 Open Journal of Polymer Chemistry, 2013, 3, 92-98 Published Online November 2013 (http://www.scirp.org/journal/ojpchem) http://dx.doi.org/10.4236/ojpchem.2013.34016 Open Access OJPChem Thermal and Photo Alignment Behavior of Polyethylene Imine Having Methoxy Substituent Azobenzene Side Chain Group Mohammad Kamruzzaman1, Sun-nam Kim1, Yutaka Kuwahara1, Tomonari Ogata2, Seiji Kurihara1,3,4* 1Department of Applied Chemistry and Biochemistry, Graduate School of Science and Technology, Kumamoto University, Kurokami, Kumamoto, Japan 2Innovate Collaboration Organization, Kumamoto University, Kurokami, Kumamoto, Japan 3PHOENICS, 3-11-38 Higashimachi, Higashi-ku, Kumamoto, Japan 4JST-CREST, 5 Sanbancho, Chiyoda-ku, Tokyo, Japan Email: *kurihara@gpo.kumamoto-u.ac.jp Received July 10, 2013; revised August 15, 2013; accepted September 2, 2013 Copyright © 2013 Mohammad Kamruzzaman et al. This is an open access article distributed under the Creative Commons Attribu- tion License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Novel type of polyethylene imine having methoxy (–OCH3) substituent azobenzene side chain group through eight me- thylene spacer group (PEI8M) was successfully synthesized and characterized by the polymer by 1H NMR, differential scanning calorimetry, polarized optical microscopic and X-ray diffraction analysis. Synthesized polymer possessed liq- uid crystalline (LC) properties. Spin coated PEI8M film showed out-of-plane molecular orientation on annealing and non-polarized visible light irradiation. PEI8M in solid film exhibited photoresponsive properties upon irradiation of UV and visible light. PEI8M film also exhibited reversible molecular orientation from random state to out-of-plane and from out-of-plane to random state on annealing, non-polarized UV and visible light irradiation. Keywords: Azobenzene Polymer; Thermal Alignment Behavior; Liquid Crystalline Properties; Out-of-Plane Ordering 1. Introduction In recent years, azobenzene containing polymers have attracted considerable attention for the possibility of changing the molecular orientation by irradiation with an appropriate wavelength of light, and the potential appli- cations including reversible optical storage, holographic grating and optical switching [1,2]. In addition, the azo- benzene derivatives are known to undergo trans/cis photo- isomerization upon irradiation. This is not a simple swi- tching process between the two photochromic isomers, because a steady state is strongly dependent on the sub- stitution and the wavelength of the excitation. In literature so far very few papers are published on spontaneous out-of-plane molecular orientation of poly- meric liquid crystals (PLCs) [3-7]. Bobrovsky group re- ported that polyacrylate copolymer containing 4-ethoxy- 4’-hexoxyazobenzene and cholesterol groups as side chains showed spontaneous out-of-plane molecular ori- entation on a glass substrate by annealing at 75˚C, whereas only partial out-of-plane molecular orientation was observed for polyacrylate homopolymer [3]. Our group also reported that PEI having nitro substituent azobenzene side chain group showed out-of-plane mo- lecular orientation on a glass substrate by annealing but not upon non-polarized visible light irradiation [8]. In addition, Ujiie group reported that some polyethylene imines (PEIs) having azobenzene groups as side groups show liquid crystalline phases and align perpendicular direction to the substrate spontaneously by heating and following cooling without any alignment process [9,10]. So, by photochemically controlling the molecular orien- tation of PEIs between random state and out-of-plane structures, one can fabricate the optical switching system showing excellent memory stability by coating PEIs on a substrate without any alignment process. In this article, we synthesized polyethylene imine having methoxy sub- *Corresponding author.  M. KAMRUZZAMAN ET AL. 93 stituent azobenzene side chain group through eight me- thylene spacer group (PEI8M) and investigated its pho- tochemical as well as thermal alignment behavior on annealing, UV and non-polarized visible light irradiation. And, we also demonstrated the multiple reorientations of the azobenzene chromophores in PEI8M. 2. Experimental 2.1. Materials p-Anisidine was purchased from Sigma-Aldrch and used as received. 1,8-dibromooctane was purchased from To- kyo Chemical Industry and polyethylene imine of low molecular weight (Mn ≈ 1800) and high molecular weight (Mn ≈ 10,000) were purchased from Polysciences Inc. Reagents were used without further purification, unless stated. N,N-dimethylformamide (DMF) was pur- chased from Wako Pure Chemical Industries Ltd and was dried up with molecular sieves prior to use. 2.2. Synthesis of Azobenzene Monomer and Polymer Synthesis of 4-methoxy-4’-hydroxyazobenzene (MAz-OH) p-Anisidine (0.05 mol, 6.16 g) was dissolved in 3 mol/L hydrochloric acid (50 ml). After complete dissolu- tion, the solution was cooled with ice to a temperature below 5˚C. With vigorous stirring, to this cold solution was added slowly a solution of 3.5 g (0.05 mol) of so- dium nitrite in 10 ml of water. The resulting diazonium solution, kept below 5˚C, was subsequently added drop- wise to a cold solution of 4.7 g (0.05 mol) of phenol in 25 ml of 10% aqueous sodium hydroxide. The dark brown suspension was acidified and the precipitate was collected. The crude product was washed with copious amount of water and dried under vacuum. Yield: 65% as solid. Melting point: 139˚C - 140˚C. 1H-NMR (CDCl3, δ): 7.88 (2H, dd, aromatic), 7.82 (2H, dd, aromatic), 6.98 (2H, d, aromatic), 6.94 (2H, d, aro- matic), 5.31 (1H, s, –OH), 3.87 (3H, s, –OCH3). Anal. Calcd for C13H12N2O2 (228.25): C—68.41; H—5.30; N—12.27. Found: C—68.82; H—6.42; N—12.01. Synthesis of 4-(8-bromo-n-octyloxy)-4’-methoxy azobenzene [MAzO8Br] 4-(8-bro mo-n-octyloxy)-4’-methoxy azobenzene was synthesized by the following procedure: To a mixture of 1.1 g (4.4 mmol) of 4-methoxy-4’-hydroxyazobenzene (MAz-OH) and 0.91 g (6.6 mmol) potassium carbonate dissolved in 150 ml of dried acetone, 6.08 g (22.3 mmol) of 1,8-dibromooctane was added. After refluxing for 48 h at 70˚C the reaction mixture was filtered and evaporated the solvent from filtrate and the product MAzO8Br was re-crystallized from methanol twice. The reaction scheme is shown in Figure 1. Yield: 81.4% as solid. Melting point: 107˚C - 109˚C. Anal. Calcd for C21H27BrN2O2 (419.36): C—60.15; H— 6.49; N—6.68. Found: C—60.25; H—6.35; N—6.70. Synthesis of polyethylene imine having azobenzene side chain group (PEI8M) Polyethylene imines having methoxy substituent azobenzene side chain group with eight methylene spacer groups was synthesized by using different reaction con- ditions. The typical procedure is as follows: 4-(8-bromo- n-octyloxy)-4’-methoxy azobenzene 1.76 g (4.2 mmol), polyethylene imine 0.18 g (4.0 mmol), potassium car- bonate 1.8 g (6.0 mmol) and 50 ml dry DMF were added into a round bottom flask equipped with a condenser. With continuous stirring the reaction mixture was re- fluxed at 100˚C for 72 h. After the reaction, the mixture was filtered and evaporated the half amount of DMF Figure 1. Synthetic route for monomer and polymer. Copyright © 2013 SciRes. OJPChem  M. KAMRUZZAMAN ET AL. 94 from filtrate and then poured into methanol. The result- ing polymer (PEI8M) was purified by precipitation from chloroform/THF into methanol two to three times. The removal of the monomer was monitored using thin-layer chromatography. Finally, the product was dried in vac- uum for 24 h (Figure 1). 2.3. Characterization To identify the structure and composition of the synthe- sized monomers and polymer, elemental analyses were performed with a YANAKO CHN CORDER MT-6. The structures of the compounds were also determined using 1H NMR spectroscopy with CDCl3 or acetone as solvent and tetramethylsilane as internal standard. The spectra were recorded at ambient temperature with a JEOL JNM-EX400, 400MHz NMR-spectrometer. The phase transition behavior of the polymer was stu- died by differential scanning calorimetry (DSC; Seiko SSC-5020) with a heating rate of 10 K/min and polariz- ing optical microscopy (Olympus BHSP polarizing mi- croscope; Mettler FP-80 and FP 82 hot stage and con- troller). X-ray diffraction was used to confirm the nature of the LC phases and to determine the spacing of the smectic layers. Rigaku, RINT 2100/PC XRD machine (X-Ray, 40 kV/200 mA) equipped with a θ-θ wide angle go- niometer and scintillation detector was used for X-ray diffraction (XRD) measurement. Photoirradiation was performed by using a 500 W high-pressure Hg lamp with adequate cut filter for UV and visible light at room temperature. The orientational order was studied using polarized UV-Vis spectroscopy (Perkin Elmer Lambda 650 UV/Vis Spectroscopy) and the angular dependence of the absorbance was measured. The values of order parameter determined by spectro- scopic method were calculated by Equation (1) [3]. 2 A SA ║┴ ║┴ (1) where A║ is the absorbance at the preferred direction; A┴ is the absorbance perpendicular to this direction. 3. Results and Discussion 3.1. Synthesis and Characterization of PEI8M In this study, liquid crystalline polyethylene imine PEI8M was successfully synthesized. For good photo alignment behavior, polyethylene imines should have high degree of substitution. It is assumed that as the in- troduction of azobenzene group into polyethylene imine main chain increased the out-of-plane alignment behav- ior of the polymers also increased. So in synthesis step, to achieve higher degree of substitution in PEI, reaction conditions were optimized by changing the reaction pa- rameters such as temperature, reaction time, molar ratio of azobenzene monomer and PEI, molecular weight of PEI and reaction solvent. The degree of substitution of PEI8M has considerable effect on all of these parameters and finally we synthesized PEI8M having 70.2% degree of substitution and 86.4% yield by optimizing the reac- tion conditions as temperature 100˚C, reaction time 72 h, molar ratio of azobenzene monomer to PEI (low mol. wt.) = 4.2:4.0, and solvent (DMF) = 50 ml. Synthesized poly- mer showed good solubility in DMF, chloroform, THF and cyclohexanone. Synthesized polymer, PEI8M was characterized using 1H NMR spectroscopy. The structure and 1H NMR spec- trum of the polymer with assignments are shown in Fig- ure 2. The resonances corresponding to the protons of –OCH3 (a) appeared at 3.75 ppm, while those of –CH2O– (d) at 3.99 ppm. The resonances corresponding to phenyl Figure 2. Structure and 1H NMR spectrum of synthesized polyethylene imine PEI8M. Open Access OJPChem  M. KAMRUZZAMAN ET AL. 95 protons appeared at 6.97 and 7.85 ppm while resonances due to six methylene (–CH2–) (e, f, g) protons in azo- benzene side chain and one methylene (–CH2–) (f) pro- tons in polyethylene unit were observed at 1.28 - 1.92 ppm. The signal at 2.59 ppm was attributed to methylene protons of –N–CH2– (i+j) and at 2.43 ppm was observed for methylene (–CH2–N–) (h) protons. The chemical shifts and relative intensity of 1H NMR resonances agreed well with the structure shown in Figure 2. Thermal characteristics of PEI8M were studied using DSC and polarized optical microscopy (POM) analysis. In DSC thermogram, there is a peak at around 116 degree, it is expected for phase transition from nematic to iso- tropic temperature. In addition, the peak at 98 degree is assessed as phase transition temperature for smectic to nematic. Therefore, the phase transition temperature is determined for G 28 S 98 N 116 I. And, the polymer is stable up to 190˚C. The synthesized polymer was also characterized by X-ray diffraction measurement. The X-ray diffraction patterns for synthesized polyethylene imine film con- firmed that the polymer is smectic layer structures and exhibited strong few diffraction peaks in small angle re- gion. On the basis of X-ray diffraction patterns, the sme- ctic layer spacings were calculated from the XRD peaks using Bragg’s law and the results are 2θ(1) = 2.46; 2θ(2) = 5.06; dist.1(Å) = 33.08 and dist.2(Å) = 16.45. 3.2. Thermooptical Alignment Behavior of PEI8M in Solid Film To study the thermal induced optical anisotropy in PEI8M solid film, the changes in absorbance of spin coated polymeric films on annealing were demonstrated. In this study, all experiments were performed for poly- meric films with a thickness ranging from 200 to 300 nm. The films were prepared by spin coating technique. Upon annealing the test film at room temperature, no marked changes took place. However, when the film was kept at the temperature, which is above its glass transition tem- perature corresponding to LC state, observed the signifi- cant spectral changes (Figure 3(a)). On annealing, the absorption in the region of the π-π* transition is related to out-of-plane ordering was decreased by about 80%. The position of the absorption maximum remained almost unchanged (Figure 3(a)). It should be noted that, the profiles of the corresponding spectra and absorption in the region of the φ-φ* transitions were preserved. In addition, the relative intensity of π-π* absorption band to absorption band around 250 nm which is as- signed to the φ-φ* transition of the aromatic ring [4,11], was varied as shown in Figure 3(b). It has been reported that the φ-φ* transition is insensitive to the molecular orientation, consequently, the change in the relative in- tensity of π-π* absorption band to φ-φ* absorption band (a) (b) Figure 3. (a) UV-vis. absorption spectra of PEI8M single layer film before and after annealing at 70˚C during 5 min. Longer time of annealing does not lead to additional changes. (b) Changes in Aπ-π*/Aφ-φ* values of PEI8M single layer film on annealing time. (Aπ-π*/Aφ-φ*) is related to the out-of-plane molecular ori- entation [4,5]. From Figure 3(b), it is also clear that the out-of-plane molecular orientation proceeded in a few minutes. Therefore, to quantify the thermal induced anisotropy in PEI8M solid film, polarized absorption spectra were measured and explored the angular dependency of ab- sorbance before and after annealing of the film. Anneal- ing of the PEI8M film brought about not only decrease in absorbance corresponding to the π-π* transition without polarizer, but also change in the polarized absorption spectra as shown in Figure 4. The order parameter (S), the degree of out-of-plane ordering, was calculated from polarized absorption spectra by using Equation (1). The polarized absorption spectra were recorded at an angle of 45˚ to the normal of the film. From polarized absorption spectra, it is clear that, before annealing, little angular dependency was observed, indicating that the azobenzene chromophores were slightly orientated per- pendicularly on glass substrate in solid film. But after Copyright © 2013 SciRes. OJPChem 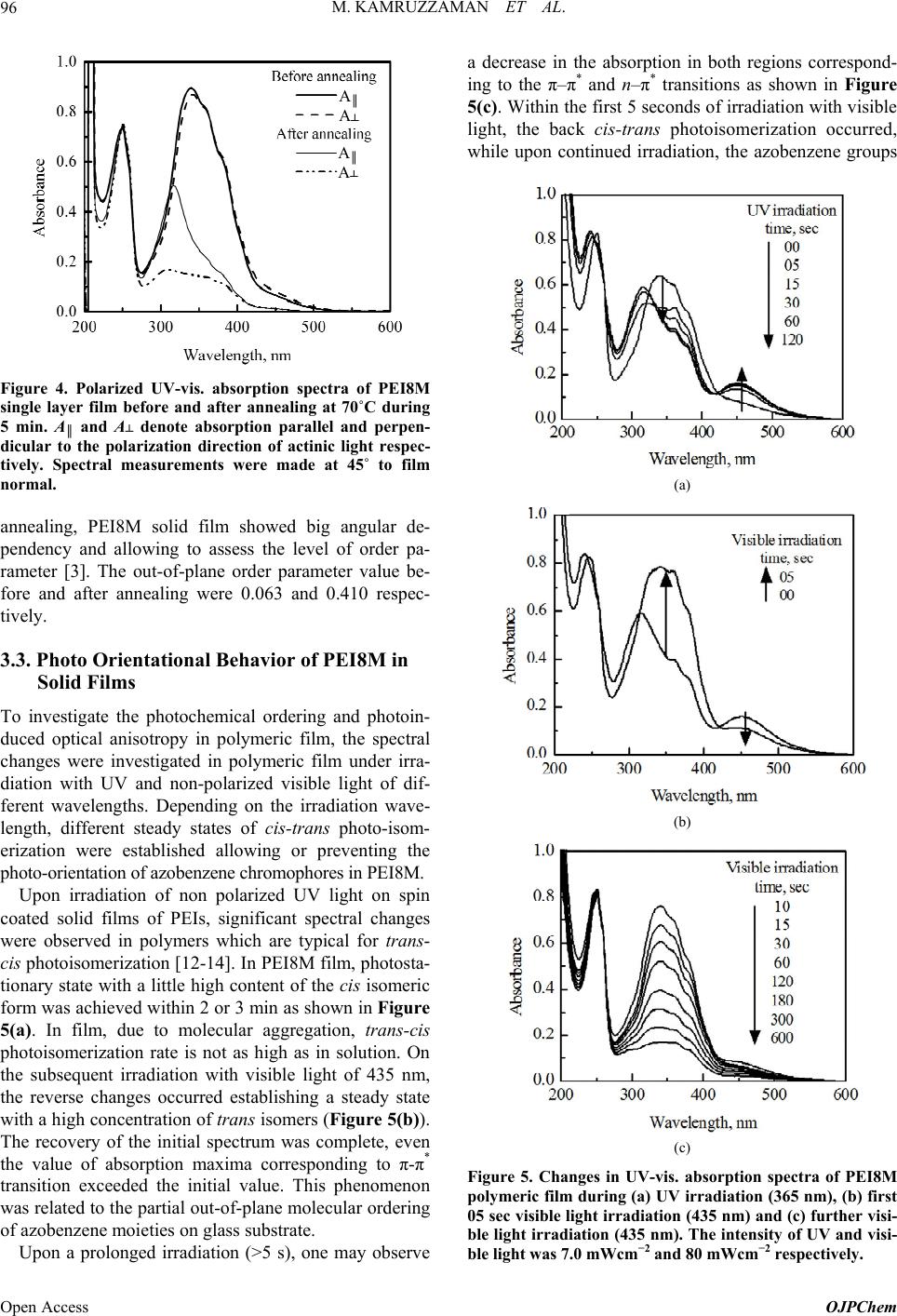 M. KAMRUZZAMAN ET AL. 96 A ║ A A ║ A Figure 4. Polarized UV-vis. absorption spectra of PEI8M single layer film before and after annealing at 70˚C during 5 min. A║ and A┴ denote absorption parallel and perpen- dicular to the polarization direction of actinic light respec- tively. Spectral measurements were made at 45˚ to film normal. annealing, PEI8M solid film showed big angular de- pendency and allowing to assess the level of order pa- rameter [3]. The out-of-plane order parameter value be- fore and after annealing were 0.063 and 0.410 respec- tively. 3.3. Photo Orientational Behavior of PEI8M in Solid Films To investigate the photochemical ordering and photoin- duced optical anisotropy in polymeric film, the spectral changes were investigated in polymeric film under irra- diation with UV and non-polarized visible light of dif- ferent wavelengths. Depending on the irradiation wave- length, different steady states of cis-trans photo-isom- erization were established allowing or preventing the photo-orientation of azobenzene chromophores in PEI8M. Upon irradiation of non polarized UV light on spin coated solid films of PEIs, significant spectral changes were observed in polymers which are typical for trans- cis photoisomerization [12-14]. In PEI8M film, photosta- tionary state with a little high content of the cis isomeric form was achieved within 2 or 3 min as shown in Figure 5(a). In film, due to molecular aggregation, trans-cis photoisomerization rate is not as high as in solution. On the subsequent irradiation with visible light of 435 nm, the reverse changes occurred establishing a steady state with a high concentration of trans isomers (Figure 5(b)). The recovery of the initial spectrum was complete, even the value of absorption maxima corresponding to π-π* transition exceeded the initial value. This phenomenon was related to the partial out-of-plane molecular ordering of azobenzene moieties on glass substrate. Upon a prolonged irradiation (>5 s), one may observe a decrease in the absorption in both regions correspond- ing to the π–π* and n–π* transitions as shown in Figure 5(c). Within the first 5 seconds of irradiation with visible light, the back cis-trans photoisomerization occurred, while upon continued irradiation, the azobenzene groups (a) (b) (c) Figure 5. Changes in UV-vis. absorption spectra of PEI8M polymeric film during (a) UV irradiation (365 nm), (b) first 05 sec visible light irradiation (435 nm) and (c) further visi- ble light irradiation (435 nm). The intensity of UV and visi- ble light was 7.0 mWcm−2 and 80 mWcm−2 respectively. Open Access OJPChem  M. KAMRUZZAMAN ET AL. 97 were oriented along the normal of film due to photo- orientation [3]. In this case, the transition moment of these groups become oriented perpendicular to the plane of film; as a result, the probability of light absorption and, thus, optical density markedly decreased [3]. So irradia- tion with non-polarized visible light (435 nm) caused the induction of anisotropy in the films by photoorientation of azobenzene side groups. 3.4. Reversibility of Alignment Ordering of PEI8M in Solid Film In order to show the possibility of reversible reorienta- tion of azobenzene chromophores in PEI8M, molecular ordering behaviors were observed at different conditions. And synthesized polymer showed reversible molecular alignment behavior from random state to out-of-plane order and from out-of-plane order to random state. In all cases we observed the angular dependency on absorb- ance at different conditions and plotted the polar plots. Figure 6 illustrates the polar plots for PEI8M, showing the changes in absorbance on annealing, UV and non- polarized visible light irradiation. The shape of the cor- responding polar plots at various conditions has demon- strated the nature of the alignment behaviors. The reversibility of the alignment behavior of PEI8M was also demonstrated at different conditions in terms of order parameter, estimated by measuring polarized ab- sorption spectra. In fresh film, polymer showed no out-of-plane ordering (S = 0.04). Upon annealing, poly- meric film showed high order parameter value (S = 0.41) showing out-of-plane molecular alignment. Irradiation with the 365 nm light brings the film always into the iso- tropic state and erases any previously induced anisotropy. So after the irradiation of UV light on annealed films, thevalue of order parameter decreased via cis-trans photoi- Figure 6. Polar plot for PEI8M (λ = 317 nm) single layer film: □—fresh film, Ο—after annealing, Δ—after non-po- larized UV light irradiation and ▽—after non-polarized visible light irradiation. Spectral measurements were made at 45˚ to film normal. somerization and reached to almost initial level (S = 0.024). Namely the azobenzene molecules aligned ran- domly on the glass substrate. Following non-polarized visible light irradiation again increased the order pa- rameter value from 0.024 to 0.44, indicating the trans- formation of molecular orientation of azobenzene mole- cules from random state to out-of-plane order due to photo-orientation. The efficiency of the induction of ani- sotropy in the polymeric film was higher for the 435 nm non-polarized visible light compared with that of an- nealing because the pre-irradiation with the 365 nm light strongly increased the effectiveness of photoorientation process [15]. Again upon UV irradiation, the azobenzene group aligned randomly on the glass substrate (S = 0.02). It should be noted that after each step of irradiation with the 365 nm UV light the angular-dependent spectra were exactly the same. This indicates a complete erasure of the previously written orientation was achieved. Again, after annealing the out-of-plane order parameter value in- creased from 0.02 to 0.41 and after irradiation of 435 nm non-polarized visible light order parameter value changed from 0.01 to 0.46. In this way, the reversibility of the alignment behavior of azobenzene groups in polyethyl- ene imine could be achieved by changing the conditions such as annealing, UV and non-polarized visible light irradiation. All these results indicated that the optical information in the polymer can be rewritten in the system without any memory effects of previous irradiation or annealing. 4. Conclusion Liquid crystalline polyethylene imine (PEI8M) having methoxy (−OCH3) substituent azobenzene side chain group through eight methylene spacer group was suc- cessfully synthesized and characterized. Photochemical, thermooptical as well as photoorientational behavior of the polymer were investigated elaborately. Spin coated PEI8M film showed out-of-plane molecular ordering on annealing and exhibited photoresponsive properties upon irradiation of UV and visible light. PEI8M film also ex- hibited reversible molecular orientation from random state to out-of-plane and from out-of-plane to random state on annealing, non-polarized UV and visible light irradiation. This reversibility of molecular ordering has been achieved by the combination of thermal and photo- chemical processes. REFERENCES [1] K. Ichimura, “Photoalignment of Liquid-Crystal Systems,” Chemical Review, Vol. 100, No. 5, 2000, pp. 1847-1873. http://dx.doi.org/10.1021/cr980079e [2] T. Ikeda, “Photomodulation of Liquid Crystal Orienta- tions for Photonic Applications,” Journal of Materials Copyright © 2013 SciRes. OJPChem  M. KAMRUZZAMAN ET AL. Open Access OJPChem 98 Chemistry, Vol. 13, No. 9, 2003, pp. 2037-2057. http://dx.doi.org/10.1039/b306216n [3] A. Bobrovsky, N. Boiko, V. Shibaev and J. Stumpe, “Com- parative Study of Photoorientation Phenomena in Photo- sensitive Azobenzene-Containing Homopolymers and Co- polymers,” Journal of Photochemistry and Photobiology A: Chemistry, Vol. 163, No. 3, 2004, pp. 347-358. http://dx.doi.org/10.1016/j.jphotochem.2004.01.021 [4] B. Sapich, A. B. E. Vix, J. P. Rabe, J. Stumpe, G. Wilbert and R. Zentel, “Ordering and Dewetting in Spin-Coated Films of a Liquid Crystalline Main Chain Polymer,” Thin Solid Films, Vol. 514, No. 1, 2006, pp. 165-173. http://dx.doi.org/10.1016/j.tsf.2006.02.021 [5] T. Uekusa, S. Nagano and T. Seki, “Highly Ordered In- Plane Photoalignment Attained by the Brush Architecture of Liquid Crystalline Azobenzene Polymer,” Macromo- lecule, Vol. 42, No. 1, 2009, pp. 312-318. [6] S. Ujiie and K. Iimura, “Thermal Properties and Orienta- tional Behavior of a Liquid-Crystalline Ion Complex Po- lymer,” Macromolecules, Vol. 25, No. 12, 1992, pp. 3174- 3178. http://dx.doi.org/10.1021/ma00038a024 [7] S. Ujiie and K. Iimura, “Formation of Smectic Orienta- tional Order in an Ionic Thermotropic Liquid-Crystalline Side-Chain Polymer,” Polymer Journal, Vol. 25, No. 4, 1993, pp. 347-354. http://dx.doi.org/10.1295/polymj.25.347 [8] M. Kamruzzaman, Y. Kuwahara, T. Ogata, S. Ujiie and S. Kurihara, “Synthesis, Thermal, and Photo Alignment Be- havior of Polyethylene Imines Having Nitro Substituent Azobenzene Side Chain Group,” Journal of Applied Po- lymer Science, Vol. 120, No. 2, 2011, pp. 950-959. http://dx.doi.org/10.1002/app.33138 [9] S. Ujiie and K. Iimura, “Ammonium Halide Type Ther- motropic Liquid-Crystalline Polyethyleneimines and Those Low-Mass Model Compounds,” Chemistry Letters, Vol. 19, No. 6, 1990, pp. 995-998. http://dx.doi.org/10.1246/cl.1990.995 [10] S. Ujiie and Y. Yano, “Thermotropic and Lyotropic Be- havior of Novel Amphiphilic Liquid Crystals Having Hy- drophilic Poly(ethyleneimine) Units,” Chemistry Commu- nications, Vol. 1, 2000, pp. 79-80. [11] J. Fabian and H. Hartmann, “Light Absorption of Organic Colarants,” Springer-Verlag, Berlin, 1980, pp. 32-79. http://dx.doi.org/10.1007/978-3-642-67587-4 [12] A. Natansohn and P. Rochon, “Photoinduced Motions in Azo-Containing Polymers,” Chemical Reviews, Vol. 102, No. 11, 2002, pp. 4139-4175. http://dx.doi.org/10.1021/cr970155y [13] M. Z. Alam, T. Ohmachi, T. Ogata, T. Nonaka and S. Ku- rihara, “Photoisomerization Behavior and Photoinduced Surface Relief Gratings on Azopolymer Film by a Mono- chromatic Light Irradiation,” Optical Materials, Vol. 29, No. 4, 2006, pp. 365-370. http://dx.doi.org/10.1016/j.optmat.2005.10.005 [14] M. Ishiguro, D. Sato, A. Shishido and T. Ikeda, “Bragg- Type Polarization Gratings Formed in Thick Polymer Films Containing Azobenzene and Tolane Moieties,” Langmuir, Vol. 23, No. 1, 2007, pp. 332-338. http://dx.doi.org/10.1021/la061587j [15] D. Rais, Y. Zakrevskyy, J. Stumpe, S. Nešpůrek and Z. Sedláková, “Photoorientation of Azobenzene Side Groups in a Liquid-Crystalline Polybutadiene-Based Polymer,” Optical Materials, Vol. 30, No. 8, 2008, pp. 1335-1342. http://dx.doi.org/10.1016/j.optmat.2007.07.001
|