Paper Menu >>
Journal Menu >>
 Journal of Biomaterials and Nanobiotechnology, 2011, 2, 144-149 doi:10.4236/jbnb.2011.22018 Published Online April 2011 (http://www.scirp.org/journal/jbnb) Copyright © 2011 SciRes. JBNB Synthesis, Structure Analysis and Antibacterial Activity of New Potent Sulfonamide Derivatives Abdulhakeem Alsughayer1, Abdel-Zaher A. Elassar2, Seham Mustafa1 , Fakhreia Al Sagheer2 1Pharmaceutical Science Department, College of Health Science, The Public Authority for Applied Education and Training, Adaili- yah, Kuwait; 2Chemistry Department, Faculty of Science, Kuwait University, Kuwait City, Kuwait. E-mail: aelassar@yahoo.com Received October 6th, 2010; revised January 9th, 2011; accepted January 10th, 2011. ABSTRACT Modification of sulfonamide drug using different principles of chemical reactions was investigated. These reactions involve the condensa tion of an amino group with triethyl ortho formate and dimethylformamide d imethyl acetal. Ability of sulfa to condense with active keto compounds, like ethyl pyruvate and piprazine carboxyaldehye was studied. Alkyla- tion of sulfa with different chloro deriva tives was also reported. The structur e of the isolated compound was elucidated and confirmed using elemen tal analysis and spectral data. The bioactivity of the obtain ed compounds was investigated against different gram positive and g ram negative bacteria. The study reveals that most of the modified drugs show high to moderate antiba c terial activity. Keywords: Sulfonamide, Sulfa Drug, Gram Positive, Gram Negative Bacteria 1. Introduction The demand for novel chemotherapeutic antibacterial remains attractive in the field of medicinal chemistry. The discovery of sulfonamides as antibacterial in the early 30s was the beginning of the most fascinating era of chemotherapeutic agents [1-4]. Since the introduction of prontosil over 70 years ago, sulfa drugs have been widely used to treat a broad spectrum of microbial dis- eases [5]. However, due to the rapid emergence of sul- fonamide resistance organisms and the development of more potent drugs have limited their clinical use. The sulfonamide group is considered as a pharmacophore which is present in a number of biologically active molecules, particularly in antimicrobial agents [6-10]. In addition, numerous sulfonamide derivatives have been reported as carbonic anhydrase inhibitors [11-15], anti- cancer [16], and anti-inflammatory agents [17]. Some organisms are resistant to all approved antibiotics and can only be treated with experimental and potentially toxic drugs. Therefore, there is an overwhelming need to develop more effective antibacterial agents to treat infec- tions caused by antibiotic resistant bacterial pathogens. Sulfonamides exert their effect by targeting on dihy- dropteroate synthase (DHPS) enzyme, which catalyzes folic acid pathway in bacteria and some eukaryotic cells [18] but is not present in human cells [19]. This is the basis for the selective effect of sulfonamides on bacteria and for their broad spectrum of antibacterial activity. Since sulfanilamide first came into use, different deriva- tives have appeared on the market. Chemically modified sulfanilamide is prepared to achieve more effective anti- bacterial activity, wider spectrum of microorganisms affected, or more prolonged action. Because of their low cost they are still used in many parts of the world. The substances are still used to treat some urinary tract infec- tions, leprosy, and in combination with other drugs, fun- gal diseases such as toxoplasmosis. The pharmaceutical industry has responded with new classes of drugs, thus a great insight to search for potential pharmacologically active sulfanilamide and its derivatives is still of inter- esting. This study deals with the synthesis of N-substituted sulfonamide derivatives. The structure was established and confirmed using elemental analysis and spectral data e.g. IR, 1H NMR, 13C NMR and MS spectra. Biological activity of the synthesized compounds against gram posi- tive and gram negative bacteria has been investigated. 2. Materials and Methods Sulfanilamide, triethyl orthoformate, ethyl pyruvate, 3- chloro-2,4-pentanedione, dimethylformamide dimethy- lacetal (DMFDMA), piprazinecarboxyaldehyde, chloro-  Synthesis, Structure Analysis and Antibacterial Activity of New Potent Sulfonamide Derivatives 145 acetonitrile and chloroacetone (aldrich, milwaukee, wi) were used as received. All other chemicals were reagent grade and were used without further purification. 2.1. Elemental Analysis and Physical Measurements All melting points are uncorrected. IR spectra were re- corded in KBr with a IR spectrophotometer Shimadzu 408. 1H NMR and 13C NMR spectra were recorded on Varian EM-390 MHz spectrometer using TMS as an in- ternal reference with the chemical shifts expressed as δ ppm. Mass spectra were measured on a Shimadzu GCMS-QP 1000 Ex mass spectrometer. Microanalytical data were obtained from the ANALAB Unit at chemistry depart- ment, Kuwait University. 2.2. Synthesis of N, N`-(4-sulfonamido) forma- midine (2b); of N, N`-Bis (4-sulfonamido) for- mamidine (3); Ethyl N-(4-benzenesulfonaMido) -2-Iminopropanoate (4); 4-(Pipra- Zin-1-ylme- thyleneamino) benzenesulfonamide (7); 4-(Cya- nomethylamino) benzenesulfonamide (8); 4- (2- Oxopropylamino) benzene-Sulfonamide (9);4- (2,4-Dioxopentan-3-ylamino)benzenesulfonamide (10) To a solution of 1 (0.01 mol) in toluene (20 ml) and DMF (10 ml) mixture, dimethyl formaminde dimethyl acetal or triethyl orthoformate or ethyl pyruvate or pip- razine carboxyaldehyde or chloroacetonitrile or 1-chloro- 2-propanone or 3-chloro-2,4-pentanedione (0.01 mol) was added. The reaction mixture was heated under reflux for 3 h. The solvent was evaporated under vacuum and the solid product formed after cooling was collected by filtration, washed by ether and crystallized from proper solvent (cf. Table 1). 2.3. 4-(3,5-Dimethyl-1H-pyrazol-4-ylamino) benzenesulfonamide (11) To a solution of 10 (0.01 mol) in DMF (20 ml), hydrazine hydrate (0.01 mol) was added. The reaction mixture was heated under reflux for 3h. The solvent was evaporated under vacuum and the yellow solid product so formed after cooling was collected by filtration, washed by ether and crystallized from ethanol. Compound 11 was col- lected as pale brown crystal 66% yield (cf. Table 1). 2.4. Drug Susceptibility Test The drugs were tested by disc-diffusion method. Diluted bacterial cultures (100 mL) were spread on sterile Muel- ler-Hinton agar plates, after which 8 mm diameter discs (sterile blank) impregnated with drug for testing (10-100 mg) were placed on the plates. The plates were incubated for 24 h at 37℃ under aerobic conditions and the diame- ter of the inhibition zone around each disc was then measured and recorded. If the drugs were found to be active in the disc diffusion test (inhibition zone > 10 mm), they were further evaluated for determining minimum inhibitory concentration (MIC) values. 2.5. Minimum Inhibitory Concentration (MIC) The drugs were screened for their antibacterial activity against E. coli, P. aeruginosa, B. subtilis and S. aureus. MIC was evaluated by turbidity method. A loop full of bacteria was inoculated in 100 mL of nutrient broth at 37℃ for 20 h in a test-tube shaker at 150 rev min-1. The test compounds were prepared by dissolving in a mini- mal volume of DMSO and were serially diluted in Muel- ler-Hinton broth at concentrations in the range of 1 - 100 mg/mL. The 24-h bacterial cultures were then transferred into 10 mL of Muller-Hinton broth (control and test compounds) and incubated at 37℃ for 24 h. The growth of the bacteria was determined by measuring the turbid- ity after 24 h. Thus, the MIC was generally read as the smallest concentration of drug in the series that prevents the development of visible growth of test organism. All the experiments were done in triplicate. 2.6. Statistical Analysis The MIC value and modified drug susceptibility test were measured in triplicate. Statistical analysis of the MIC value was performed using the unpaired Student’s t-test. Differences were considered significant when P < 0.01. 3. Results and Discussion 3.1. Chemistry Sulfanilamide 1 reacted with triethyl orthoformate to give 4-(ethoxymethyleneamino) benzenesulfonamide 2a or N,N`-bis (4-sulfonamido)formamidine 3 (cf. scheme 1). Compound 2a was ruled out based on accurate mass m/z 354.04 which referred that the reaction took place with 1:2 molar ratios and was in agreement with the mo- lecular formula C13H14N4O4S2 (cf. Table 1). While in the case of dimethyl formamide dimethyl acetal (DMFDMA) N,N`-(4-sulfonamido)formamidine 2b was isolated. Fur- ther reaction of 2b with 1 gives 3. The structure of com- NH 2 SO 2 NH 2 N SO 2 NH 2 XN SO 2 NH 2 HN SO 2 NH 2 H 2 NSO 2 NH 2 (EtO)2CH 12a: X=OEt b: X=NMe 2 3 OR DMFDMA Scheme 1 C opyright © 2011 SciRes. JBNB 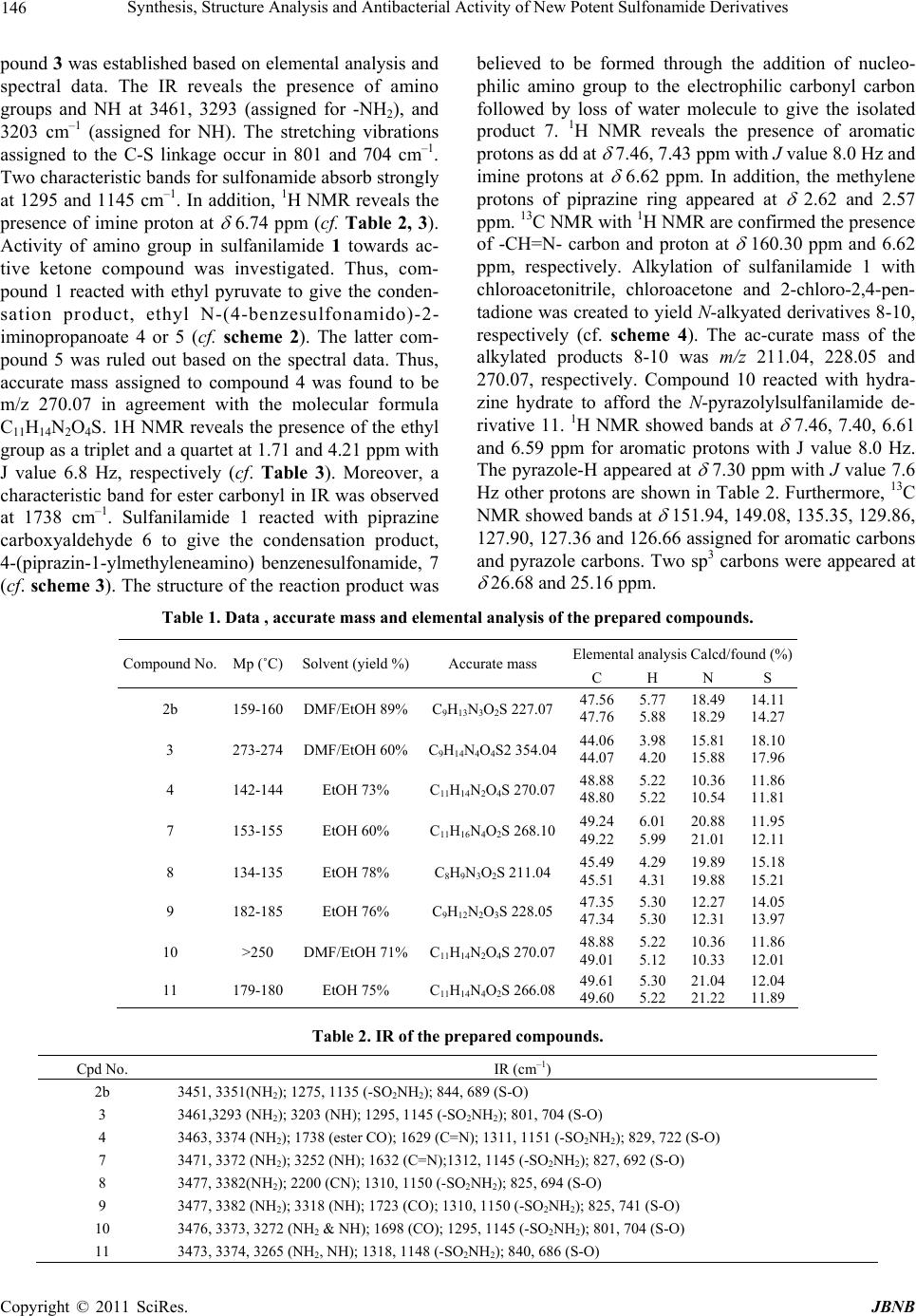 Synthesis, Structure Analysis and Antibacterial Activity of New Potent Sulfonamide Derivatives Copyright © 2011 SciRes. JBNB 146 pound 3 was established based on elemental analysis and spectral data. The IR reveals the presence of amino groups and NH at 3461, 3293 (assigned for -NH2), and 3203 cm–1 (assigned for NH). The stretching vibrations assigned to the C-S linkage occur in 801 and 704 cm–1. Two characteristic bands for sulfonamide absorb strongly at 1295 and 1145 cm–1. In addition, 1H NMR reveals the presence of imine proton at 6.74 ppm (cf. Table 2, 3). Activity of amino group in sulfanilamide 1 towards ac- tive ketone compound was investigated. Thus, com- pound 1 reacted with ethyl pyruvate to give the conden- sation product, ethyl N-(4-benzesulfonamido)-2- iminopropanoate 4 or 5 (cf. scheme 2). The latter com- pound 5 was ruled out based on the spectral data. Thus, accurate mass assigned to compound 4 was found to be m/z 270.07 in agreement with the molecular formula C11H14N2O4S. 1H NMR reveals the presence of the ethyl group as a triplet and a quartet at 1.71 and 4.21 ppm with J value 6.8 Hz, respectively (cf. Table 3). Moreover, a characteristic band for ester carbonyl in IR was observed at 1738 cm–1. Sulfanilamide 1 reacted with piprazine carboxyaldehyde 6 to give the condensation product, 4-(piprazin-1-ylmethyleneamino) benzenesulfonamide, 7 (cf. scheme 3). The structure of the reaction product was believed to be formed through the addition of nucleo- philic amino group to the electrophilic carbonyl carbon followed by loss of water molecule to give the isolated product 7. 1H NMR reveals the presence of aromatic protons as dd at 7.46, 7.43 ppm with J value 8.0 Hz and imine protons at 6.62 ppm. In addition, the methylene protons of piprazine ring appeared at 2.62 and 2.57 ppm. 13C NMR with 1H NMR are confirmed the presence of -CH=N- carbon and proton at 160.30 ppm and 6.62 ppm, respectively. Alkylation of sulfanilamide 1 with chloroacetonitrile, chloroacetone and 2-chloro-2,4-pen- tadione was created to yield N-alkyated derivatives 8-10, respectively (cf. scheme 4). The ac-curate mass of the alkylated products 8-10 was m/z 211.04, 228.05 and 270.07, respectively. Compound 10 reacted with hydra- zine hydrate to afford the N-pyrazolylsulfanilamide de- rivative 11. 1H NMR showed bands at 7.46, 7.40, 6.61 and 6.59 ppm for aromatic protons with J value 8.0 Hz. The pyrazole-H appeared at 7.30 ppm with J value 7.6 Hz other protons are shown in Table 2. Furthermore, 13C NMR showed bands at 151.94, 149.08, 135.35, 129.86, 127.90, 127.36 and 126.66 assigned for aromatic carbons and pyrazole carbons. Two sp3 carbons were appeared at 26.68 and 25.16 ppm. Table 1. Data , accurate mass and elemen tal analysis of the prepared compounds. Elemental analysis Calcd/found (%) Compound No. Mp (˚C) Solvent (yield %)Accurate mass C H N S 2b 159-160 DMF/EtOH 89%C9H13N3O2S 227.0747.56 47.76 5.77 5.88 18.49 18.29 14.11 14.27 3 273-274 DMF/EtOH 60%C9H14N4O4S2 354.0444.06 44.07 3.98 4.20 15.81 15.88 18.10 17.96 4 142-144 EtOH 73% C11H14N2O4S 270.0748.88 48.80 5.22 5.22 10.36 10.54 11.86 11.81 7 153-155 EtOH 60% C11H16N4O2S 268.1049.24 49.22 6.01 5.99 20.88 21.01 11.95 12.11 8 134-135 EtOH 78% C8H9N3O2S 211.04 45.49 45.51 4.29 4.31 19.89 19.88 15.18 15.21 9 182-185 EtOH 76% C9H12N2O3S 228.0547.35 47.34 5.30 5.30 12.27 12.31 14.05 13.97 10 >250 DMF/EtOH 71%C11H14N2O4S 270.0748.88 49.01 5.22 5.12 10.36 10.33 11.86 12.01 11 179-180 EtOH 75% C11H14N4O2S 266.0849.61 49.60 5.30 5.22 21.04 21.22 12.04 11.89 Table 2. IR of the prepared compounds. Cpd No. IR (cm–1) 2b 3451, 3351(NH2); 1275, 1135 (-SO2NH2); 844, 689 (S-O) 3 3461,3293 (NH2); 3203 (NH); 1295, 1145 (-SO2NH2); 801, 704 (S-O) 4 3463, 3374 (NH2); 1738 (ester CO); 1629 (C=N); 1311, 1151 (-SO2NH2); 829, 722 (S-O) 7 3471, 3372 (NH2); 3252 (NH); 1632 (C=N);1312, 1145 (-SO2NH2); 827, 692 (S-O) 8 3477, 3382(NH2); 2200 (CN); 1310, 1150 (-SO2NH2); 825, 694 (S-O) 9 3477, 3382 (NH2); 3318 (NH); 1723 (CO); 1310, 1150 (-SO2NH2); 825, 741 (S-O) 10 3476, 3373, 3272 (NH2 & NH); 1698 (CO); 1295, 1145 (-SO2NH2); 801, 704 (S-O) 11 3473, 3374, 3265 (NH2, NH); 1318, 1148 (-SO2NH2); 840, 686 (S-O)  Synthesis, Structure Analysis and Antibacterial Activity of New Potent Sulfonamide Derivatives 147 Table 3. 1H NMR and 13C NMR of the prepared compounds. Cpd No. 1H NMR (ppm) 13C NMR (ppm) 2b 11.40, 11.37 (br 2H, NH2, D2O-exchange)); 8.54-6.76 (dd, 4H,C6H4, J=8.8); 6.74 (s, 1H, CH=) 2.75, 2.74(s, 6H, 2Me). 162.35(imine carbon); 157.96, 141.71, 128.07, 127.27, 121.26, 121.13 (aromatic carbons) 3 11.38 (d, 1H, NH, D2O-exchange); 7.88, 7.77 (br, 4H, 2NH2, D2O-exchange); 8.52-7.30(m, 8H, 2 C6H4); 6.74 (d, 1H, -CH=N, J=7.6 Hz) 162.38 (imine carbon); 158.03, 155.75, 140.91, 140.85 (C and C4 in two benzene rings); 121.27, 121.34, 128.88, 128.78 (other aromatic-C). 4 9.00 (br, 2H, NH2, D2O-exchange ); 7.95-6.57(dd, 4H,C6H4, J=8.8); 4.21(q, 2H, CH2, J=6.8Hz); 1.71 (t, 3H, Me, J=6.8 Hz) 163.45 (CO); 161.12 (imine carbon); 152.36, 138.42, 128.01, 123.29 (aromatic carbons); 61.24 (CH2); 15.62, 14.35 (2 Me). 7 8.00 (d, 2H, NH2); 7.46, 7.43 (dd, 4H, C6H4, J=8.4Hz); 6.62 (s, 1H, CH=); 5.72 (s, 1H, NH); 2.62, 2.57 (tt, 8H, 4CH2, J=5.6Hz) 160.3 (imine carbon); 152.11, 131.01, 128.21, 112.64 (aromatic carbons); 5.32, 47.01 (piprazine carbons). 8 7.51, 7.49 (d, 2H, C6H4, J=8.0 Hz); 6.95 ( br, 1H, NH, D2O-exchange); 6.65, 6.63 (d, 2H, Ar-H, J=8.0 Hz); 5.80 (br, 2H, NH2, D2O-exchange); 2.86 (s, 2H, CH2) 151.88, 132.70, 127.44, 112.53 (aromatic carbons); 129.90 (CN); 30.80 (CH2). 9 7.56 (d, 2H, C6H4 , J=8.4 Hz); 6.75 (d, 2H, C6H4 , J=8.8 Hz), 6.91 (br, 2H, NH2, D2O-exchange); 8.02 (br, 1H, NH, D2O-exchange); 4.45 (s, 2H, CH2); 2.91(s, 3H, Me) 162.45 (CO); 152.67, 130.84, 127.86, 112.90 (aromatic carbons); 35.49 (CH2); 30.35 (Me). 10 7.46 (d, 2H, C6H4 , J=8.4 Hz); 6.93 (br, 2H, NH2, D2O-exchange); 6.60 (d, 2H, C6H4 , J=8.0 Hz); 5.83 (s, 1H, NH, D2O-exchange); 3.42 (s, 1H, CH); 2.50 (s, 6H, 2Me) 169.00 (CO); 151.99, 130.00, 127.47, 112.46 (aromatic carbons); 85.00 (CH); 25.50 (Me) 11 7.46, 7.40 (d, 2H, C6H4 , J=8 Hz); 7.30 (d, 1H, pyrazole-H, J=7.6 Hz); 6.97 (br, 1H, NH, D2O-exchange); 6.61, 6.59 (d, 2H, C6H4 , J=8 Hz); 5.51 (br, 2H, NH2, D2O-exchange); 1.67 (s, 6H, 2Me) 151.94, 149.08, 135.35, 129.86, 127.90, 127.36, 126.66 (aromatic & pyrazole carbons); 26.68, 25.16 (2Me) 3.2. Antimicrobial Activity Substitution on the sulfanilamide nitrogen is referred to as N1-substitution and N4-substitution on the 4-amino group as N-substitution. The therapeutically active de- rivatives are usually N1-substitutes. The MIC values (average of triplicates) of the sulfanilamide and sulfa- nilamide derivatives are shown in Table 4 and Figures 1-5. It can be observed from Figures 1 and 2 that the antimicrobial results of compound 2b has high effect on S. Aureus and E. coli, while compound 9 and 4 have a low effect. In the case of E. coli compound 8 showed high effects as compared to compound 4 at low concen- tration (10-40 mg mL-1). In contrast at high concentration (70-100 mg) compound 4 showed greater effects than compound 8. Similar behavior was observed in the case of E. coli with compound (3 and 8) and (7 and 9). Severe effect was observed by compound 9 toward P. Aerugi- nosa and B. subtilis (cf. Figure 3 and 4). In general most compounds showed better effect with increase its con- centration. In contrast to this, for compounds 2b and 7 at high concentration (more than 80 mg mL–1), the effect of compound 2b was more than compound 7, while at low concentration (less than 80 mg mL-1) compound 7 showed greater effect than compound 2b. Figure 5 represents the average zone of inhibition against sample number. It shows that the effect of compound 2a on microor- ganism can be arranged as follows: E. coli > S. Aureus > P. Aeruginosa > B. subtilis (from highest to lowest); in the case of compound 3 the only observed effect is on E. coli followed by S. Aureus. Compound 4 showed severe NH 2 SO 2 NH 2 1 N CH 3 CO 2 CH 2 CH 3 CH 3 COCO 2 Et H 2 NO 2 S H N O H 2 NO 2 S O CH 3 5 4 Scheme 2 N SO 2 NH 2 7 N NH NH 2 SO 2 NH 2 1 HNN CHO 6 Scheme 3 C opyright © 2011 SciRes. JBNB 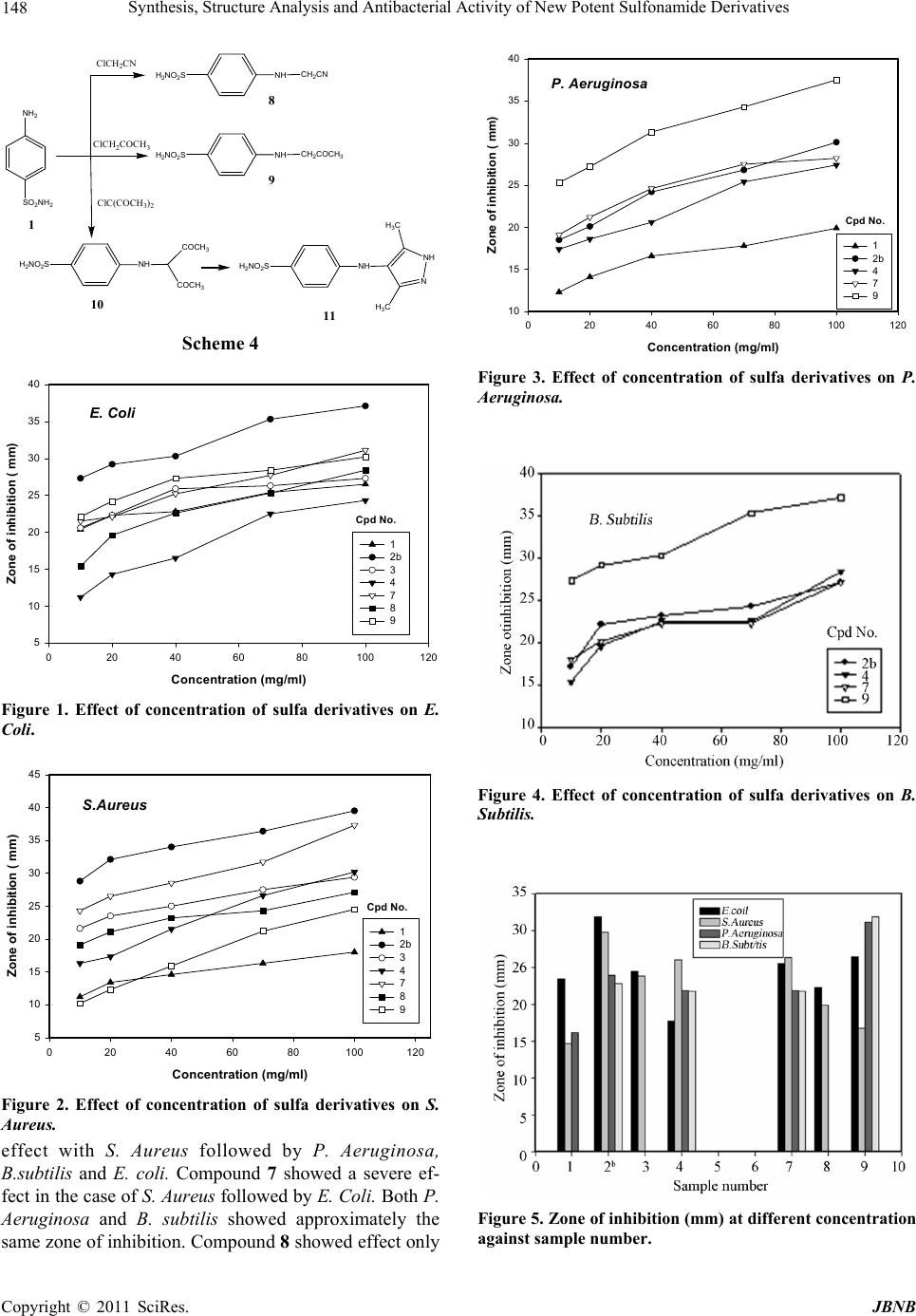 148 Synthesis, Structure Analysis and Antibacterial Activity of New Potent Sulfonamide Derivatives NHH 2 NO 2 S NH 2 SO 2 NH 2 1 10 8 CH 2 CN NHH 2 NO 2 S 9 CH 2 COCH 3 NHH 2 NO 2 S COCH 3 COCH 3 ClCH 2 CN ClCH 2 COCH 3 ClC(COCH 3 ) 2 NHH 2 NO 2 S N NH H 3 C H 3 C 11 Scheme 4 E. Coli Concentration (mg/ml) 020 40 60 80100120 Zone of inhibition ( mm) 5 10 15 20 25 30 35 40 1 2b 3 4 7 8 9 Cpd No. Figure 1. Effect of concentration of sulfa derivatives on E. Coli. S.Aureus Concentr at ion (mg/ml) 0 20406080100120 Zone of inhibition ( mm) 5 10 15 20 25 30 35 40 45 1 2b 3 4 7 8 9 Cpd No. Figure 2. Effect of concentration of sulfa derivatives on S. Aureus. effect with S. Aureus followed by P. Aeruginosa, B.subtilis and E. coli. Compound 7 showed a severe ef- fect in the case of S. Aureus followed by E. Coli. Both P. Aeruginosa and B. subtilis showed approximately the same zone of inhibition. Compound 8 showed effect only P. Aeruginosa Concentration (mg/ml) 0 20406080100120 Zone of inhibition ( mm) 10 15 20 25 30 35 40 1 2b 4 7 9 Cpd No. Figure 3. Effect of concentration of sulfa derivatives on P. Aeruginosa. Figure 4. Effect of concentration of sulfa derivatives on B. Subtilis. Figure 5. Zone of inhibition (mm) at different concentration against sample number. C opyright © 2011 SciRes. JBNB  Synthesis, Structure Analysis and Antibacterial Activity of New Potent Sulfonamide Derivatives Copyright © 2011 SciRes. JBNB 149 on E. coli and S. Aureus. Compound 9 showed a severe effect on P. Aeruginosa and B. subtilis. A moderate ef- fect on E. coli and low effect was observed on S. Aureus. 4. Acknowledgements The authors would like to acknowledge the Public Au- thority for Applied Education and Training for the provi- sion of grant No. HS-07-01 and the authors would like also to acknowledge Kuwait University for the general facility projects grant Nos. GS 01/01 and GS 03/01. REFERENCES [1] N. Hen, M. Bialer, B. Wlodarczyk, R. H. Finnell and B. Yagen, “Syntheses and Evaluation of Anticonvulsant Pro- file and Teratogenicity of Novel Amide Derivatives of Branched Aliphatic Carboxylic Acids with 4-Aminobenz- ene sulfonamide,” Journal Medic al Chemistry, Vol. 53, No. 10, 2010, pp. 4177-4186. doi:10.1021/jm100170w [2] F.Carta, A. Maresca, A. Scozzafava, D. Vullo and C. T. Supuran, “Carbonic Anhydrase Inhibitors. Diazenylben- zenesulfonamides are Potent and Selective Inhibitors of the Tumor-Associated Isozymes IX and XII over the Cytosolic Isoforms I and II,” Bioorg anic & Medical Chemistry, 2009, Vol. 17, No.20, 7093-7099. doi:10.1016/j.bmc.2009.09.003 [3] K. Namba, X. Zheng, K. Motoshima, H. Kobayashi, A. Tai, E. Takahashi, K. Sasaki, K. Okamoto and H. Kakuta, “De- sign and Synthesis of Benzenesulfonanilides Active against Methicillin-Resistant Staphylococcus Aureus and Vanco- mycin-Resistant,” Enterococcus Bioorganic & Medical Chemistry, Vol. 16, No. 11, 2008, pp. 6131-6144. [4] P. Joseph, F. Turtaut, S. Ouahrani-Bettache, J. L. Montero, I. Nishimori, T. Minakuchi, D. Vullo, A. Scozzafava, S. Kohler, J.-Y. Winum and C. T. Supuran. “Cloning, Char- acterization, and Inhibition Studies of a β-Carbonic Anhy- drase from Brucella suis,” Journal Medical Chemistry, 2010, Vol. 53, No. 5, pp 2277-2285. doi:10.1021/jm901855h [5] B. L. Wilkinson, L. F. Bornaghi, A. D. Wright, T. A. Houston and S. A. Poulsen, “Anti-Mycobacterial Activity of a Bis-Sulfonamide,” Bioorganic & Medical Chemistry Letter, Vol. 17, No. 5, 2007, pp. 1355-1357. doi:10.1016/j.bmcl.2006.11.079 [6] B. G. Katzung, “Basic and Clinical Pharmacology,” 6th Edition, University of California, San Francisco, 1995. [7] S. Joshi and N. Khosla, “QSAR Study on Anti Bacterial Activity of Sulfonamides and Derived Mannich Bases,” Bioorganic Medical Chemistry Letter, Vol. 13, No. 21, 2003, pp. 3747-3751. doi:10.1016/j.bmcl.2003.08.017 [8] S. Joshi, N. Khosla and P. Tiwari, “In Vitro Study of Some Medicinally Important Mannich Bases Derived from an An- titubercular Agent,” Bioorganic & Medical Chemistry, Vol. 12, No. 3, 2004, pp. 571-576. doi:10.1016/j.bmc.2003.11.001 [9] N. Anand, “Sulfonamides and Sulfones,” In: M. E. Wolff, Ed., Burger’s Medicinal Chemistry and Drug Discovery, John Wiley & Sons, Inc., New York, 1996, pp. 527-573. [10] A. Kamal, M. N. A. Khan, K. S. Reddy, K. Rohini, G. N. Sastry, B. Sateesh and B. Sridhar, “Synthesis, Structure Analysis and Antibacterial Activity of Some Novel 10-Substituted 2-(4-Piperidyl/Phenyl) -5, 5-Dioxo [1,2,4] triazolo [1,5b] [1,2,4] Benzothiadiazine Derivatives,” Bio- organic & Medical Chemistry Letter, Vol. 17, 2007, pp. 5400-5405. doi:10.1016/j.bmcl.2007.07.043 [11] S. Zimmerman, A. Innocenti, A. Casini, J. G. Ferry, A. Scozzafava and C. T. Supuran, “Carbonic Anhydrase In- hibitors. Inhibition of the Prokaryotic Beta and Gamma- Class Enzymes from Archaea with Sulfonamides,” Bioor- ganic & Medical Chemistry Letter, Vol. 14, 2004, pp. 6001-6006. doi:10.1016/j.bmcl.2004.09.085 [12] V. Garaj, L. Puccetti, G. Fasolis, J-Y. Winum, J-L. Mon- tero, A. Scozzafava, D. Vullo, A. Innocentia and C. T. Su- purana, “Carbonic Anhydrase Inhibitors: Synthesis and In- hibition of Cytosolic/Tumor-Associated Carbonic Anhy- drase Isozymes I, Ii, and Ix with Sulfonamides Incorporat- ing 1,2,4-Triazine Moieties,” Bioorganic Medical Chemis- try Letter, Vol. 14, 2004, pp. 5427-5433. doi:10.1016/j.bmcl.2004.07.087 [13] L. Puccetti, G. Fasolis, D. Vullo, Z. H. Chohan, A. Scoz- zafavab and C. T. Supuranb, “Carbonic Anhydrase Inhibitors. Inhibition of Cytosolic/Tumor-Associated Carbonic Anhy- drase Isozymes I, II, IX, and XII with Schiff Bases Incorpo- rating Chromone and Aromatic Sulfonamide Moieties, and Their Zinc Complexes,” Bioorganic & Medical Chemistry Letter, Vol. 15, 2005, pp. 3096-3101. doi:10.1016/j.bmcl.2005.04.055 [14] J. M. Lehtonen, S. Parkkila, D. Vullo, A. Casini, A. Scoz- zafavac and C. T. Supuranc, “Carbonic Anhydrase Inhibi- tors. Inhibition of Cytosolic Isozyme XIII with Aromatic and Heterocyclic Sulfonamides: A Novel Target for the Drug Design,” Bioorganic& Medical Chemistry Letter, Vol. 14, 2004, pp. 3757-3762. doi:10.1016/j.bmcl.2004.04.106 [15] O. Güzel, A. Innocenti, A. Scozzafava, A. Salman and C. T. Supuran, “Carbonic Anhydrase Inhibitors. Phenacetyl-, Pyridylacetyl- and Thienylacetyl-Substituted Aromatic Sulfonamides Act as Potent and Selective Isoform Vii In- hibitors,” Bioorganic & Medical Chemistry Letter, Vol. 19, 2009, pp. 3170-3173. doi:10.1016/j.bmcl.2009.04.123 [16] A. Scozzafava, T. Owa, A. Mastrolorenzo and C. T. Supu- ran, “Anticancer and Antiviral Sulfonamides,” Current Medi ca l C h em is tr y , Vol. 10, No. 11, 2003, pp. 925-953. doi:10.2174/0929867033457647 [17] A. Weber, A. Casini, A. Heine, D. Kuhn, C. T. Supuran, A. Scozzafava and G. Kiebe, “Unexpected Nanomolar Inhibi- tion of Carbonic Anhydrase by COX-2-Selective Celecoxib: New Pharmacological Opportunities Due to Related Bind- ing Site Recognition,” Journal of Medicinal Chemistry, Vol. 47, No.3, 2004, pp. 550-557. doi:10.1021/jm030912m [18] G. M. Brown, “Biosynthesis of Folic Acid. II. Inhibition by Sulfonamides,” Journal of Biological Chemistry, Vol. 237, No. 1962, pp. 536-540. [19] P. Huovinen, L. Sundström, G. Swedberg, O. Skold and Antimicrob, “Agents Chemother Trimethoprim and Sul- fonamide Resistance,” Antimicrobiology Agents and Che- motherapy, Vol. 39, No.2, 1995, pp. 279-289. |

