 Journal of Water Resource and Protection, 2013, 5, 792-800 http://dx.doi.org/10.4236/jwarp.2013.58080 Published Online August 2013 (http://www.scirp.org/journal/jwarp) Assessment of Acid Deposition Effects on Water Quality of the Upper Rio Grande River Section in Texas Qin Qian1*, Badri Parajuli1, Qi Fu2, Kaiming Yan1, John L. Gossage3, Thomas Ho3 1Department of Civil Engineering, Lamar University, Beaumont, USA 2Department of Earth and Atmospheric Sciences, University of Houston, Houston, USA 3Department of Chemical Engineering, Lamar University, Beaumont, USA Email: *qin.qian@lamar.edu Received June 4, 2013; revised July 5, 2013; accepted August 6, 2013 Copyright © 2013 Qin Qian et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Airborne pollutants such as and 2 4 SO 3 O that cause acid rain may pollute water resources via acid deposition. However, such effects on the water quality of the upper Rio Grande River section in Texas have not been systematically studied. The objective of this study is to collect and analyze field data, and perform hydrological and water chemistry analyses to assess acid deposition effects on the river water quality. The analysis of the precipitation data indicates that the concentrations of ions decrease as the quantity of precipitation increases. The precipitation with higher concentra- tions of and 2 4 SO 3 O has a lower pH while that with higher concentrations of Ca2+ and Na+ has a relatively higher pH value. The analysis of river data demonstrates that the pH value, Dissolved Oxygen (DO), and Total Dissolved Solid (TDS) generally decrease when the flow rate increases immediately following precipitation events. The drop in pH fol- lowing a precipitation event is due to the low pH in the precipitation. The DO and TDS decrease after the precipitation due to the increased flow rate. The slightly higher pH and lower DO values in the eastern section of the river (where the basin is limestone-dominated) as compared to the western section is due to the limestone erosion caused by the acid deposition. The annual stone loss by the acid deposition is about 72,000 m3. The fluctuation between the pH value and the temperature suggests the effect of CaCO3 solubility on the pH value. The water chemistry analysis using Geochem- ist’s Work Bench (GWB) has been performed to estimate the effect on the oscillation of CaCO3 dissolution-precipita- tion process. The equilibrium pH decreases with decreasing temperature, but increases as the CaCO3 concentration de- creases. The effect of limestone on observed daily pH fluctuations appears to be supported by the simulation. Keywords: Acid Deposition; Precipitation; Rio Grande River; Water Quality 1. Introduction Acid deposition, commonly known as acid rain, is one of the important culprits for water quality and ecology sys- tem degradation in the USA [1-3]. Acid deposition is formed from airborne particulate pollutants, such as SO2 and NOx, which react with water, oxygen, carbon dioxide, and sunlight in the atmosphere to result in sulfuric (H2SO4) and nitric (HNO3) acids, the primary agents of acid deposition. Airborne pollutants are transported into the river basin along with the air mass and deposit in the basin as dry and wet deposition [4]. Before getting into the water body, acid deposition within the catchment flows through forests, fields, buildings, and roads, mixes with impurities and undergoes a series of chemical reac- tions on its course. In areas where buffering capacity is low, acid rain releases aluminum from soils into lakes and streams, which is highly toxic to many species of aquatic organisms [5]. Acidity of the water body affects the aquatic life inhabiting it since the water pH deter- mines the solubility and biological availability of chemi- cal constituents such as nutrients and heavy metals [6]. Solubility of many metals increases at lower pH resulting in the increasing toxicity [7]. Water with excessively high pH causes a bitter taste, encrusts water pipes and water-using appliances with deposits, and depresses the effectiveness of chlorine disinfection, thereby causing the need for additional chlorine [8]. Only a limited pH range between 6.5 and 8.5 of water is useful for drinking, irri- gation, industrial use if other water quality parameters are within the normal values and this pH range is typical of most major drainage basins of the world [9]. Data provided by the National Atmospheric Deposi- *Corresponding author. C opyright © 2013 SciRes. JWARP  Q. QIAN ET AL. 793 tion Program (NADP) showed more acid rain events in the Rio Grande River basin in recent years. With in- creasing population and urbanization in the Rio Grande basin, the recent Texas Commission on Environmental Quality (TCEQ) studies have reported high concentra- tions of sulfates and nitrates in some segments of the Upper Rio Grande River in Texas. The results from Community Multi-scale Air Quality (CMAQ) model version 4.6 [10] indicate that the deposition of pollutants, sulfate in particular, can be a serious problem on water sustainability in the Rio Grande River in Texas [11]. The major sources of airborne particulate pollution that pose risks to the sustainable use of Rio Grande River water may be vehicular activities and industrial activities (re- fineries) from Mexico and/or the Texas Gulf Coast re- gion [11]. The acid deposition attaches to the beautifully etched limestone cliffs in the Sierra del Caballo Muerto and in Big Bend’s canyons to dissolve a tiny bit of calcite, which is transported away by rapid runoff and flash-flood- ing following summer thunderstorms [12]. Such impact caused by the acid deposition in streams is a complex physical and chemical process [13]. The objective of this study is to assess the impact of acid deposition on the water quality in the Rio Grande River section between El Paso and the Amistad Reser- voir in Texas. It is accomplished through collecting and analyzing field measurement data and performing hy- drological and water chemical analyses on river water. The field data collected and analyzed include the amount of precipitation and the corresponding water chemicals in the precipitation, including pH and concentrations of , 3 2 4 SO O, Ca2+, Na+, and other species, as well as the corresponding water pH, total dissolved solid (TDS), and Dissolved Oxygen (DO) in the river water after precipi- tation events. The hydrological analysis demonstrates the relationship between flow rates and precipitations, in turn to evaluate the acid deposition impact on the river water quality. To assess the limestone erosion due to acid de- position, the total stone loss has been estimated. A geo- chemical analysis by Geochemist’s Work Bench (GWB) has been performed to estimate the effect of the oscilla- tion of calcite dissolution-precipitation process on the equilibrium pH in the river. 2. Study Region The Rio Grande (Figure 1) is the fifth longest river sys- tem in North America. It runs 1800 miles from its origin in the southern Colorado Rocky Mountains before it drains into the Gulf of Mexico. The Upper Rio Grande River Section in Texas between El Paso and the Amistad reservoir serves as the border between Mexico and Texas. It is arid to semi-arid climate desert ecosystems. It en- compasses a total of 51,475 square miles of which 16,845 square miles are upstream of the Rio Conchos and 34,630 Figure 1. The geography map of the Rio Grande River Ba- sin. are downstream of the Rio Conchos [14]. The three ma- jor tributaries are the Rio Conchos from Mexico with a watershed area of 26,404 mi2, the Pecos River with a wa- tershed area of 35,308 mi2, and the Devils River with a watershed area of 4305 mi2 [15]. The watershed of Pecos River and the Devils River are located in Texas and sup- ply the water to the Amistad reservoir. The first reach between El Paso and Presidio, upstream of the Rio Con- chos, contains about 55% alluvial tar, 40% desert moun- tain terrain, and 5% aquifer recharge zone. The second reach between Presidio and Amistad Dam, below the Rio Conchos, contains 40% Desert Mountain and canyon land (volcanic rock), 55% massive limestone, and 5% chalk [16]. The Rio Conchos enters the Rio Grande near Presidio, Texas, just upstream of Big Bend National Park and Ojinaga, Mexico, in a region of mountains and can- yons. Along the entire river, water lost through evapora- tion exceeds water gained from precipitation. Because of the dry, desiccated state in the Rio Grande watershed, most rainfall events are absorbed by the watershed with- out creating any appreciable runoff. By some accounts, less than four percent of the precipitation that falls on this watershed reaches the Rio Grande [17]. 3. Analysis of Field Measurement Data 3.1. Characteristics of Wet Deposition The wet deposition data in the Rio Grande River basin Copyright © 2013 SciRes. JWARP  Q. QIAN ET AL. 794 from 1994 to 2011 at the Big Bend National Park (TX04, Brewster County), Sonora (TX16, Edwards County), and Guadalupe Mountains National Park Frijole Ranger Sta- tion (TX22, Culberson County) were collected from the National Atmospheric Deposition Program (NADP). Sample handling procedures at all NADP sites were changed substantially on 01/11/1994 to reduce contami- nation from the sample shipping container. Therefore, the annual precipitation quantity and pH as well as deposi- tion data (kg/ha) of nitrate, sulfate, ammonium, calcium, magnesium, potassium, sodium, and chloride ions after 01/11/1994 were retrieved. The average annual precipita- tion of these three stations is 42.6 cm/yr. The maximum quantity (70.9 cm/yr) occurred in 2004 and the minimum quantity (25.4 cm/yr) occurred in 2011. The annual pre- cipitation and the number of raining day have been plot- ted in Figure 2 (no daily data recorded after 05/08/2007 in 2007). It shows TX16 is the wettest station, while the TX04 is the driest station. However, TX22 has more raining days than TX16. The monthly precipitation (Figure 3) shows higher precipitation quantity occurs in summer for all three stations. The maximum daily pre- cipitation at each station occurred on 08/17/2006 at TX 04, 10/09/2011 at TX16 and 10/7/2003 at TX22. The average, maximum and minimum field pH of annual pre- cipitations is 5.24, 5.41 and 5.03 respectively, which in- dicates the basin is experiencing acid deposition. The annual deposition mass was calculated graphically by multiplying the deposition by the corresponding drai- nage area. Figure 4 shows the maximum depositions of sulfate (5267 tons) and nitrate (4227 tons) occurred in 2001, the maximum depositions of ammonium (1678 tons) and calcium (3688 tons) occurred in 2011, and the maximum quantities of chloride (1141 tons), sodium (845 tons), magnesium (249 tons) and potassium (135 tons) were deposited in 1996. In addition, for each of these eight species the annual deposition in 2011 was higher than in any other year from 2003 to 2010 despite the fact that the precipitation quantity is the lowest, i.e. the drought condition may cause acid pulse because sul- phur dioxide (SO2) deposited onto the soil is reduced to sulphur and oxygen; then this is re-oxidised in combina- tion with runoff to form acids in the soil or discharge to the river [18]. The newest data in 2012 indicate the drought in the basin is continuing. To illustrate the precipitation chemistry, the statistical analysis was applied to the weekly weighted mean wet deposition and weekly precipitation quantity data at the three stations. The different ion concentrations of a typi- cal acid rain (pH < 5.6) and normal rain (pH > 5.6) is graphed in Figure 5. It indicates the acid rain has higher concentrations of anions such as sulfate and nitrate but the normal rain has higher concentrations of cations such as calcium and sodium. With the weekly precipitation Figure 2. The annual precipitation and the number of rain- ing day at three stations. Figure 3. The monthly precipitation at the three stations. data at each station between 1994 and 2011, the number of weekly precipitation events was counted for every 10 mm interval. There are more than 200 events at each sta- tion with less than 10 mm quantity and the number of events decreases with increasing precipitation quantity as shown in Figure 6. The weekly weighted ion concentra- tions of ammonium, calcium, chloride, magnesium, ni- trate, potassium, sodium and sulfate at 95% confidence interval were plotted against the 10 mm interval weekly precipitations. The result, that less than 10 mm weekly precipitations have much higher ion concentrations than other events, is observed for different ions at three sta- Copyright © 2013 SciRes. JWARP  Q. QIAN ET AL. 795 Figure 4. The annual amount wet deposition from 1994 to 2011 in the study area. Figure 5. The ion concentrations of one typical acid rain and normal rain. Figure 6. The number of weekly precipitation events at three stations. tions. It indicates the dilution effect of precipitations on the dissolved ions extracted from the airborne pollutants. Figure 7 shows such observations for sulfate and cal- cium ions at the TX04 station. The precipitation pH value fluctuates at the stations. the maximum, average, and minimum values of the field measured pH were 7.95, 5.50, and 4.31 for the TX04 station, 7.82, 5.18, and 3.97 for the TX16 station and 8.12, 5.44, and 4.05 for the TX22 station, respectively. To derive a reasonable relationship between pH and the quantity of precipitation is difficult. The deviation ap- pears to be caused by the concentration of cations and anions in the precipitation [19]. Sulfate, nitrate, and chlo- ride ions presented in the precipitation yield H+ resulting in low-pH precipitations. On the other hand, calcium, magnesium, potassium and sodium ions yield OH− re- sulting in relatively high-pH precipitations. As shown above in Figure 5, concentrations of sulfate and nitrate are higher in acid rains (pH ≤ 5.6). On the contrary, higher concentrations of calcium and sodium are ob- served to be associated with normal rains (pH > 5.6). It is worth reporting that the effect of the precipitation quan- tity on pH is different from acid rain (sulfate-rich) and normal rain (calcium-rich). As indicated in upper panel of Figure 8, the measured pH increases with the precipi- >8070- 8060-7050-6040-5030-4020-3 010-20<10 2.0 1.5 1.0 0.5 0.0 Pre cipitatio n (mm) Co n cent ration (mg/l) 95% CI for the Mean Concent ationo Sul ate ion o n P ecipitation >8070-8060-7050-6 040-5030-4020- 3 010-20<10 1.8 1.6 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 Precipitatio n (mm) Concentrat ion (mg/l) 95% CI for the Mean Concent ation o Calc ium i on on P ecipitation Figure 7. The weekly weighted ion concentration of sulfate and calcium at 95% confidence interval mean versus 10 mm interval we ekly preci pitations events. Copyright © 2013 SciRes. JWARP 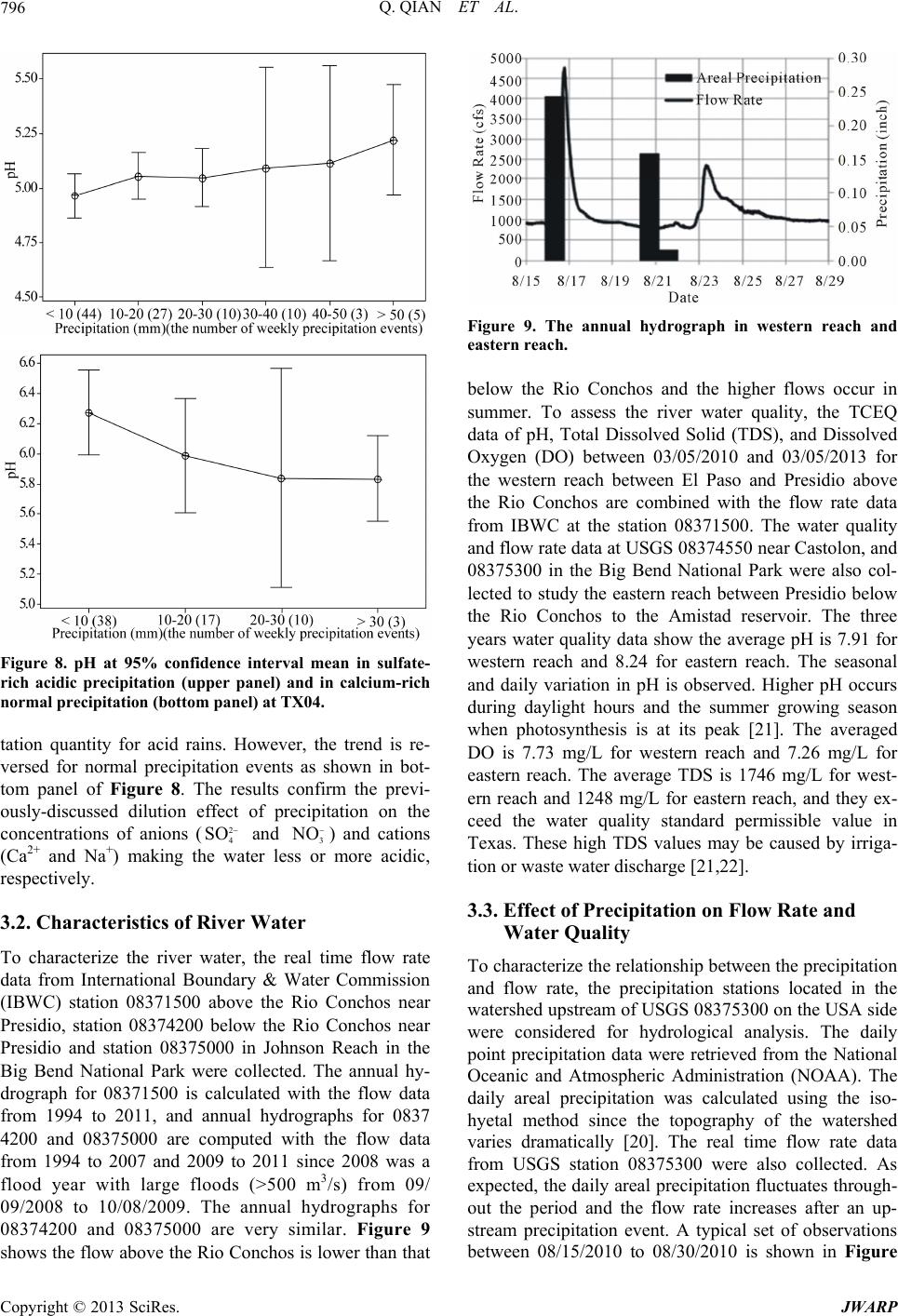 Q. QIAN ET AL. 796 Figure 8. pH at 95% confidence interval mean in sulfate- rich acidic precipitation (upper panel) and in calcium-rich normal precipitation (bottom panel) at TX04. tation quantity for acid rains. However, the trend is re- versed for normal precipitation events as shown in bot- tom panel of Figure 8. The results confirm the previ- ously-discussed dilution effect of precipitation on the concentrations of anions (2 4 SO and 3 O) and cations (Ca2+ and Na+) making the water less or more acidic, respectively. 3.2. Characteristics of River Water To characterize the river water, the real time flow rate data from International Boundary & Water Commission (IBWC) station 08371500 above the Rio Conchos near Presidio, station 08374200 below the Rio Conchos near Presidio and station 08375000 in Johnson Reach in the Big Bend National Park were collected. The annual hy- drograph for 08371500 is calculated with the flow data from 1994 to 2011, and annual hydrographs for 0837 4200 and 08375000 are computed with the flow data from 1994 to 2007 and 2009 to 2011 since 2008 was a flood year with large floods (>500 m3/s) from 09/ 09/2008 to 10/08/2009. The annual hydrographs for 08374200 and 08375000 are very similar. Figure 9 shows the flow above the Rio Conchos is lower than that Figure 9. The annual hydrograph in western reach and eastern reach. below the Rio Conchos and the higher flows occur in summer. To assess the river water quality, the TCEQ data of pH, Total Dissolved Solid (TDS), and Dissolved Oxygen (DO) between 03/05/2010 and 03/05/2013 for the western reach between El Paso and Presidio above the Rio Conchos are combined with the flow rate data from IBWC at the station 08371500. The water quality and flow rate data at USGS 08374550 near Castolon, and 08375300 in the Big Bend National Park were also col- lected to study the eastern reach between Presidio below the Rio Conchos to the Amistad reservoir. The three years water quality data show the average pH is 7.91 for western reach and 8.24 for eastern reach. The seasonal and daily variation in pH is observed. Higher pH occurs during daylight hours and the summer growing season when photosynthesis is at its peak [21]. The averaged DO is 7.73 mg/L for western reach and 7.26 mg/L for eastern reach. The average TDS is 1746 mg/L for west- ern reach and 1248 mg/L for eastern reach, and they ex- ceed the water quality standard permissible value in Texas. These high TDS values may be caused by irriga- tion or waste water discharge [21,22]. 3.3. Effect of Precipitation on Flow Rate and Water Quality To characterize the relationship between the precipitation and flow rate, the precipitation stations located in the watershed upstream of USGS 08375300 on the USA side were considered for hydrological analysis. The daily point precipitation data were retrieved from the National Oceanic and Atmospheric Administration (NOAA). The daily areal precipitation was calculated using the iso- hyetal method since the topography of the watershed varies dramatically [20]. The real time flow rate data from USGS station 08375300 were also collected. As expected, the daily areal precipitation fluctuates through- out the period and the flow rate increases after an up- stream precipitation event. A typical set of observations between 08/15/2010 to 08/30/2010 is shown in Figure Copyright © 2013 SciRes. JWARP  Q. QIAN ET AL. 797 10. The precipitation depth = 0.24 inch on 08/16/2010 caused the peak flow rate on 08/17/2010, and the pre- cipitation depth = 0.16 inch on 08/21/2010 and 0.02 inch on 08/22/2010 lead to the peak flow rate on 08/23/2010. The pH = 5.16 for the 0.24 inch event, and pH = 6.19 for the 0.16 inch and 0.02 inch events can be estimated from NADP station TX04 weekly precipitation data since the NOAA precipitation stations are close to TX04 station. To assess the acid precipitation impacts in the study area, the three years water quality data analysis indicates that the pH, DO and TDS decrease with the increasing flow rate followed by the precipitation event. Moreover, the DO value is low along with the low pH value, but no clear correlation between the TDS and pH is observed. Therefore, the acid deposition decreases the water pH and may also decrease the DO. Figure 11 shows the wa- ter pH, DO and TDS values vary with flow rates after two precipitation events as demonstrated in Figure 10. The pH and DO values decrease sharply with the in- crease of the flow rate on 08/17/2010 indicating the acid precipitation (pH = 5.16) impact on the water quality. The effect, however, is not as obvious at the precipitation event (pH = 6.19) on 8/21/2010 and 8/22/2010. The DO is an important parameter in defining the health of aquatic ecosystems [23]. DO concentrations below 5.0 mg/L can produce adverse impacts on aquatic life [23]. Factors affecting DO are climate or season, the type and number of organisms and nutrients, organic wastes, and dissolved or suspended solids [24]. The drought climate in the watershed can decrease the DO because water levels decrease and the flow rate of the river slows down. Low DO after the acid precipitation event primarily results from the runoff because the runoff washes out nutrients, dissolved or suspended solids [24]. Nutrients including nitrate and phosphate are found in fertilizer runoff. Runoff from roads and other surfaces can bring salts and sediments into stream water, increas- ing the dissolved and suspended solids in the water. Moreover, bottom sediments are stirred by the runoff to increase the suspended solids in the river [24]. Figure 10. The relationship between the precipitation and the flow rate from 08/15/2010 to 08/30/2010. Figure 11. The pH, DO and TDS values versus the flow rate from 08/15/2010 to 08/30/2010 at the USGS 08375300. Following precipitation events, the lowest pH between 03/05/2010 and 03/05/2013 in study area is 6.02, still close to 6.5, but the lowest DO value is 0.9 mg/L, much lower than 5 mg/L. Such a low DO can cause serious water quality problems and can kill the fish in a few hours. In addition, the slightly higher annual pH and lower annual DO values are observed in limestone- dominated eastern reach compared with western reach. These differences between eastern reach and western reach may be due to the acid deposition causing the ero- sion of carbonates, in turn increasing the Ca2+ in the wa- ter of eastern reach and thereby increasing the pH value. Copyright © 2013 SciRes. JWARP  Q. QIAN ET AL. 798 At the same time, the erosion of carbonates adds sus- pended solids into the runoff, thus in turn decreasing the DO value. Therefore, the impact of the acid deposition on the water quality in the eastern reach (limestone- dominated) is different from the western reach. The impact of the acid deposition on the water quality in the limestone basin of eastern reach is a complex physical and chemical process. To understand better the limestone erosion from the runoff, an estimation of the stone loss due to the acid deposition is necessary. After the runoff reaches the river, the calcium carbonate disso- lution-precipitation process affects the water quality pa- rameters, such as the hardness, pH, DO and temperature. The dissolution reactions of calcium carbonate (CaCO3) occurring in parallel are as follows [25]: 1 2 3 k +2+- 3 k2+ - 323 k2+ 2- 33 CaCO (s)+HCa+HCO CaCO (s)+H COCa+2HCO CaCO (s)Ca+CO 3 3 (1) The dissolution rate has been described as [26] -2 -1+ 1223 ratemol cms=kH+kHCO+k 3 (2) The dissolution rate is a function of the pH, PCO2 and the reaction rate constants. At very low pH, the rate of dissolution is fast, but within the pH range of natural waters the dissolution rate is controlled by the chemical reaction at the water-mineral interface with a very small dissolution rate (<10−8 mol cm−2sec−1). However, with wet and dry deposition of SO2 and HNO3, an important consequence is the rapid weathering of exposed lime- stone. Lipfert [27] has proposed a theoretical damage function equation to describe the weathering of limestone between pH 3 and 5: + dS 2 dN3 Stone lossμm/m/y = 18.8+0.016 H +0.18 VSO +VHNOR (3) where (H+) is in µmol/L, VdS and VdN are impact veloci- ties of SO2 and HNO3 on stone surface in cm/s, atmos- pheric concentrations of SO2 and HNO3 are in µg/m3, and R is meters of rain per year. The erosion increases with the acidity, the velocities and concentrations of SO2 and HNO3. Recalling the an- nual wet deposition data (1994 to 2011) obtained from NADP, the concentration of H+, atmospheric concentra- tion of SO2 and HNO3, and the rain per year can be cal- culated. The VdS and VdN are estimated to be around 0.2 cm/s ~ 2 cm/s with the American Society of Civil En- gineering kinematic wave equation in [20]. The average annual stone loss is about 0.02 mm. If only 10% of the limestone is bared soil, the total stone loss in the sub- basin is estimated around 0.02 mm x 34630 sq miles × 40% of limestone × 10% bared soil = 71746 m3 (72,000 m3). If only 1% of the stone loss can reach the river by the runoff, a total sediment load of 717 m3 will be added to the river. The erosion leads to increased sediment storage, decreased channel capacity and increased flood- ing frequency and the water quality (e.g. TDS) exceeds the maximum permissible value set by Texas State Water Quality Standards. The ongoing effort is to evaluate the sediment transport in the channel. 3.4. Daily pH Fluctuations Caused by Calcium Carbonate The presence of dissolved calcium carbonate causes in- creasing hardness. The Langelier Saturation Index has been developed to predict the tendency of calcium car- bonate either to precipitate or to dissolve under varying conditions [28]. With a pH of 6.5 to 9.5, the equation expresses as [29]: 2+ 2 Hs=pK-pKs+pCa+pAlk (4) where pHs = the pH at which water with a given calcium content and alkalinity is in equilibrium with calcium car- bonate, K2 = the second dissociation constant for carbo- nic acid, Ks = the solubility product constant for calcium carbonate. These terms are functions of temperature and total mineral content. Daily pH fluctuations were observed in river water under steady flow rates. The fluctuations were found to synchronize with temperature fluctuations. A typical set of such observations is displayed in Figure 12. The phe- nomenon is believed to be due to the effect of the oscilla- tion of the limestone dissolution-precipitation process. With limestone being the major component (55%) in the subbasin of the river segment, calcium carbonate (CaCO3) is expected to be abundant in the water with the dis- solved amount near the solubility amount or the 2 3 CO concentration (proportional to pH) near the saturation concentration (or saturation pH), which decreases with temperature [30]. The observed daily pH fluctuations appear to reflect the limestone dissolution process near the equilibrium concentration at the daily temperature Figure 12. The relationship between water pH and tem- perature fluctulation. Copyright © 2013 SciRes. JWARP 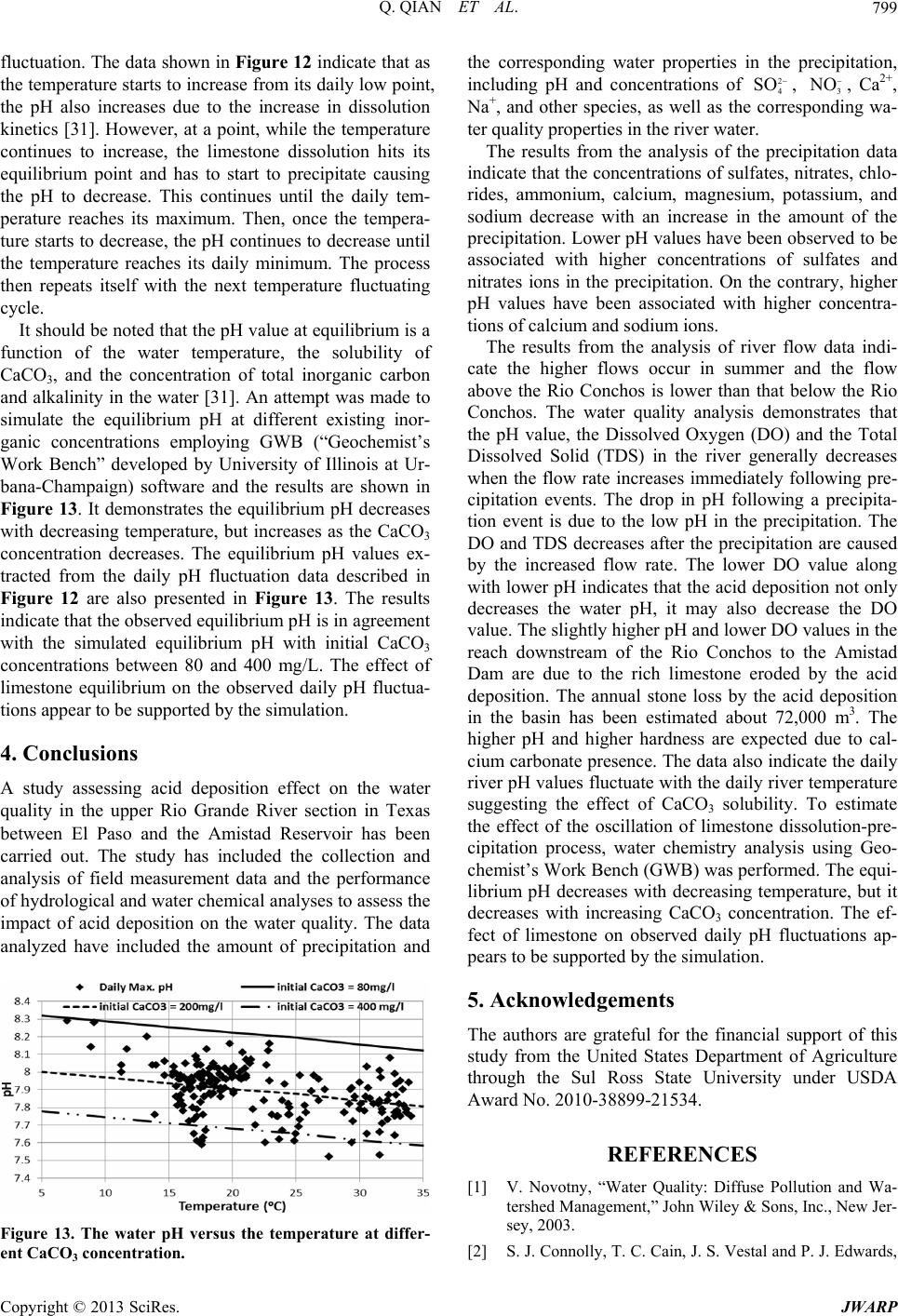 Q. QIAN ET AL. 799 fluctuation. The data shown in Figure 12 indicate that as the temperature starts to increase from its daily low point, the pH also increases due to the increase in dissolution kinetics [31]. However, at a point, while the temperature continues to increase, the limestone dissolution hits its equilibrium point and has to start to precipitate causing the pH to decrease. This continues until the daily tem- perature reaches its maximum. Then, once the tempera- ture starts to decrease, the pH continues to decrease until the temperature reaches its daily minimum. The process then repeats itself with the next temperature fluctuating cycle. It should be noted that the pH value at equilibrium is a function of the water temperature, the solubility of CaCO3, and the concentration of total inorganic carbon and alkalinity in the water [31]. An attempt was made to simulate the equilibrium pH at different existing inor- ganic concentrations employing GWB (“Geochemist’s Work Bench” developed by University of Illinois at Ur- bana-Champaign) software and the results are shown in Figure 13. It demonstrates the equilibrium pH decreases with decreasing temperature, but increases as the CaCO3 concentration decreases. The equilibrium pH values ex- tracted from the daily pH fluctuation data described in Figure 12 are also presented in Figure 13. The results indicate that the observed equilibrium pH is in agreement with the simulated equilibrium pH with initial CaCO3 concentrations between 80 and 400 mg/L. The effect of limestone equilibrium on the observed daily pH fluctua- tions appear to be supported by the simulation. 4. Conclusions A study assessing acid deposition effect on the water quality in the upper Rio Grande River section in Texas between El Paso and the Amistad Reservoir has been carried out. The study has included the collection and analysis of field measurement data and the performance of hydrological and water chemical analyses to assess the impact of acid deposition on the water quality. The data analyzed have included the amount of precipitation and Figure 13. The water pH versus the temperature at differ- ent CaCO3 concentration. the corresponding water properties in the precipitation, including pH and concentrations of , 3 2 4 SO O , Ca2+, Na+, and other species, as well as the corresponding wa- ter quality properties in the river water. The results from the analysis of the precipitation data indicate that the concentrations of sulfates, nitrates, chlo- rides, ammonium, calcium, magnesium, potassium, and sodium decrease with an increase in the amount of the precipitation. Lower pH values have been observed to be associated with higher concentrations of sulfates and nitrates ions in the precipitation. On the contrary, higher pH values have been associated with higher concentra- tions of calcium and sodium ions. The results from the analysis of river flow data indi- cate the higher flows occur in summer and the flow above the Rio Conchos is lower than that below the Rio Conchos. The water quality analysis demonstrates that the pH value, the Dissolved Oxygen (DO) and the Total Dissolved Solid (TDS) in the river generally decreases when the flow rate increases immediately following pre- cipitation events. The drop in pH following a precipita- tion event is due to the low pH in the precipitation. The DO and TDS decreases after the precipitation are caused by the increased flow rate. The lower DO value along with lower pH indicates that the acid deposition not only decreases the water pH, it may also decrease the DO value. The slightly higher pH and lower DO values in the reach downstream of the Rio Conchos to the Amistad Dam are due to the rich limestone eroded by the acid deposition. The annual stone loss by the acid deposition in the basin has been estimated about 72,000 m3. The higher pH and higher hardness are expected due to cal- cium carbonate presence. The data also indicate the daily river pH values fluctuate with the daily river temperature suggesting the effect of CaCO3 solubility. To estimate the effect of the oscillation of limestone dissolution-pre- cipitation process, water chemistry analysis using Geo- chemist’s Work Bench (GWB) was performed. The equi- librium pH decreases with decreasing temperature, but it decreases with increasing CaCO3 concentration. The ef- fect of limestone on observed daily pH fluctuations ap- pears to be supported by the simulation. 5. Acknowledgements The authors are grateful for the financial support of this study from the United States Department of Agriculture through the Sul Ross State University under USDA Award No. 2010-38899-21534. REFERENCES [1] V. Novotny, “Water Quality: Diffuse Pollution and Wa- tershed Management,” John Wiley & Sons, Inc., New Jer- sey, 2003. [2] S. J. Connolly, T. C. Cain, J. S. Vestal and P. J. Edwards, Copyright © 2013 SciRes. JWARP  Q. QIAN ET AL. Copyright © 2013 SciRes. JWARP 800 “The Potential Effects of Acid Deposition: What’s a Na- tional Forest to Do?” Proceedings of the Forest Service National Earth Sciences Conference, San Diego, 18-22 October 2004. [3] P. A. Siver, “Distribution of Scaled Chrysophytes in 17 Adirondack (New York) Lakes with Special Reference to pH,” Canadian Journal of Botany, Vol. 66, No. 7, 1988, pp. 1391-1403. doi:10.1139/b88-195 [4] J. W. Deuller, “Hydrological Response to Acid Rain,” Monitoring to Detect Changes in Water Quality Series. Proceedings of Budapest Symposium, Hungary, 2-4 July 1986, pp. 175-184. [5] C. N. Lane, “Acid Rain Overview and Abstracts,” Nova Science Publishers, Inc., New York, 2003. [6] J. P. Michaud, “A Citizen’s Guide to Understanding and Monitoring Lakes and Streams,” Washington State De- partment of Ecology, Olympia, 1991. [7] C. S. Carvalho and M. N. Fernandes, “Effect of Tempe- rature on Copper Toxicity and Hematological Responses in the Neotropical Fish Prochilodus scrofa at Low and High pH,” Aquaculture, Vol. 251, No. 1, 2006, pp. 109- 117. [8] United State Geology Surveying, “Water Science for School,” 2012. http://ga.water.usgs.gov/edu/earthgwquality.html [9] United Nations Environment Program GEMS Water Pro- gram, “Water Quality Outlook,” 2011. http//www.gemswater.org [10] Environmental Protection Agency (EPA), “Community Multi-Scale Air Quality (CMAQ) Model 4.6,” 2007. http://www.epa.gov/amad/Research/CMAQ/release4_6.ht ml [11] J. K. Upadhyay, S. Chandru, C. J. Lin and T. C. Ho, “Mo- deling of Long-Range Transport of the Airborne Pollut- ants on the Rio Grande Basin Watershed,” International Symposium on Air Quality and Waste Management for Agriculture, Broomfield, 16-19 September 2007. [12] Big Bend National Park, 2013. http://www.nps.gov/bibe/naturescience/geology.htm [13] D. W. Schindler, “Effects of Acid-Rain on Freshwater Ecosystems,” Science, Vol. 239, No. 4836, 1988, pp. 149- 157. doi:10.1126/science.239.4836.149 [14] United States Geology Surveying, 2012. http://water.usgs.gov/nasqan/docs/riogrndfact [15] X. Fang, R. Shrestha, A. W. Groeger, C. J. Lin and M. Jao, “Simulations of Impact of Stream Flow and Climate Con- ditions,” Journal of Contemporary Water Research and Education, Vol. 137, No. 1, 2007, pp. 14-20. doi:10.1111/j.1936-704X.2007.mp137001003.x [16] H. Passell, V. C. Tidwell, J. Brainard, M. Ennis, J. Apari- cio, A. Guitron, R. Lovato, J. Valdes, A. Serrat-Capdevila, A. M. Michelsen, Z. Sheng, W. Morrison, T. Gerik, G. Piccinni, J. Villalobos, G. Newman, K. Pallachula and J. Emery, “Data Collection for Cooperative Water Resour- ces Modeling in the Lower Rio Grande Basin: Fort Quit- man to the Gulf of Mexico,” Research Report SAND 2004-5241, Sandia National Laboratories, New Mexico, 2004. [17] C. L. Danner, D. C. McKinney, R. L. Teasley and S. San- doval-Solis, “Documentation and Testing of the WEAP Model for the Rio Grande/Bravo Basin,” Center for Re- search in Water Resources, CWRW on Line Report 06-8, University of Texas at Austin, Texas, 2006. [18] D. Langmuir, “Aqueous Environmental Geochemistry” Prentic-Hall, Inc., New Jersey, 1997. [19] J. A. Lynch, V. C. Bowersox and J. W. Grimm, “Trends in Precipitation Chemistry in the United States, 1983- 1994: An Analysis of the Effects in 1995 of Phase I of the Clean Air Act Amendments of 1990, Title IV,” Open-File Report 96-0346, US Geology Surveying. [20] W. Viessman Jr. and G. L. Lewis, “Introduction to Hy- drology,” 5th Edition, Pearson Education Inc., New York, 2003. [21] US Army Corps of Engineers, “Forgotten Reach of the Rio Grande, Fort Quitman to Presidio,” Texas Section 729 Report, TCEQ, Albuquerque District, 2008. [22] R. L. Teasley, “Modeling the Forgotten River Segment of the Rio Grande/Bravo Basin,” CRWR Online Report 05-12, 2005. [23] United States Environmental Protection Agency, 2013. http://water.epa.gov/type/rsl/monitoring/vms52.cfmrefere nce [24] American Public Health Association, “Standard Methods for the Examination of Water and Wastewater,” 18th Edition, American Public Health Association, Washing- ton DC, 1992. [25] W. Stumm and J. J. Morgan, “Aquatic Chemistry: Chemi- cal Equilibria and Rates in Natural Waters,” 3rd Edition, John Wiley & Sons, Inc., New York, 1996. [26] E. Busenberg and L. N. Plummer, “A Comparative Stu- dy of the Dissolution and Crystal Growth Kinetics of Calcite and Aragonite,” In: F. A. Mumpton, Ed., Studies in Diagenesis, USGS, Vol. 1578, 1986, pp. 139-168. [27] F. W. Lipfert, “Air Pollution and Materials Damage,” In: O. Hutzinger, Ed., The Handbook of Environmental Che- mistry, Springer-Verlag, Berlin, 1989, pp. 114-186. [28] W. F. Langlier, “The Analytical Control of Anti-Corro- sion Water Treatment,” Journal of the American Water Works Association, Vol. 28, 1936, pp. 1500-1521. [29] J. F. Dye and J. L. Tuepker, “Chemistry of the Lime-Soda Process,” In: Water Quality and Treatment: A Handbook of Public Water Supplies, 3rd Edition, American Water Works Association, McGraw-Hill, Toronto, 1971. [30] R. D. Letterman, “Water Quality and Treatment,” 5th Edition, Mcgraw-Hill, Inc., Toronto, 1999. [31] O. Levenspiel, “Chemical Reaction Engineering,” 3rd Edition, John Wiley & Sons, Inc., New York, 1999.
|