Pb at Sub-Nano Gram Levels by MC-ICPMS Employing a Newly Designed Sample Cone and a Pre-Amplifier with a 1012 Ohm Register,” Journal of Analytical Atomic Spectrometry, Vol. 25, No. 11, 2010, pp. 1712-1716. http://dx.doi.org/10.1039/c0ja00015a [16] A. Yamakawa, K. Yamashita, A. Makishima and E. Na- kamura, “Chemical Separation and Mass Spectrometry of Cr, Fe, Ni, Zn and Cu in Terrestrial and Extraterrestrial Materials Using Thermal Ionization Mass Spectrometry,” Analytical Chemistry, Vol. 81, No. 23, 2009, pp. 9787- 9794. http://dx.doi.org/10.1021/ac901762a [17] P. D. P. Taylor, R. Maeck and P. De Bievre, “Determina- tion of the Absolute Isotopic Composition and Atomic- Weight of a Reference Sample of Natural Iron,” Interna- tional Journal of Mass Spectrometry and Ion Processes, Vol. 121, No. 1-2, 1992, pp. 111-125. Open Access IJAMSC  A. MAKISHIMA Open Access IJAMSC 102 http://dx.doi.org/10.1016/0168-1176(92)80075-C [18] N. Dauphas, P. E. Janney, R. A. Mendybaev, M. Wadhwa, F. M. Richter, A. M. Davis, M. van Zuilen, R. Hines and C. N. Foley, “Chromatographic Separation and Multicol- lection-ICPMS Analysis of Iron. Investigating Mass- Dependent and -Independent Isotope Effects,” Analytical Chemistry, Vol. 76, No. 19, 2004, pp. 5855-5863. http://dx.doi.org/10.1021/ac0497095 [19] A. Makishima and E. Nakamura, “New Preconcentration Technique of Zr, Nb, Mo, Hf, Ta and W Employing Co- precipitation with Ti Compounds: Its Application to Lu- Hf System and Sequential Pb-Sr-Nd-Sm Separation,” Geochemical Journal, Vol. 42, No. 2, 2008, pp. 199-206. http://dx.doi.org/10.2343/geochemj.42.199 [20] S. G. Nielsen, M. Rehkämper, J. Baker and A. N. Halli- day, “The Precise and Accurate Determination of Thal- lium Isotope Compositions and Concentrations for Water Samples by MC-ICPMS,” Chemical Geology, Vol. 204, No. 1, 2004, pp. 109-124. http://dx.doi.org/10.1016/j.chemgeo.2003.11.006 [21] E. Nakamura, A. Makishima, K. Hagino and K. Okabe, “Accumulation of Radium in Ferruginous Protein Bodies Formed in Lung Tissue: Association of Resulting Radia- tion Hotspots with Malignant Mesothelioma and Other Malignancies,” Proceedings of the Japan Academy, Se- ries B, Vol. 85, 2009, pp. 229-239. http://dx.doi.org/10.2183/pjab.85.229 [22] P. R. Craddock and N. Dauphas, “Iron Isotopic Composi- tions of Geological Reference Materials and Chondrites,” Geostandards and Geoanalytical Research, Vol. 35, No. 1, 2011, pp. 101-123. http://dx.doi.org/10.1111/j.1751-908X.2010.00085.x [23] O. J. Rouxel, A. Bekker and K. J. Edwards, “Iron Isotope Constraints on the Archean and Paleoproterozoic Ocean Redox State,” Science, Vol. 307, No. 5712, 2005, pp. 1088- 1091. http://dx.doi.org/10.1126/science.1105692 [24] J. A. Schuessler, R. Schoenberg and O. Sigmarsson, “Iron and Lithium Isotope Systematics of the Hekla Volcano, Iceland—Evidence for Fe Isotope Fractionation during Magma Differentiation,” Chemical Geology, Vol. 258, No. 1-2, 2009, pp. 78-91. http://dx.doi.org/10.1016/j.chemgeo.2008.06.021 [25] B. L. Beard, C. M. Johnson, J. L. Skulan, K. H. Nealson, L. Cax and H. Sun, “Application of Fe Isotopes to Trac- ing the Geochemical and Biological Cycling of Fe,” Chemical Geology, Vol. 195, No. 1, 2003, pp. 87-117. http://dx.doi.org/10.1016/S0009-2541(02)00390-X [26] S. Weyer, A. D. Anbar, G. P. Brey, C. Muenker, K. Mezger and A. B. Woodland, “Iron Isotope Fractionation during Planetary Differentiation,” Earth and Planetary Science Letters, Vol. 240, No. 2, 2005, pp. 251-264. http://dx.doi.org/10.1016/j.epsl.2005.09.023 [27] X. K. Zhu, Y. Guo, R. K. O’Nions, E. D. Young and R. D. Ash, “Isotopic Heterogeneity of Iron in the Early Solar Nebula,” Nature, Vol. 412, No. 6844, 2001, pp. 311-313. http://dx.doi.org/10.1038/35085525 [28] K. Kehm, E. H. Hauri, C. M. O’D. Alexander and R. W. Carlson, “High Precision Iron Isotope Measurements of Meteoritic Material by Cold Plasma ICP-MS,” Geo- chimica Cosmochimica Acta, Vol. 67, No. 15, 2003, pp. 2879-2891. http://dx.doi.org/10.1016/S0016-7037(03)00080-2
|