Paper Menu >>
Journal Menu >>
 Advances in Chemical Engineering and Science, 2011, 1, 15-19 doi:10.4236/aces.2011.11003 Published Online January 2011 (http://www.SciRP.org/journal/aces) Copyright © 2011 SciRes. ACES Halloysite Nanotubes Supported Gold Catalyst for Cyclohexene Oxidation with Molecular Oxygen Zhen-Yu Cai1, Ming-Qiao Zhu1*, Huan Dai1, Yi Liu1, Jian-Xin Mao2, Xin-Zhi Chen1, Chao-Hong He1 1Department of Chemical and Biochemical Engineering, Zhejiang University, Hangzhou, China 2Department of Chemistry, Zhejiang University, Hangzhou, China E-mail: zhumingqiao@zju.edu.cn Received January 6, 2011; revised January 18, 2011; accepted January 20, 2011 Abstract The selective oxidation of cyclohexene to 2-cyclohexene-1-ol and 2-cyclohexene-1-one has been investi- gated over Au/HNTs (HNTs: halloysite nanotubes) catalysts with molecular oxygen in a solvent-free system. The catalysts were prepared by deposition precipitation method and characterized by ICP-AES, TEM and XRD. The results show that the catalytic performance of Au/HNTs is quite well and the catalytic activity over recycled catalyst remains highly. Moreover, the nano-size effect of gold is also reported for the reaction. Keywords: Gold Catalyst, HNTs, Cyclohexene Oxidation, Oxygen 1. Introduction The catalytic oxidation of hydrocarbons into value-added oxygenated derivatives is still a challenge in modern chemistry and industry world [1-3]. In particular, the oxidation of cyclohexene is often inefficient as there is a C = C bound and four α-H atoms in the cyclohexene molecule. Oxidation of cyclohexene is an important method for the synthesis of chemical intermediates like 2-cyclohexene-1-ol and 2-cyclohexene-1-one in the manufacture of high-value pharmaceuticals [4]. A greater demand for these oxidation products and increased envi- romental concerns warrant the introduction of catalytic systems using heterogeneous catalyst and the environ- mentally friendly oxidants such as molecular oxygen or hydrogen peroxide [5]. The use of H2O2 is atom efficient and the only by-product is water, but the relatively high cost of H2O2 severely hinders its wide application in catalytic oxidation [6]. On the other hand, catalytic sys- tems using oxygen as the oxidant instead resulted in three important advantages: the facility to separate the catalyst after the reaction, lower energy costs and a higher stability of the irreversible reaction of over- oxidantion products [7,8]. Therefore, oxidation of cyclohexene with oxygen under solvent-free condition would be valuable. In recent years, an increasing interest has been di- rected to the catalytic potential of gold catalysts [9-12]. Supported gold catalysts have been extensively studied for a wide range of oxidation reactions including CO oxidation [13], propylene epoxidation [14], the direct synthesis of hydrogen peroxide from oxygen and hydro- gen [15,16], oxidation of cyclohexane to KA oil [17-19], etc.. Particularly, the partial liquid-phase cyclohexene oxidation using gold catalysts including Au/C and Au/CNTs makes gold even more attractive [20,21]. Un- fortunately, as for the Au/C catalyst, it has good catalytic performance only with the addition of special organic solvent [20]. The performance of Au/CNTs catalyst is influenced by the amount of TBHP [21]. Compared Au/C and Au/CNTs, we can see that the structure of the carrier has great effect on the catalytic performance of supported gold. As silica and Al2O3 are quite common industrial materials as catalysts support material because of their relative stability, high surface area and low price. Au/Al2O3 and Au/SiO2 are quite effective in cyclohexane oxidation [8,22], but there is little report about alumi- nosilicate-supported Au catalyst. HNTs (halloysite nanotubes) is a special kind of aluminosilicate. The ob- jective of this work is to report catalyst Au/HNTs of very low metal loadings and the effect of nano-size of gold on catalytic performance for the selective oxidation of cyclohexene using molecular oxygen in a solvent-free system. 2. Experimental Au/HNTs catalysts with varied gold loadings were pre- 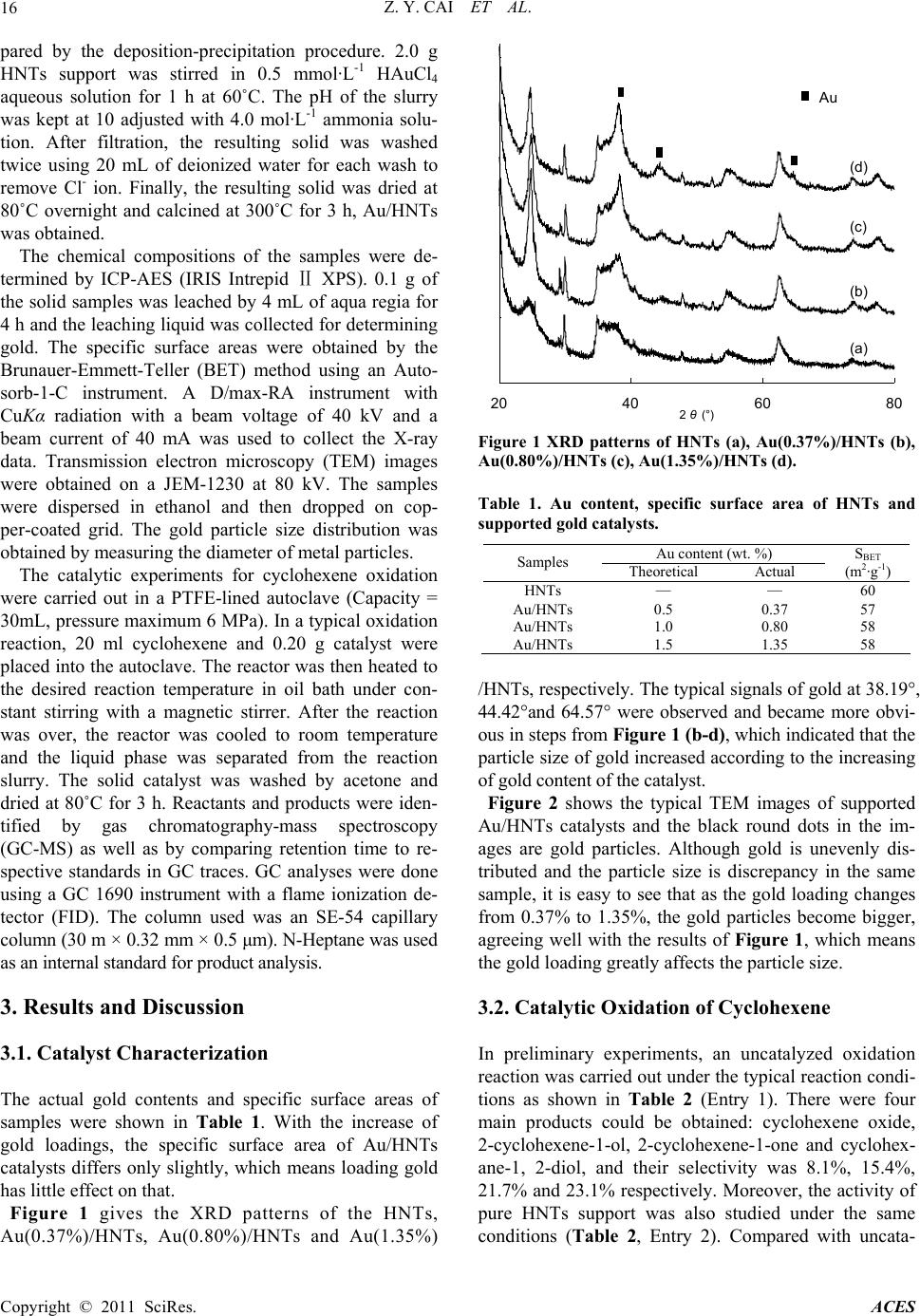 Z. Y. CAI ET AL. Copyright © 2011 SciRes. ACES 16 pared by the deposition-precipitation procedure. 2.0 g HNTs support was stirred in 0.5 mmol·L-1 HAuCl4 aqueous solution for 1 h at 60˚C. The pH of the slurry was kept at 10 adjusted with 4.0 mol·L-1 ammonia solu- tion. After filtration, the resulting solid was washed twice using 20 mL of deionized water for each wash to remove Cl- ion. Finally, the resulting solid was dried at 80˚C overnight and calcined at 300˚C for 3 h, Au/HNTs was obtained. The chemical compositions of the samples were de- termined by ICP-AES (IRIS Intrepid XPS). 0.1 g of Ⅱ the solid samples was leached by 4 mL of aqua regia for 4 h and the leaching liquid was collected for determining gold. The specific surface areas were obtained by the Brunauer-Emmett-Teller (BET) method using an Auto- sorb-1-C instrument. A D/max-RA instrument with CuKα radiation with a beam voltage of 40 kV and a beam current of 40 mA was used to collect the X-ray data. Transmission electron microscopy (TEM) images were obtained on a JEM-1230 at 80 kV. The samples were dispersed in ethanol and then dropped on cop- per-coated grid. The gold particle size distribution was obtained by measuring the diameter of metal particles. The catalytic experiments for cyclohexene oxidation were carried out in a PTFE-lined autoclave (Capacity = 30mL, pressure maximum 6 MPa). In a typical oxidation reaction, 20 ml cyclohexene and 0.20 g catalyst were placed into the autoclave. The reactor was then heated to the desired reaction temperature in oil bath under con- stant stirring with a magnetic stirrer. After the reaction was over, the reactor was cooled to room temperature and the liquid phase was separated from the reaction slurry. The solid catalyst was washed by acetone and dried at 80˚C for 3 h. Reactants and products were iden- tified by gas chromatography-mass spectroscopy (GC-MS) as well as by comparing retention time to re- spective standards in GC traces. GC analyses were done using a GC 1690 instrument with a flame ionization de- tector (FID). The column used was an SE-54 capillary column (30 m × 0.32 mm × 0.5 μm). N-Heptane was used as an internal standard for product analysis. 3. Results and Discussion 3.1. Catalyst Characterization The actual gold contents and specific surface areas of samples were shown in Table 1. With the increase of gold loadings, the specific surface area of Au/HNTs catalysts differs only slightly, which means loading gold has little effect on that. Figure 1 gives the XRD patterns of the HNTs, Au(0.37%)/HNTs, Au(0.80%)/HNTs and Au(1.35%) 20 4060 80 2 θ (°) A u (d) (c) (b) (a) Figure 1 XRD patterns of HNTs (a), Au(0.37%)/HNTs (b), Au(0.80%)/HNTs (c), Au(1.35%)/HNTs (d). Table 1. Au content, specific surface area of HNTs and supported gold catalysts. Au content (wt. %) Samples Theoretical Actual SBET (m2·g-1) HNTs — — 60 Au/HNTs 0.5 0.37 57 Au/HNTs 1.0 0.80 58 Au/HNTs 1.5 1.35 58 /HNTs, respectively. The typical signals of gold at 38.19°, 44.42°and 64.57° were observed and became more obvi- ous in steps from Figure 1 (b-d), which indicated that the particle size of gold increased according to the increasing of gold content of the catalyst. Figure 2 shows the typical TEM images of supported Au/HNTs catalysts and the black round dots in the im- ages are gold particles. Although gold is unevenly dis- tributed and the particle size is discrepancy in the same sample, it is easy to see that as the gold loading changes from 0.37% to 1.35%, the gold particles become bigger, agreeing well with the results of Figure 1, which means the gold loading greatly affects the particle size. 3.2. Catalytic Oxidation of Cyclohexene In preliminary experiments, an uncatalyzed oxidation reaction was carried out under the typical reaction condi- tions as shown in Table 2 (Entry 1). There were four main products could be obtained: cyclohexene oxide, 2-cyclohexene-1-ol, 2-cyclohexene-1-one and cyclohex- ane-1, 2-diol, and their selectivity was 8.1%, 15.4%, 21.7% and 23.1% respectively. Moreover, the activity of pure HNTs support was also studied under the same conditions (Table 2, Entry 2). Compared with uncata- 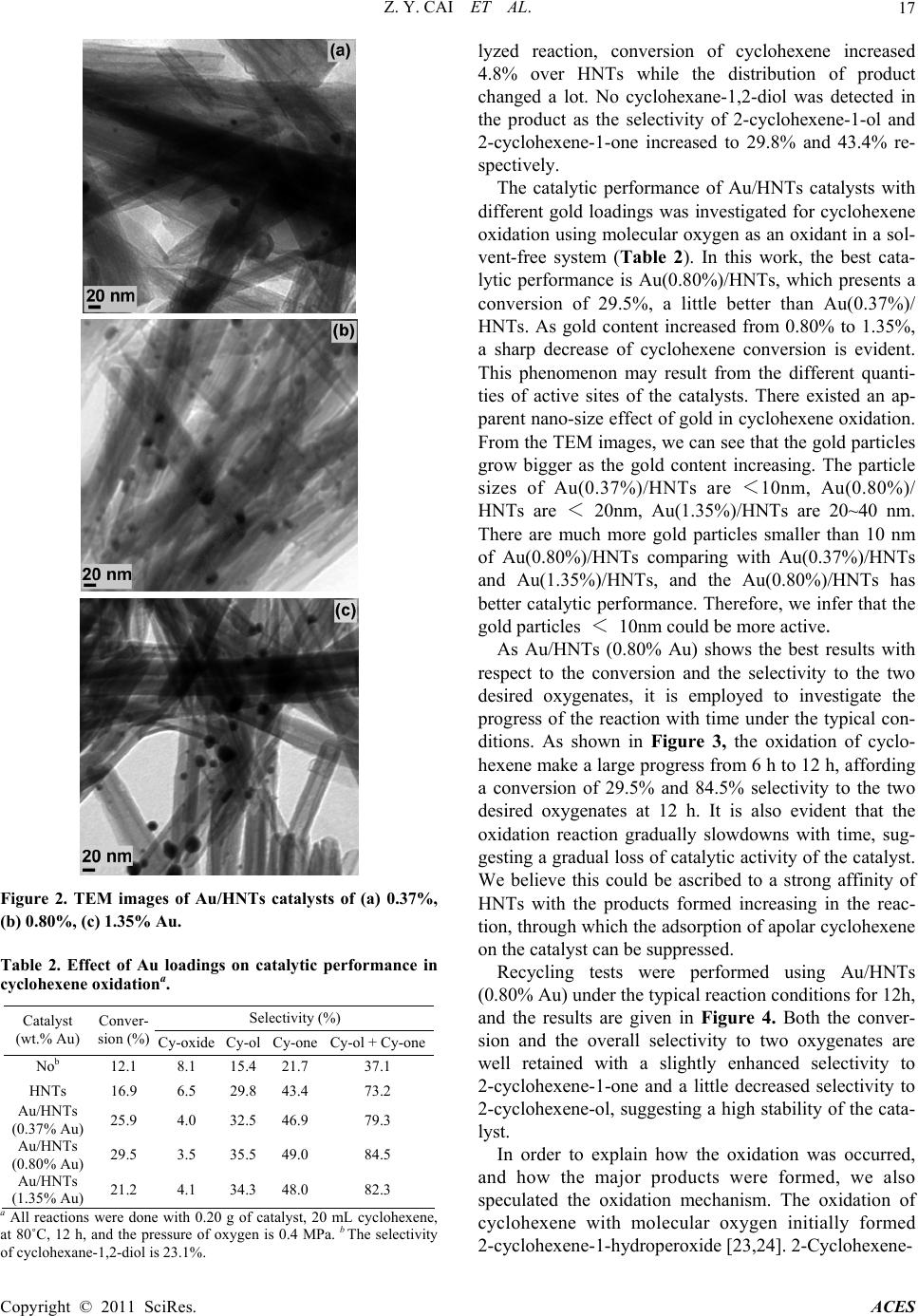 Z. Y. CAI ET AL. Copyright © 2011 SciRes. ACES 17 Figure 2. TEM images of Au/HNTs catalysts of (a) 0.37%, (b) 0.80%, (c) 1.35% Au. Table 2. Effect of Au loadings on catalytic performance in cyclohexene oxidationa. Selectivity (%) Catalyst (wt.% Au) Conver- sion (%) Cy-oxide Cy-ol Cy-one Cy-ol + Cy-one Nob 12.1 8.1 15.4 21.7 37.1 HNTs 16.9 6.5 29.8 43.4 73.2 Au/HNTs (0.37% Au) 25.9 4.0 32.5 46.9 79.3 Au/HNTs (0.80% Au) 29.5 3.5 35.5 49.0 84.5 Au/HNTs (1.35% Au) 21.2 4.1 34.3 48.0 82.3 a All reactions were done with 0.20 g of catalyst, 20 mL cyclohexene, at 80˚C, 12 h, and the pressure of oxygen is 0.4 MPa. b The selectivity of cyclohexane-1,2-diol is 23.1%. lyzed reaction, conversion of cyclohexene increased 4.8% over HNTs while the distribution of product changed a lot. No cyclohexane-1,2-diol was detected in the product as the selectivity of 2-cyclohexene-1-ol and 2-cyclohexene-1-one increased to 29.8% and 43.4% re- spectively. The catalytic performance of Au/HNTs catalysts with different gold loadings was investigated for cyclohexene oxidation using molecular oxygen as an oxidant in a sol- vent-free system (Table 2). In this work, the best cata- lytic performance is Au(0.80%)/HNTs, which presents a conversion of 29.5%, a little better than Au(0.37%)/ HNTs. As gold content increased from 0.80% to 1.35%, a sharp decrease of cyclohexene conversion is evident. This phenomenon may result from the different quanti- ties of active sites of the catalysts. There existed an ap- parent nano-size effect of gold in cyclohexene oxidation. From the TEM images, we can see that the gold particles grow bigger as the gold content increasing. The particle sizes of Au(0.37%)/HNTs are <10nm, Au(0.80%)/ HNTs are < 20nm, Au(1.35%)/HNTs are 20~40 nm. There are much more gold particles smaller than 10 nm of Au(0.80%)/HNTs comparing with Au(0.37%)/HNTs and Au(1.35%)/HNTs, and the Au(0.80%)/HNTs has better catalytic performance. Therefore, we infer that the gold particles < 10nm could be more active. As Au/HNTs (0.80% Au) shows the best results with respect to the conversion and the selectivity to the two desired oxygenates, it is employed to investigate the progress of the reaction with time under the typical con- ditions. As shown in Figure 3, the oxidation of cyclo- hexene make a large progress from 6 h to 12 h, affording a conversion of 29.5% and 84.5% selectivity to the two desired oxygenates at 12 h. It is also evident that the oxidation reaction gradually slowdowns with time, sug- gesting a gradual loss of catalytic activity of the catalyst. We believe this could be ascribed to a strong affinity of HNTs with the products formed increasing in the reac- tion, through which the adsorption of apolar cyclohexene on the catalyst can be suppressed. Recycling tests were performed using Au/HNTs (0.80% Au) under the typical reaction conditions for 12h, and the results are given in Figure 4. Both the conver- sion and the overall selectivity to two oxygenates are well retained with a slightly enhanced selectivity to 2-cyclohexene-1-one and a little decreased selectivity to 2-cyclohexene-ol, suggesting a high stability of the cata- lyst. In order to explain how the oxidation was occurred, and how the major products were formed, we also speculated the oxidation mechanism. The oxidation of cyclohexene with molecular oxygen initially formed 2-cyclohexene-1-hydroperoxide [23,24]. 2-Cyclohexene- 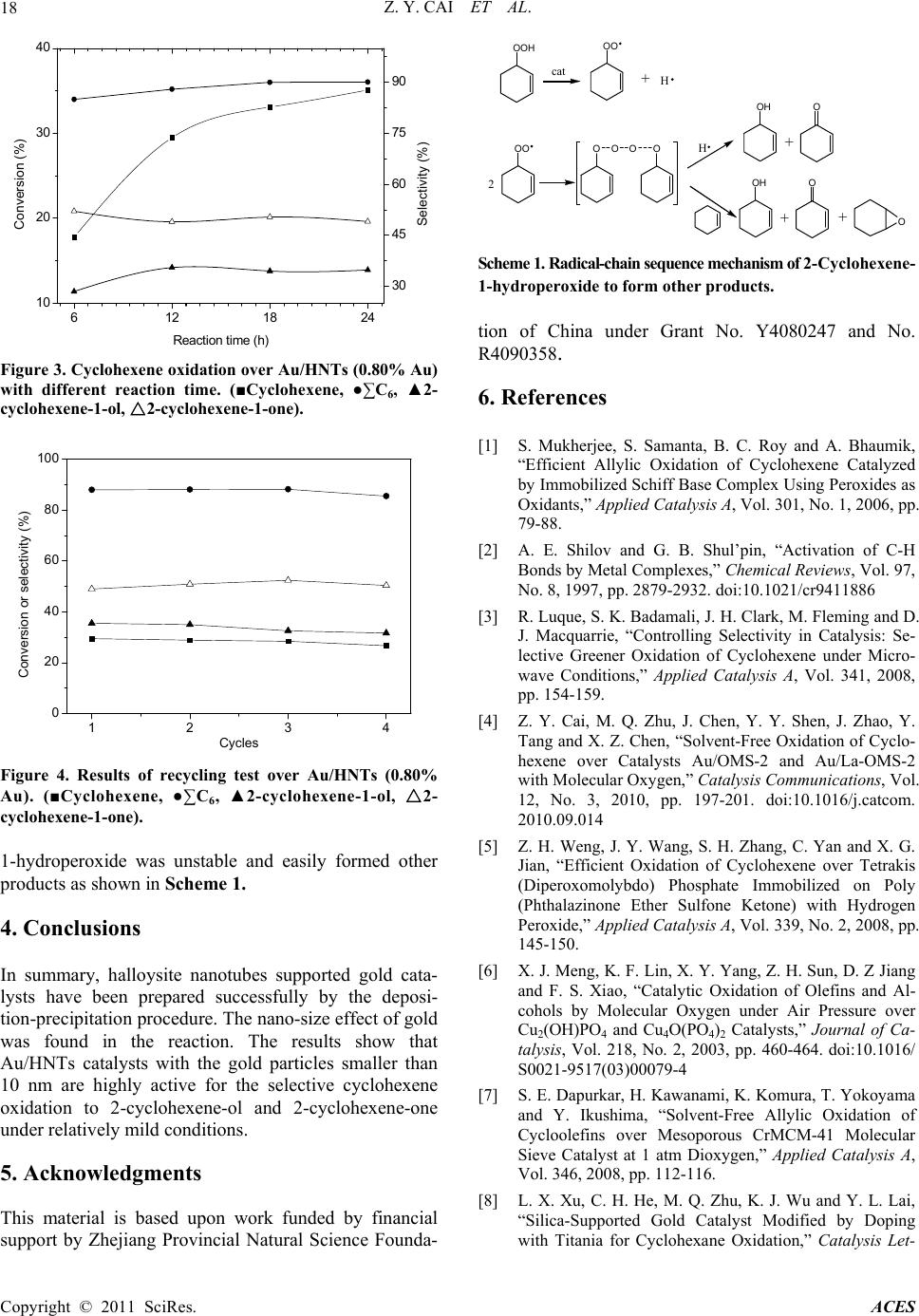 Z. Y. CAI ET AL. Copyright © 2011 SciRes. ACES 18 6 121824 10 20 30 40 Re actio n time (h) Conversion (%) 30 45 60 75 90 Selectivity (%) Figure 3. Cyclohexene oxidation over Au/HNTs (0.80% Au) with different reaction time. (■Cyclohexene, ●∑C6, ▲2- cyclohexene-1-ol, 2△-cyclohexene-1-one). 1234 0 20 40 60 80 100 Conversion or selectivity (%) Cycles Figure 4. Results of recycling test over Au/HNTs (0.80% Au). (■Cyclohexene, ●∑C6, ▲2-cyclohexene-1-ol, △2- cyclohexene-1-one). 1-hydroperoxide was unstable and easily formed other products as shown in Scheme 1. 4. Conclusions In summary, halloysite nanotubes supported gold cata- lysts have been prepared successfully by the deposi- tion-precipitation procedure. The nano-size effect of gold was found in the reaction. The results show that Au/HNTs catalysts with the gold particles smaller than 10 nm are highly active for the selective cyclohexene oxidation to 2-cyclohexene-ol and 2-cyclohexene-one under relatively mild conditions. 5. Acknowledgments This material is based upon work funded by financial support by Zhejiang Provincial Natural Science Founda- OOH cat OO + H H OO OO 2 OH + O OO O + OH + O Scheme 1. Radical-chain sequence mechanism of 2-Cyclohexene- 1-hydroperoxide to form other products. tion of China under Grant No. Y4080247 and No. R4090358. 6. References [1] S. Mukherjee, S. Samanta, B. C. Roy and A. Bhaumik, “Efficient Allylic Oxidation of Cyclohexene Catalyzed by Immobilized Schiff Base Complex Using Peroxides as Oxidants,” Applied Catalysis A, Vol. 301, No. 1, 2006, pp. 79-88. [2] A. E. Shilov and G. B. Shul’pin, “Activation of C-H Bonds by Metal Complexes,” Chemical Reviews, Vol. 97, No. 8, 1997, pp. 2879-2932. doi:10.1021/cr9411886 [3] R. Luque, S. K. Badamali, J. H. Clark, M. Fleming and D. J. Macquarrie, “Controlling Selectivity in Catalysis: Se- lective Greener Oxidation of Cyclohexene under Micro- wave Conditions,” Applied Catalysis A, Vol. 341, 2008, pp. 154-159. [4] Z. Y. Cai, M. Q. Zhu, J. Chen, Y. Y. Shen, J. Zhao, Y. Tang and X. Z. Chen, “Solvent-Free Oxidation of Cyclo- hexene over Catalysts Au/OMS-2 and Au/La-OMS-2 with Molecular Oxygen,” Catalysis Communications, Vol. 12, No. 3, 2010, pp. 197-201. doi:10.1016/j.catcom. 2010.09.014 [5] Z. H. Weng, J. Y. Wang, S. H. Zhang, C. Yan and X. G. Jian, “Efficient Oxidation of Cyclohexene over Tetrakis (Diperoxomolybdo) Phosphate Immobilized on Poly (Phthalazinone Ether Sulfone Ketone) with Hydrogen Peroxide,” Applied Catalysis A, Vol. 339, No. 2, 2008, pp. 145-150. [6] X. J. Meng, K. F. Lin, X. Y. Yang, Z. H. Sun, D. Z Jiang and F. S. Xiao, “Catalytic Oxidation of Olefins and Al- cohols by Molecular Oxygen under Air Pressure over Cu2(OH)PO4 and Cu4O(PO4)2 Catalysts,” Journal of Ca- talysis, Vol. 218, No. 2, 2003, pp. 460-464. doi:10.1016/ S0021-9517(03)00079-4 [7] S. E. Dapurkar, H. Kawanami, K. Komura, T. Yokoyama and Y. Ikushima, “Solvent-Free Allylic Oxidation of Cycloolefins over Mesoporous CrMCM-41 Molecular Sieve Catalyst at 1 atm Dioxygen,” Applied Catalysis A, Vol. 346, 2008, pp. 112-116. [8] L. X. Xu, C. H. He, M. Q. Zhu, K. J. Wu and Y. L. Lai, “Silica-Supported Gold Catalyst Modified by Doping with Titania for Cyclohexane Oxidation,” Catalysis Let-  Z. Y. CAI ET AL. Copyright © 2011 SciRes. ACES 19 ters, Vol. 118, 2007, pp. 248-253. doi:10.1007/s10562- 007-9178-6 [9] N. R. Shiju and V. V. Guliants, “Recent Developments in Catalysis Using Nanostructured Materials,” Applied Ca- talysis A, Vol. 356, No. 1, 2009, pp. 1-17. [10] G. J. Hutching, “Gold Catalysis in Chemical Processing,” Catalysis Today, Vol. 72, 2002, pp. 11-17. doi:10.1016 /S0920-5861(01)00473-4 [11] J. H. Clark, “Catalysis for Green Chemistry,” Pure and Applied Chemistry, Vol. 73, No. 1, 2001, pp. 103-111. doi:10.1351/pac200173010103 [12] A. S. K. Hashmi, “The Catalysis Gold Rush: New Claims,” Angewandte Chemie International Edition, Vol. 44, No. 43, 2005, pp. 6990-6993. doi:10.1002/anie.2005 02735 [13] M. Haruta, T. Kobayashi, H. Sano and N. Yamada, “Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature Far Below 0˚C,” Chemistry Letters, 1987, pp. 405-408. doi:10.1246/cl.1987.405 [14] A. K. Sinha, S. Seelan, S. Tsubota and M. Haruta, “Catalysis by Gold Nanoparticles: Epoxidation of Propene,” Topics in Catalysis, Vol. 29, 2004, pp. 95-102. doi:10.1023/B:TOCA.0000029791.69935.53 [15] P. Landon, P. J. Collier, A. F. Carley, D. Chadwick, A. J. Papworth, A. Burrows, C. J. Kiely and G. J. Hutchings, “Direct Synthesis of Hydrogen Peroxide from H2 and O2 Using Pd and Au Catalysts,” Physical Chemistry Chemi- cal Physics, Vol. 5, 2003, pp. 1917-1923. doi:10.1039/ b211338b [16] F. Menegazzo, M. Signoretto, M. Manzoli, F. Boccuzzi, G. Cruciani, F. Pinna and G. Strukul, “Influence of the Preparation Method on the Morphological and Composi- tion Properties of Pd-Au/ZrO2 Catalysts and Their Effect on the Direct Synthesis of Hydrogen Peroxide from Hy- drogen and Oxygen,” Journal of Catalysis, Vol. 268, No. 1, 2009, pp. 122-130. doi:10.1016/j.jcat.2009.09.010 [17] L. X. Xu, C. H. He, M. Q. Zhu, K. J. Wu and Y. L. Lai, “Surface Stabilization of Gold by Sol-Gel Post-modification of Alumina Support with Silica for Cyclohexane Oxidation,” Catalysis Communications, Vol. 9, No. 5, 2008, pp. 816-820. doi:10.1016/j.catcom.2007.09.005 [18] R. Zhao, D. Ji, G. Lv, G. Qian, L. Yan, X. Wang and J. Suo, “A Highly Efficient Oxidation of Cyclohexane over Au/ZSM-5 Molecular Sieve Catalyst with Oxygen as Oxidant,” Chemical Communications, 2004, pp. 904-905. doi:10.1039/b315098d [19] K. Zhu, J. C. Hu and R. Richards, “Aerobic Oxidation of Cyclohexane by Gold Nanoparticles Immobilized upon Mesoporous Silica,” Catalysis Letters, Vol. 100, 2005, pp. 195-199. doi:10.1007/s10562-004-3454-5 [20] M. D. Hughes, Y. J. Xu , P. Jenkins, P. McMorn, P. Landon, D. I. Enache, A. F. Carley, G. A. Attard, G. J. Hutchings, F. King, E. H. Stitt, P. Johnston, K. Griffin and C. J. Kiely, “Tunable Gold Catalysts for Selective Hydrocarbon Oxidation under Mild Conditions,” Nature, Vol. 437, 2005, pp. 1132-1135. doi:10.1038/nature04190 [21] B. D. Li, P. He, G. Q. Yi, H. Q. Lin and Y. Z. Yuan, “Performance of Gold Nanoparticles Supported on Carbon Nanotubes for Selective Oxidation of Cyclooctene with Use of O2 and TBHP,” Catalysis Letters, Vol. 133, 2009, pp. 33-40. doi:10.1007/s10562-009-0171-0 [22] L. X. Xu, C. H. He, M. Q. Zhu and S. Fang, “A Highly Active Au/Al2O3 Catalyst for Cyclohexane Oxidation Using Molecular Oxygen,” Catalysis Letters, Vol. 114, 2007, pp. 202-205. doi:10.1007/s10562-007-9058-0 [23] H. Weiner, A. Trovarelli and R. G. Finke, “Expanded Product, Plus Kinetic and Mechanistic, Studies of Poly- oxoanion-based Cyclohexene Oxidation Catalysis: The Detection of ~70 Products at Higher Conversion Leading to a Simple, Product-based Test for the Presence of Ole- fin Autoxidation,” Journal of Molecular Catalysis A: Chemical, Vol. 191, No. 2, 2003, pp. 217-252. doi: 10.1016/S1381-1169(02)00344-8 [24] G. B. Shul’pin, Y. N. Kozlov, S. N. Kholuiskaya and M. I. Plieva, “Oxidations by the System ‘Hydrogen Peroxide- [Mn2L2O3]2+ (L=1,4,7-trimethyl-1,4,7-tri-azacyclononane)- oxalic acid’. Part 11. Degradation of Dye Rhodamine 6G and Oxygenation of Cyclohexene,” Journal of Molecular Catalysis A: Chemical, Vol. 299, No. 2, 2009, pp. 77-87. |

