 International Journal of Organic Chemistry, 2013, 3, 214-219 http://dx.doi.org/10.4236/ijoc.2013.33029 Published Online September 2013 (http://www.scirp.org/journal/ijoc) Synthesis of 5-Substituted 2,9-Dimethyl-1,10-Phenanthroline Dialdehydes and Their Schiff Bases with Sulfur-Containing Amines Zinia Jaman1, Mohammad R. Karim1*, Tasneem A. Siddiquee1, Aminul H. Mirza2, Mohamad A. Ali2 1Department of Chemistry, Tennessee State University, Nashville, USA 2Department of Chemistry, Faculty of Science, University Brunei Darussalam, Gadong, Brunei Darussalam Email: *mkarim@tnstate.edu Received July 4, 2013; revised August 14, 2013; accepted August 31, 2013 Copyright © 2013 Zinia Jaman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Eight new Schiff bases of 5-nitro and 5-bromo-substituted 1,10-phenanthro line-2,9-dicarboxaldehydes with sulfur-con- taining amines, thiosemicarbazide, S-alkyl/aryl dithiocarbazates and 2-mercaptoaniline have been synthesized and cha- racterized by a variety of spectroscopic methods. The condensation reactions of the dialdehydes with the amines were carried out both in the presence and absence of conc. sulfuric acid. A significant increase in yield of the Schiff bases was observed when the reactions were carried out in the presence of sulfuric acid. Keywords: 5-Nitro-1,10-Phenanthroline Dialdehyde; 5-Bromo-1,10-Phenanthroline Dialdehyde; Schiff Bases; S-Alkyl/Aryldithiocarbazates; Thiosemicarbazide 1. Introduction Recently, nitrogen and sulfur-containing organic chelat- ing agents such as the Schiff bases derived from 2,9-di- methyl-1,10-phenanthroline dialdehyde and sulfur-con- taining amines and their metal complexes have received considerable attention because of their important roles in synthetic and medicinal chemistry[1]. By properly de- signing this type of compounds and studying their struc- ture-activity relationships, potentially useful antibacterial, antifungal and anticancer agents can also be synthesized [2]. 2,9-Dimethyl-1,10-phenanthroline and its derivatives from which 2,9-dimethyl-1,10-phenanthroline dialdehy- des are prepared, are themselves important ligands for complexation with many metal ions [3]. This property has made them important in different areas like self-as- sembly and catalysis. It has also played a significant role in both analytical and preparative coordination chemistry as well as in the preparation of many mixed-ligand com- plexes [4]. Although a large number of Schiff bases containing “hard” nitrogen and “soft” sulfur donor atoms have been synthesized using S-alkyl/aryl dithiocarbazates and het- erocyclic aldehydes and ketones, which are able to form stable complexes with a variety of metal ions [5], less work has been reported on Schiff bases formed by con- densation of 1,10-phenanthroline dialdehydes with sufur- containing amines such as S-alkyl/aryl dithioicarbazates, thiosemi-carbazide and aminobenzenethiol. In view of the importance of Schiff bases derived from 1,10-phenanthroline dialdehyde and sulfur-containing amines in coordination chemistry and biology, we report here the synthesis and characterization of eight new Schiff bases formed by condensation of 5-nitro-1,10-phe- nanthroline-2,9-dialdehyde and 5-bromo-1,10-phenan- throline-2,9-dialdehyde with different types of sulfur- containing amines. 2. General Methods and Procedures HPLC grade solvents were used in all the reactions. The conventional method of synthesis of the Schiff bases involves refluxing the reaction mixture containing the dialdehydes and amines for 1 hour followed by filtration of the solid products using suction filtration. In all the reactions, 2 - 3 drops of conc. sulfuric acid were used. The solid product that had formed was filtered off using suction filtration. All the NMR data *Corresponding author. C opyright © 2013 SciRes. IJOC 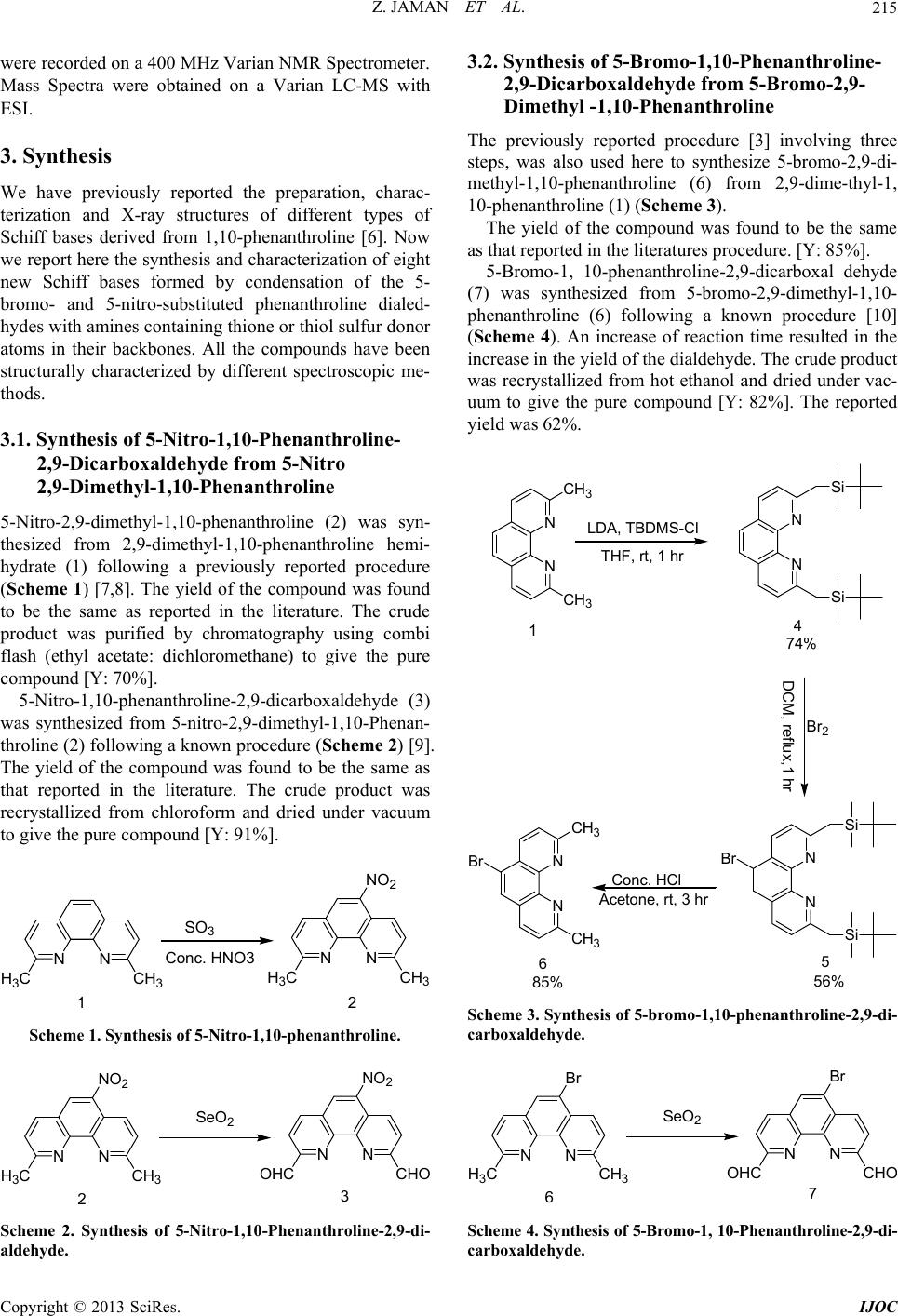 Z. JAMAN ET AL. 215 were recorded on a 400 MHz Varian NMR Spectrometer. Mass Spectra were obtained on a Varian LC-MS with ESI. 3. Synthesis We have previously reported the preparation, charac- terization and X-ray structures of different types of Schiff bases derived from 1,10-phenanthroline [6]. Now we report here the synthesis and characterization of eight new Schiff bases formed by condensation of the 5- bromo- and 5-nitro-substituted phenanthroline dialed- hydes with amines containing thione or thiol sulfur donor atoms in their backbones. All the compounds have been structurally characterized by different spectroscopic me- thods. 3.1. Synthesis of 5-Nitro-1,10-Phenanthroline- 2,9-Dicarboxaldehyde from 5-Nitro 2,9-Dimethyl-1,10-Phenanthroline 5-Nitro-2,9-dimethyl-1,10-phenanthroline (2) was syn- thesized from 2,9-dimethyl-1,10-phenanthroline hemi- hydrate (1) following a previously reported procedure (Scheme 1) [7,8]. The yield of the compound was found to be the same as reported in the literature. The crude product was purified by chromatography using combi flash (ethyl acetate: dichloromethane) to give the pure compound [Y: 70%]. 5-Nitro-1,10-phenanthroline-2,9-dicarboxaldehyde (3) was synthesized from 5-nitro-2,9-dimethyl-1,10-Phenan- throline (2) following a known procedure (Scheme 2) [9]. The yield of the compound was found to be the same as that reported in the literature. The crude product was recrystallized from chloroform and dried under vacuum to give the pure compound [Y: 91%]. NN CH 3 H 3 C SO 3 NN CH 3 H 3 C Conc. HNO3 NO 2 12 Scheme 1. Synthesis of 5-Nitro-1,10-phe nanth r oline. NN CH 3 H 3 CNN CH OHC NO 2 NO 2 SeO 2 23 Scheme 2. Synthesis of 5-Nitro-1,10-Phenanthroline-2,9-di- aldehyde. 3.2. Synthesis of 5-Bromo-1,10-Phenanthroline- 2,9-Dicarboxaldehyde from 5-Bromo-2,9- Dimethyl -1,10-Phenanthroline The previously reported procedure [3] involving three steps, was also used here to synthesize 5-bromo-2,9-di- methyl-1,10-phenanthroline (6) from 2,9-dime-thyl-1, 10-phenanthroline (1) (Scheme 3). The yield of the compound was found to be the same as that reported in the literatures procedure. [Y: 85%]. 5-Bromo-1, 10-phenanthroline-2,9-dicarboxal dehyde (7) was synthesized from 5-bromo-2,9-dimethyl-1,10- phenanthroline (6) following a known procedure [10] (Scheme 4). An increase of reaction time resulted in the increase in the yield of the dialdehyde. The crude product was recrystallized from hot ethanol and dried under vac- uum to give the pure compound [Y: 82%]. The reported yield was 62%. N N N N CH3 CH3 Br LDA, TBDMS-Cl THF, rt, 1 hr Si Si N N Si Si Br Br2 DCM, reflux,1 hr Conc. HCl Acetone, rt, 3 hr 74% 56% 85% N N CH3 CH3 14 65 Scheme 3. Synthesis of 5-br omo-1,10-phenanthroline-2,9-di- carboxaldehyde. NN CH 3 H 3 CNN CH OHC B B SeO 2 67 Scheme 4. Sy nthesis of 5-Bromo-1, 10-P henanthroline-2,9-di- carboxaldehyde. Copyright © 2013 SciRes. IJOC 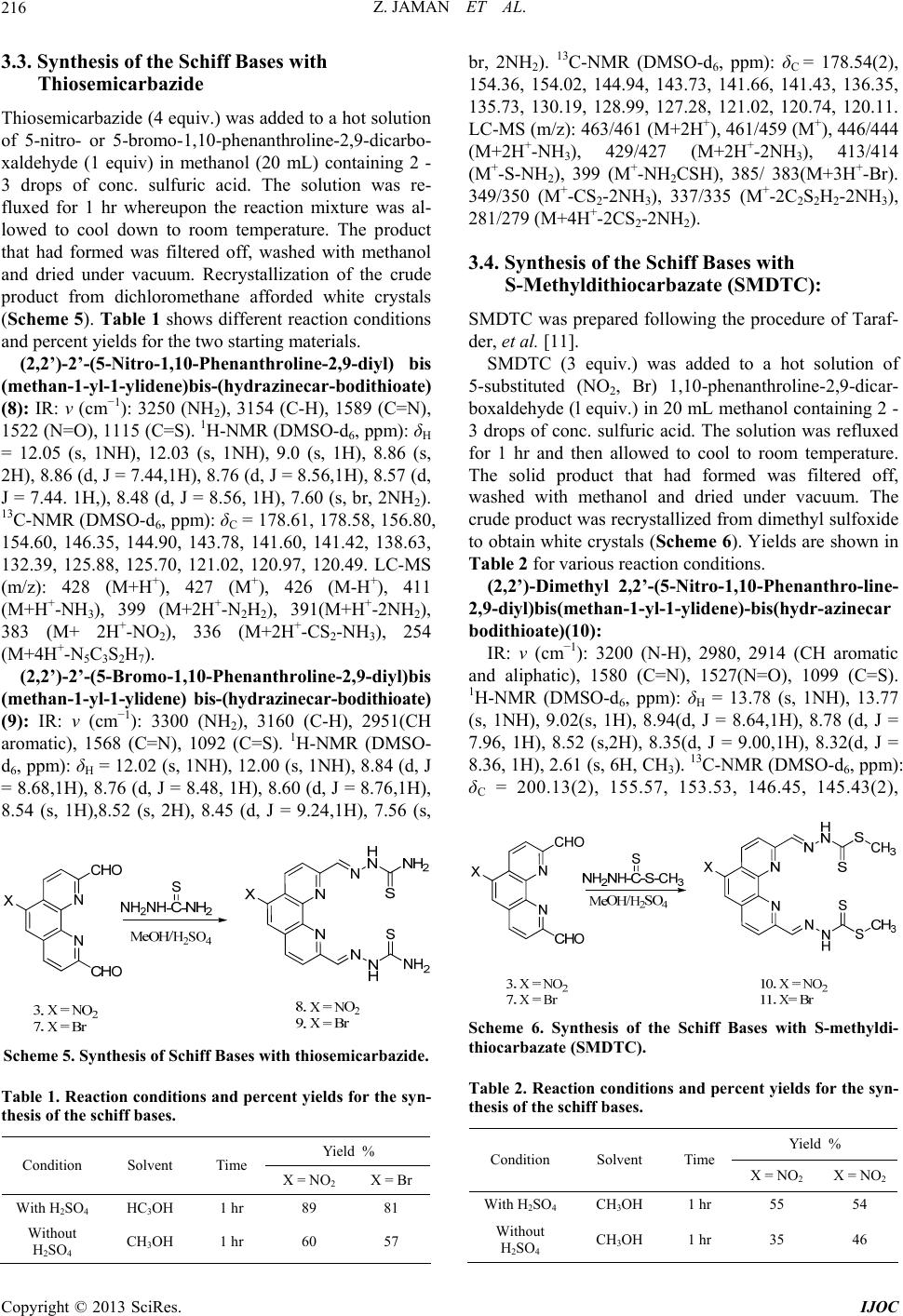 Z. JAMAN ET AL. 216 3.3. Synthesis of the Schiff Bases with Thiosemicarbazide Thiosemicarbazide (4 equiv.) was added to a hot solution of 5-nitro- or 5-bromo-1,10-phenanthroline-2,9-dicarbo- xaldehyde (1 equiv) in methanol (20 mL) containing 2 - 3 drops of conc. sulfuric acid. The solution was re- fluxed for 1 hr whereupon the reaction mixture was al- lowed to cool down to room temperature. The product that had formed was filtered off, washed with methanol and dried under vacuum. Recrystallization of the crude product from dichloromethane afforded white crystals (Scheme 5). Ta ble 1 shows different reaction conditions and percent yields for the two starting materials. (2,2’)-2’-(5-Nitro-1,10-Phenanthroline-2,9-diyl) bis (methan-1-yl-1-ylidene)bis-(hydrazinecar-bodithioate) (8): IR: ν (cm−1): 3250 (NH2), 3154 (C-H), 1589 (C=N), 1522 (N=O), 1115 (C=S). 1H-NMR (DMSO-d6, ppm): δH = 12.05 (s, 1NH), 12.03 (s, 1NH), 9.0 (s, 1H), 8.86 (s, 2H), 8.86 (d, J = 7.44,1H), 8.76 (d, J = 8.56,1H), 8.57 (d, J = 7.44. 1H,), 8.48 (d, J = 8.56, 1H), 7.60 (s, br, 2NH2). 13C-NMR (DMSO-d6, ppm): δC = 178.61, 178.58, 156.80, 154.60, 146.35, 144.90, 143.78, 141.60, 141.42, 138.63, 132.39, 125.88, 125.70, 121.02, 120.97, 120.49. LC-MS (m/z): 428 (M+H+), 427 (M+), 426 (M-H+), 411 (M+H+-NH3), 399 (M+2H+-N2H2), 391(M+H+-2NH2), 383 (M+ 2H+-NO2), 336 (M+2H+-CS2-NH3), 254 (M+4H+-N5C3S2H7). (2,2’)-2’-(5-Bromo-1,10-Phenanthroline-2,9-diyl)bis (methan-1-yl-1-ylidene) bis-(hydrazinecar-bodithioate) (9): IR: ν (cm−1): 3300 (NH2), 3160 (C-H), 2951(CH aromatic), 1568 (C=N), 1092 (C=S). 1H-NMR (DMSO- d6, ppm): δH = 12.02 (s, 1NH), 12.00 (s, 1NH), 8.84 (d, J = 8.68,1H), 8.76 (d, J = 8.48, 1H), 8.60 (d, J = 8.76,1H), 8.54 (s, 1H),8.52 (s, 2H), 8.45 (d, J = 9.24,1H), 7.56 (s, Scheme 5. Synthesis of Schiff Bases w i th thiosemic arbazide. Table 1. Reaction conditions and percent yields for the syn- thesis of the schiff bases. Yield % Condition Solvent Time X = NO2 X = Br With H2SO4 OH 3 CH 1 hr 89 81 Without H2SO4 OH 3 CH 1 hr 60 57 br, 2NH2). 13C-NMR (DMSO-d6, ppm): δC = 178.54(2), 154.36, 154.02, 144.94, 143.73, 141.66, 141.43, 136.35, 135.73, 130.19, 128.99, 127.28, 121.02, 120.74, 120.11. LC-MS (m/z): 463/461 (M+2H+), 461/459 (M+), 446/444 (M+2H+-NH3), 429/427 (M+2H+-2NH3), 413/414 (M+-S-NH2), 399 (M+-NH2CSH), 385/ 383(M+3H+-Br). 349/350 (M+-CS2-2NH3), 337/335 (M+-2C2S2H2-2NH3), 281/279 (M+4H+-2CS2-2NH2). 3.4. Synthesis of the Schiff Bases with S-Methyldithiocarbazate (SMDTC): SMDTC was prepared following the procedure of Taraf- der, et al. [11]. SMDTC (3 equiv.) was added to a hot solution of 5-substituted (NO2, Br) 1,10-phenanthroline-2,9-dicar- boxaldehyde (l equiv.) in 20 mL methanol containing 2 - 3 drops of conc. sulfuric acid. The solution was refluxed for 1 hr and then allowed to cool to room temperature. The solid product that had formed was filtered off, washed with methanol and dried under vacuum. The crude product was recrystallized from dimethyl sulfoxide to obtain white crystals (Scheme 6). Yields are shown in Table 2 for various reaction conditions. (2,2’)-Dimethyl 2,2’-(5-Nitro-1,10-Phenanthro-line- 2,9-diyl)bis(methan-1-yl-1-ylidene)-bis(hydr-azinecar bodithioate)(10): IR: ν (cm−1): 3200 (N-H), 2980, 2914 (CH aromatic and aliphatic), 1580 (C=N), 1527(N=O), 1099 (C=S). 1H-NMR (DMSO-d6, ppm): δH = 13.78 (s, 1NH), 13.77 (s, 1NH), 9.02(s, 1H), 8.94(d, J = 8.64,1H), 8.78 (d, J = 7.96, 1H), 8.52 (s,2H), 8.35(d, J = 9.00,1H), 8.32(d, J = 8.36, 1H), 2.61 (s, 6H, CH3). 13C-NMR (DMSO-d6, ppm): δC = 200.13(2), 155.57, 153.53, 146.45, 145.43(2), Scheme 6. Synthesis of the hiff Bases with S-methyldi- able 2. Reaction conditions and percent yields for the syn- Yield % Sc thiocarbazate (SMDTC). T thesis of the schiff bases. Condition Solvent Time X = N NO2 O2 X = With H2SO4 OH 3 CH 1 hr 55 54 Without H2SO4 3OHCH 1 hr 35 46 Copyright © 2013 SciRes. IJOC  Z. JAMAN ET AL. 217 145145.0.35, 13.26, , 1.16, 12120.38, 162). LC-MS (M+): 492 is(methan-1-yl-1-ylidene)-bis(hydr-azinecar bo 7 (C=N), 1056(C=S). H-NMR (D e Schiff Bases with S-Benzyldithiocarbazate (SBDTC) scribed by Aud ed to a hot ethan-1-yl-1-ylidene)-bis(hydrazinecarbodit hi 2PhCH2SCS), 279 (M+2H-2PhCH2SCSNH). .27(2), .01, 1 5, 139 .95( 3 126.39 m/z, 26 (M+3H+), 491 (M+2H+), 490 (M+H+), 489(M+), 474(M+- CH3), 459(M+-2CH3), 443 (M+-NO2), 397 (M+2H+- 2CH3S), 384 (M+H+-CH3SCSNH), 308 (M+H+- 2CH3SCS), 307 (M+-2CH3SCS), 303 (M+-2CH3SH- C2H2S2). (2,2’)-Dimethyl 2,2’-(5-Bromo-1,10-Phenanthro-line- 2,9-diyl)b dithioate)(11): IR: ν (cm−1): 3240 (N-H), 3160, 2915 (CH aromatic and aliphatic), 1561 MSO-d6, ppm): δH = 13.79 (s, 1NH), 13.76 (s, 1NH), 8.74 (d, J = 8.60, 1H), 8.61 (s, 1H), 8.57 (s, 2H), 8.56 (d, J = 8.56,1H), 8.39 (d, J = 8.84,1H),, 8.29 (d, J = 8.40,1H), 2.61 (s, 3H, CH3), 2.60 (s, 3H, CH3). 13C-NMR (DMSO-d6, ppm): δC = 199.89, 199.77, 153.30, 153.07, 145.87, 145.46, 144.34, 136.72, 136.68, 130.73, 130.72, 129.37, 127.93, 120.70, 120.31, 119.91, 16.93(2). LC-MS (m/z, M+): 525/523 (M+H+), 524/522 (M+), 477/475 (M+-CH3S), 450/448 (M+2H+-CS2), 443/441 (M+-Br), 429/427 (M+-2CH3S), 366(M+H+-Br-CS2), 338 (M+-Br-CH3S-2N2). 3.5. Synthesis of th SBDTC was prepared using a procedure de rieth et al. [12]. SBDTC (3 equiv) was add solution of 5-substituted (NO2, Br) 1,10-phenanthroline- 2,9-dicarboxaldehyde (1 equiv.) in 20 mL methanol fol- lowed by the addition of two drops of acetic acid. The reaction mixture was refluxed for 1 h, then left to cool to room temperature. The product that had formed was fil- tered off, washed with methanol and dried in vacuum. White crystals of the compound were obtained by re- crystallizing the crude product from dimethylsulfoxide (Scheme 7). Yields with and without acid are given in Table 3. (2,2’)-Benzyl 2,2’-(5-Nitro-1,10-Phenanthroline-2,9- diyl)bis(m oate)(12): IR: ν (cm−1): 3150 (N-H), 3026 (C-H aro- matic), 2920 (C-H aliphatic), 1566 (C=N), 1605,1494 (C=C aromatic),1526 (N=O), 1094(C=S). 1H-NMR (DMSO-d6, ppm): δH = 13.79 (s, 1NH), 13.78 (s, 1NH), 8.99(s, 1H), 8.88(d, J = 8.80,1H), 8.71 (d, J = 8.32, 1H), 8.48(s,1H), 8.47(s,1H), 8.29(d, J = 8.88,1H), 8.25(d, J = 8.44, 1H), 7.48 - 7.31(m, 10H), 4.54 (s, 4H, 2xCH2). 13C-NMR (DMSO-d6, ppm): δC = 198.25, 198.14, 155.51, 153.41, 146.41, 145.62, 139.36, 136.38, 129.32(6), 129.12(8), 128.56(2), 127.38, 126.19, 121.03, 120.39, 37.87(2). LC-MS (m/z): 642 (M+H+), 641 (M+), 597(M+H+-NO2), 479 (M+3H+-PhCH2S), 393 (M+2H+- 2PhCH2S), 356 (M+4H+-2PhCH 2-CS2-S), 303 (M+2H+- + N N CHO CHO N N MeOH/H 2SO4 NH2NH-C-S-CH2Ph SN H NSCH2Ph S XX S NN HSCH2Ph 12. X = NO2 13. X = B 3. X = NO2 7. X = B Scheme 7. Synthesis of the Schiff Bases with S-benzyldi- thiocarbazate (SBDTC). Table 3. Reaction Conditions and Percent Yields for the Synthesis of Schiff Bases. Yield % Condition Solvent Time X = NO2 X = NO2 With HSO OHCH 1 2 43hr 65 96 Without H2SO4 OH 3 CH 1 hr 60 92 (nzyl 2,rom,10-Phenanthro ne- 2,9-s(meth l-1-ylidene)-bis drazinecarb dithioate)(13): , R (DMSO-d6, ppm): δH = 13.76 (s, 1N e procedure e (4 equiv.) and ) 1,10-phenanthroline-2,9-di- ic), 1579 (C=N), 1555 (C=C) 1528 (N=O). H 2,2’)-Be diyl)bi 2’-(5-B an-1-y o-1 li (hy o IR: ν (cm−1): 3180 (N-H), 3027 (=CH aromatic), 2925 (C-H aliphatic), 1566 (C=N), 1600, 1479 (C=C aromatic) 1095 (C=S). 1H-NM H), 13.73 (s, 1NH), 8.58 (d, J = 8.24,1H), 8.51 (s, 1H), 8.49 (s, 1H) ,8.43 (s, 1H), 8.41 (d, J = 8.42, 1H), 8.24 (d, J = 8.36,1H), 8.15 (d, J = 7.96, 1H), 7.48 - 7.27(m, 10 H), 4.54 (s, 4H). 13C-NMR (DMSO-d6, ppm): δC = 198.04(2), 153.35, 153.30, 146.28, 145.13, 143.59, 136.74, 136.46, 129.29(6), 129.08(8), 129.56(2), 128.33, 127.37, 120.70, 52.69, 52.79. LC-MS (m/z): 677/675 (M+H+), 676/674 (M+), 597/595 (M+-Br), 553/551 (M+- PhCH2S), 526/524 (M+-2CH3S-2N2), 403/401 (M+H+- 2PhCH2S-N2), 391 (M+4H+-Br-2PhCH2-N 2). 3.6. Synthesis of the Schiff Bases with 2-Mercaptoaniline The compound was prepared using the sam as described in 3.3, using 2-mercaptoanilin 5-substituted (NO2, Br carboxaldehyde (1 equiv.). Table 4 shows different re- action conditions and percent yields. Cyclized products were obtained in both cases as shown in the equation (Scheme 8). 2,9-Di-(benzo[d]thiazol-2-yl)-5-Nitro-1,10-Phenant- hroline (14): IR: ν (cm−1): 3062 (CH aromatic), 1610 (C=C aromat 1-NMR (DMSO-d6,): H = 8.89 (s,1H), 8.83 (d, J = 7.28, 1H), 8.68(d, J = 7.56, 1H), 8.61(2d, app t, J = 8.76, 2H), 8.33 - 8.23 (m, 4H), 7.64(m, 4H). 13C-NMR (DMSO-d6, ppm): δC = 168.77, 168.60, 154.89, 154.89, 153.99, 152.07, 149.23, 147.15 145.54, 144.20, 136.36(2), 136.21, 134.30, 126.90(2), 126.72(3), 126.40, 126.33, Copyright © 2013 SciRes. IJOC 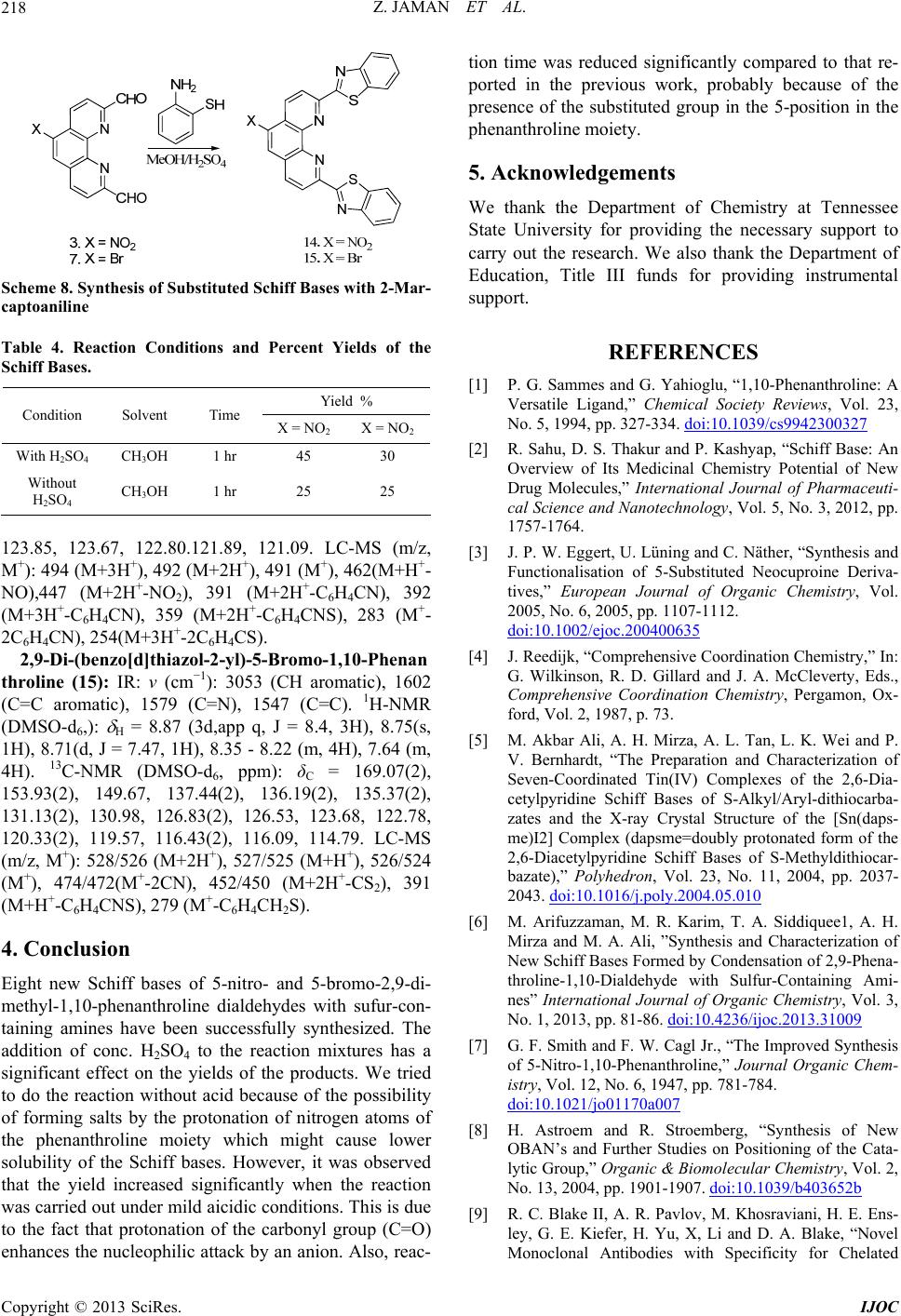 Z. JAMAN ET AL. 218 Scheme 8. Synthesis of Substitute d Schiff Bas es wi th 2-Mar- captoaniline Table 4. Reaction Conditions and Percent Yields of the Schiff Bases. Yield % Condition Solvent Time X = NO X = NO 2 2 With H2SO4 OH 3 CH 1 hr 45 30 Without H2SO4 3OHCH 1 hr 25 25 1233.670.12 121.0LC-MS/z, M+)M+3H 2 (M+2H), 491 (M+), 462(MH+- O),447 (M+2H+-NO2), 391 (M+2H+-C6H4CN), 392 7 (C=C). H-NMR (D -bromo-2, nthroline dialdehydes with sufur-con- e been successfully synthesized. The ent of Chemistry at Tennessee g the necessary support to so thank the Department of [1] P. G. Sammes and G. Yahioglu, “1,10-Phenanthroline: A Versatile LigaReviews, Vol. 23, No. 5, 1994, pcs9942300327 .85, 12 : 494 ( , 122.8 +), 49 1.89, + 9. (m + N (M+3H+-C6H4CN), 359 (M+2H+-C6H4CNS), 283 (M+- 2C6H4CN), 254(M+3H+-2C6H4CS). 2,9-Di-(benzo[d]thiazol-2-yl)-5-Bromo-1,10-Phenan throline (15): IR: ν (cm−1): 3053 (CH aromatic), 1602 (C=C aromatic), 1579 (C=N), 1541 MSO-d6,): H = 8.87 (3d,app q, J = 8.4, 3H), 8.75(s, 1H), 8.71(d, J = 7.47, 1H), 8.35 - 8.22 (m, 4H), 7.64 (m, 4H). 13C-NMR (DMSO-d6, ppm): δC = 169.07(2), 153.93(2), 149.67, 137.44(2), 136.19(2), 135.37(2), 131.13(2), 130.98, 126.83(2), 126.53, 123.68, 122.78, 120.33(2), 119.57, 116.43(2), 116.09, 114.79. LC-MS (m/z, M+): 528/526 (M+2H+), 527/525 (M+H+), 526/524 (M+), 474/472(M+-2CN), 452/450 (M+2H+-CS2), 391 (M+H+-C6H4CNS), 279 (M+-C6H4CH2S). 4. Conclusion Eight new Schiff bases of 5-nitro- and 59-di- methyl-1,10-phena taining amines hav addition of conc. H2SO4 to the reaction mixtures has a significant effect on the yields of the products. We tried to do the reaction without acid because of the possibility of forming salts by the protonation of nitrogen atoms of the phenanthroline moiety which might cause lower solubility of the Schiff bases. However, it was observed that the yield increased significantly when the reaction was carried out under mild aicidic conditions. This is due to the fact that protonation of the carbonyl group (C=O) enhances the nucleophilic attack by an anion. Also, reac- tion time was reduced significantly compared to that re- ported in the previous work, probably because of the presence of the substituted group in the 5-position in the phenanthroline moiety. 5. Acknowledgements We thank the Departm State University for providin carry out the research. We al Education, Title III funds for providing instrumental support. REFERENCES nd,” Chemical Society p. 327-334. doi:10.1039/ uti- pean Journal of Organic Chemistry, Vol. [2] R. Sahu, D. S. Thakur and P. Kashyap, “Schiff Base: An Overview of Its Medicinal Chemistry Potential of New Drug Molecules,” International Journal of Pharmace cal Science and Nanotechnology, Vol. 5, No. 3, 2012, pp. 1757-1764. [3] J. P. W. Eggert, U. Lüning and C. Näther, “Synthesis and Functionalisation of 5-Substituted Neocuproine Deriva- tives,” Euro 2005, No. 6, 2005, pp. 1107-1112. doi:10.1002/ejoc.200400635 [4] J. Reedijk, “Comprehensive Coordination Chemistry,” In: G. Wilkinson, R. D. Gillard and J. A Comprehensive Coordination . McCleverty, Eds., Chemistry, Pergamon, Ox- ) Complexes of the 2,6-Dia- ford, Vol. 2, 1987, p. 73. [5] M. Akbar Ali, A. H. Mirza, A. L. Tan, L. K. Wei and P. V. Bernhardt, “The Preparation and Characterization of Seven-Coordinated Tin(IV cetylpyridine Schiff Bases of S-Alkyl/Aryl-dithiocarba- zates and the X-ray Crystal Structure of the [Sn(daps- me)I2] Complex (dapsme=doubly protonated form of the 2,6-Diacetylpyridine Schiff Bases of S-Methyldithiocar- bazate),” Polyhedron, Vol. 23, No. 11, 2004, pp. 2037- 2043. doi:10.1016/j.poly.2004.05.010 [6] M. Arifuzzaman, M. R. Karim, T. A. Siddiquee1, A. H. Mirza and M. A. Ali, ”Synthesis and Characterization of New Schiff Bases Formed by Condensation of 2,9-Phena- throline-1,10-Dialdehyde with Sulfur-Containing Ami- nes” International Journal of Organic Chemistry, Vol. 3, No. 1, 2013, pp. 81-86. doi:10.4236/ijoc.2013.31009 [7] G. F. Smith and F. W. Cagl Jr., “The Improved Synthesis of 5-Nitro-1,10-Phenanthroline,” Journal Organic Chem- istry, Vol. 12, No. 6, 1947, pp. 781-784. doi:10.1021/jo01170a007 [8] H. Astroem and R. Stroemberg, “Synthesis of New OBAN’s and Further Studies on Positioni lytic Group,” Organic & B ng of the Cata- iomolecular Chemistry, Vol. 2, No. 13, 2004, pp. 1901-1907. doi:10.1039/b403652b [9] R. C. Blake II, A. R. Pavlov, M. Khosraviani, H. E. Ens- ley, G. E. Kiefer, H. Yu, X, Li and D. A. Blake, “Novel Monoclonal Antibodies with Specificity for Chelated Copyright © 2013 SciRes. IJOC  Z. JAMAN ET AL. Copyright © 2013 SciRes. IJOC 219 on-Uranium(VI): Isolation and Binding Properties,” Bioc jugate Chemistry, Vol. 15, No. 5, 2004, pp. 1125-1136. doi:10.1021/bc049889p [10] J. P. W. Eggert and U. Lüning, “Towards A,D-(1,10-Phe- nanthroline)-Bridged Calix[6]arene Dendrimers,” Euro- pean Journal of Organic Chemistry, Vol. 2007, No. 36, 2007, pp. 6046-6052. doi:10.1002/ejoc.200700639 [11] M. T. H. Tarafder, K. B. Chew, K. A. Crouse, A. S. M. Ali, B. M. Yamin and H. K. Fun, “Synthesis and Charac- terization of Cu(II), Ni( Bidentate NS Isomeric Schiff II) and Zn(II) Metal complexes of Bases Derived from S-Me- thyldithiocarbazate (SMDTC): Bioactivity of the Biden- tate NS Isomeric Schiff bases, Some of Their Cu(II), Ni(II) and Zn(II) Complexes and the X-ray Structure of the Bis[S-methyl-β-N-(2-furylmethyl) Methylenedithio- carbazato]Zinc(II) Complex,” Polyhedron, Vol. 21, No. 27-28, 2002, pp. 2683-2690. doi:10.1016/S0277-5387(02)01285-8 [12] Mughrabi, et al., “Cytoprotective Ef (indol-3-ylmethylidene)-Hyrazinecarbo fect of Benzyl-N’- dithioate against Ethanol-Induced Gastric Mucosal Injury in Rats,” Journal of Pure and Applied Chemistry, Vol. 5, No. 3, 2011, pp. 34-42.
|