 Advances in Microbiology, 2013, 3, 326-332 http://dx.doi.org/10.4236/aim.2013.34046 Published Online August 2013 (http://www.scirp.org/journal/aim) Cellulase Producing Bacteria from the Wood-Yards on Kallai River Bank Sasidharan Sreedevi, Sreedharan Sajith, Sailas Benjamin* Enzyme Technology Laboratory, Biotechnology Division, Department of Botany, University of Calicut, Malappuram, India Email: *benjamin@uoc.ac.in; sailasben@yahoo.co.in Received May 25, 2013; revised June 25, 2013; accepted July 10 2013 Copyright © 2013 Sasidharan Sreedevi et al. This is an open access article distributed under the Creative Commons Attribution Li- cense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT This study evaluates the influence of growth parameters such as pH, temperature, Carboxy Methyl Cellulose (CMC) concentration and agitation on cellulase production from three bacterial strains, viz., Achromobacter xylosoxidans BSS4, Bacillus sp. BSS3 and Pseudomonas sp. BSS2 isolated from the wood-yards on Kallai river bank in Kerala. Production of cellulase by these isolates was detected using basal salt medium (BSM) with 0.5% CMC as supplement, and CMCase activity was confirmed by iodine test. Dinitrosalicylic acid method was employed for assaying the cellulase production by measuring the amount of glucose liberated in µmol/mL/min. Maximum enzyme production from Pseudomonas sp. BSS2 was at pH 8, 37˚C with 1% CMC and 150 rpm, and cellulase production increased from initial 49.84 U/mL to 91.28 U/mL after optimization. The highest enzyme activity from Bacillus sp. BSS3 was at pH 9, 37˚C with 1% CMC, 150 rpm, and cellulase production increased from initial 26.05 U/mL to 104.68 U/mL after optimization. The maximum enzyme production from A. xylosoxidans BSS4 was at pH 7, 40˚C with 0.5% CMC and 150 rpm, and cellulase produc- tion increased from initial 55.28 U/mL to 68.37 U/mL after optimization. Thus among the three isolates, Bacillu s sp. BSS3 showed maximum enzyme yield which can be explored for further scale up studies with an industrial perspective. Keywords: Cellulase; Carboxy Methyl Cellulose; Dinitrosalicylic Acid; Optimization; Submerged Fermentation 1. Introduction Lignocellulose, the leading bio-residue from agricultural sector is the predominant renewable biopolymer in the world which comprises of celluloses, hemicelluloses and lignin. A promising strategy for the efficient utilization of this renewable resource is to use it as a base material for the production of desired metabolites. Apart from the production of value-added products, its bioconversion offers an effective solution for the abatement of pollution due to solid-waste and their utilization, which would al- low sustainable process and products. Numerous pro- ducts of high economic value like alcohols, acids, single cell proteins, paper, etc., are produced by the effective bioconversion of lignocellulosics [1]. Cellulose is the primary product of photosynthesis in plants which is a polymer having D-anhydroglucopyranose molecules lin- ked by β-1,4-glycosidic bonds [2]. Eventhough bulk quantity of cellulosic residues gets accumulated in the terrestrial ecosystem, they are actively degraded by nu- merous bacteria and fungi, then contributing to main- taining the carbon cycle. Cellulose is degraded by an enzyme system called cellulases produced by fungi, bacteria and actinomycetes. The cellulase system constitute three major enzymes; i.e., endoglucanase (endo-1,4-β-D-glucanase, EC3.2.1.4), exo- glucanase (exo-1,4-β-D-glucancellobiohydrolase, EC3.2. 1.91), and β-glucosidase (β-D-glucoside glucanohydro- lase, EC 3.2.1.21), which act synergistically towards the complete breakdown of cellulose. Endo-glucanases make nicks within the cellulose biomolecule thereby exposing their reducing and non-reducing ends, cellobiohydrolases release cellobiose units—a disaccharide of two glucose molecules linked by a β-1,4 linkage—the repeating units of cellulose from the chain ends; and finally β-glucosi- dases act on cellobiose to liberate glucose [3]. A wide variety of microorganisms have the ability to degrade cellulose, which include aerobic and anaerobic bacteria, white-rot and soft-rot fungi. Fungi are the most studied organisms with respect to the production of cel- lulolytic enzymes. Compared to fungi, bacteria have nu- merous advantages on an industrial view point like its *Corresponding author. C opyright © 2013 SciRes. AiM  S. SREEDEVI ET AL. 327 high growth rate, easy handling and adaptability to vari- ous genetic manipulations [4]. Production of extracellular enzymes, especially carboxymethyl cellulase (CMCase) by aerobic bacteria like Bacillus and Cellulomonas [5], are advantageous for large-scale applications. Bacterial cellulases are constitutively produced by submerged fer- mentation (SmF) in industries employing mainly geneti- cally modified strains. Most importantly, thermophilic, psychrophilic, alkalophilic, acidophilic and halophilic bacteria inhabit a wide variety of environmental and in- dustrial niches that are extremely resistant to environ- mental stress and they can produce enzymes which are stable under extremely harsh conditions [6]. As a result, isolation and characterization of cellulase-producing bac- teria will continue to be a principal component of enzy- me research. Compared to solid-state fermentation (SSF), SmF is widely used in industries since it is easy to operate with control over various process parameters, coupled with easy downstream processing [7]. Various process para- meters like incubation time, temperature, pH, agitation, etc., seem to influence microbial growth and production of cellulase; thus a judicious selection of these parame- ters can dramatically improve the enzyme yield. The centuries-old wood-yards on the Kallai river banks, in the suburbs of Kozhikode City, Kerala State, India, are famous for timber-based industries and allied business. Pursuant to that, enormous quantity of lignoce- llulolytic wastes are being created day-by-day in the form of carpentry waste, sawdust, wood chips etc. Being marshy and almost anoxic environment with wide varia- tion in pH, it is expected that extremophiles could be isolated and characterized from this habitat, and that by centuries-old natural processes, enormous microbial wealth (bacteria, fungi and yeast) thriving on wood would have evolved in this environment, and thus the importance of this study. In the light of this background, the present study focuses on the isolation and characteri- zation of novel bacteria from this wood-yard with poten- tials for producing cellulase. 2. Materials and Methods 2.1. Sample Collection Samples rich in cellulose content like wood bark of tim- ber on river bank side, water-logged wood, sawdust, and sludge from sawdust dumping site were collected from five different locations near the milling areas of wood- yards on Kallai river side. 2.2. Isolation Samples were transported to the lab under sterile condi- tions and one gram of the sample was transferred in a 250 mL flask containing sterile double distilled water (ddH2O), which was made up to 100 mL. The mixture in the flask was shaken for 15 - 20 min (150 rpm at 37˚C) for getting detached the attached surface microflora. One mL of this sample was plated after sufficient serial dilu- tions (up to 10−7) on Mullen Hinton Agar (MHA) plates and incubated for 24 - 48 h at 37˚C. 2.3. Screening for Cellulolytic Activity Bacterial cultures grown on MHA slant were cultured on basal mineral salt medium (BSM) containing (g/L) 2.0 NaNO3; 1.0 K2HPO4; 0.5 KCl; 0.5 MgSO47H2O; 2.0 proteose peptone; 20 agar and 0.5% CMC as additional nutrient, i.e., for the microbial screening for cellulase activity. Detection of CMCase activity was performed on the culture plate using iodine test (solution containing 1% iodine crystals and 2% potassium iodide), which would in 15 min form a bluish-black complex with un- used CMC, demarcating a clear zone around the colonies [8,9]. 2.4. Characterisation of Bacteria Characterization of bacterial isolates was based on cell morphology and biochemical tests. Gram staining, pro- duction of endospore, motility, IMViC, catalase, produc- tion of H2S, carbohydrate fermentation (glucose, lactose, sucrose and mannose [G, L, S, M]) starch and casein hydrolyses were the tests employed for the characteriza- tion of bacteria. 2.5. Molecular Characterization The isolates were confirmed by the PCR-amplification of 16S rDNA gene from the isolated genomic DNA with 8F, and 1492R primers using BDT v3.1 cycle sequence kit on ABI 3730 × 1 genetic analyser (Xcelris Labs, Ahme- dabad, India). 2.6. Optimization of Cellulase Production Different parameters applied for SmF were optimized for enhancing cellulase production. 2.6.1. Effect of pH To determine the optimum pH for cellulase production, BSM containing 0.5% CMC with different pH, i.e., 4, 5, 6, 7, 8, 9 or 10 was inoculated and incubated in the shak- er at 37˚C. Whole flask samples were withdrawn and cel- lulase activity in the supernatant was assayed at every 6 h interval. 2.6.2. Effect of Temperature To determine the optimum temperature for cellulase pro- duction, BSM containing 0.5% CMC was prepared with Copyright © 2013 SciRes. AiM  S. SREEDEVI ET AL. 328 optimum pH fixed earlier for each culture was incubated at 35˚C, 37˚C or 40˚C in a shaker. Whole-flask samples were withdrawn at every 6 hours interval for the cellulase assay. 2.6.3. Effect of CMC Concentration To determine the optimum CMC concentration for cellu- lase production, BSM with different concentrations of CMC (0.5%, 1% or 1.5%) was prepared and inoculated with bacterial cultures and incubated at pH and tempera- ture fixed earlier for each culture, and whole flask sam- ples were withdrawn at every 6 h interval for the cellu- lase assay. 2.6.4. Effect of Agitation To determine the effect of agitation in the production of cellulase, BSM having CMC was prepared with optimum pH, temperature and CMC concentration fixed for each culture and kept along with appropriate controls with agitations (50, 100, 150 or 200 rpm). 2.7. Enzyme Assay (CMC as Substrate) by DNS Method Cellulase activity was determined by extracting the su- pernatant by centrifugation at 8944 g at 4˚C. The reaction mixture contained 0.5 mL of 1% CMC (1 g CMC in 100 mL of 0.1 M citrate buffer, pH 4.8) as substrate; 0.5 mL of crude enzyme (supernatant) was added to it and incu- bated at 50˚C for 30 min in a water bath. At the end of the incubation period, 3 mL of 3,5-dinitrosalicylic acid (DNS) was added and incubated for 5 min in a boiling waterbath for color development and cooled rapidly. The reducing sugar was measured by the method of Miller [10]. The activity was measured against a reagent blank at 540 nm in a UV-Vis spectrophotometer (Shimadzu, Japan). One unit of cellulase activity is defined as the quantity of cellulase required to liberate 1 µmol of glu- cose equivalents per minute under the assay conditions. Cellulase activity was calculated using the formula, ΔE × Vf/Δt × ∑ × Vs × d; where, ΔE = absorbance at 540 nm, Vf = final volume of reaction mixture, including DNS, Vs = crude supernatant (mL) containing cellulase used, Δt = incubation time for hydrolysis, ∑ = extinction coef- ficient of glucose (0.0026), d = diameter of cuvette. 2.8. Statistics All experiments were repeated 5 times, and average val- ues with SD were plotted on the graphs. 3. Results In the present study, we isolated more than hundred bac- teria from the wood-yards on Kallai river bank. Among them, three potent cellulolytic bacterial cultures were screened out for further studies. Characterization of the cultures was done at morphological, biochemical and molecular levels. Based on molecular characterization, the cultures were identified and named as Achromobacter xylosoxidans BSS4 (GenBank Accession No. JQ 407052), Bacillus sp. BSS3 (GenBank Accession No. JQ 407051) and Pseudomonas sp. BSS2 (GenBank Accession No. JQ 407050). Pseudomonas sp. BSS2 was obtained from the wood bark of water-logged wood, while Bacillus sp. BSS3 and A. xylosoxidans BSS4 were isolated from the bark of the woods kept partially immersed in the river bank. No potent cellulolytic isolates were obtained from other samples like sawdust and sludge collected. The bacterial cultures were also characterized based on colony nature, shape of colony margine, size, transpar- ency, color, shape and elevation (Table 1). The cultures were stored initially on MHA and later they were main- tained in MSM supplied with CMC to induce cellulase production. The morphological characterization studies included Gram’s reaction, and examining cell shape, en- dospore formation, spore position and motility. Pseudo- monas sp. BSS2 is Gram −ve, motile rod without en- dospores; Ba cillus sp. BSS3 is Gram +ve, motile rod with endospores, while A. xylosoxidans BSS4 is Gram −ve, motile rod with endospores (Figure 1). The bio- chemical characterization of all the three cultures showed similar response for catalase, H2S production, hydrolysis of starch and casein, while showed variations in IMViC and carbohydrate fermentation reactions (Table 2). The iodine test (plate assay) for screening of cellulase activity was performed on BSM with CMC agar, in which all the three cultures showed well-defined clear zone around the colony, which indicates that the CMC in the clear zone area was hydrolyzed by the cellulase produced by the bacteria. The unutilized CMC around the clear zone formed dark colored complex with iodine. Among them, Pseudomonas sp. BSS2 showed wider hydrolytic zone (32 mm) (Figure 2). The optimization of process parameters for cellulase production by each culture included effects of pH, tem- perature, CMC concentration and agitation. Our strategy was to fix one parameter first (with other parameters ar- bitrary), and use this fixed parameter for fixing the se- cond one and so on. Initially, effect of pH on the enzyme Table 1. Culture characteristics of bacterial isolates on MHA plates. Culture name Characteristics Pseudomonas sp. BSS2 Creamy, opaque, mucoidal colonies, circular and convex Bacillus sp. BSS3 Widely spreading, opaque, mucoidal colonies with entire margin Achromobacter xylosoxidans BSS4 Creamy, opaque, circular shaped colonies with convex elevation Copyright © 2013 SciRes. AiM 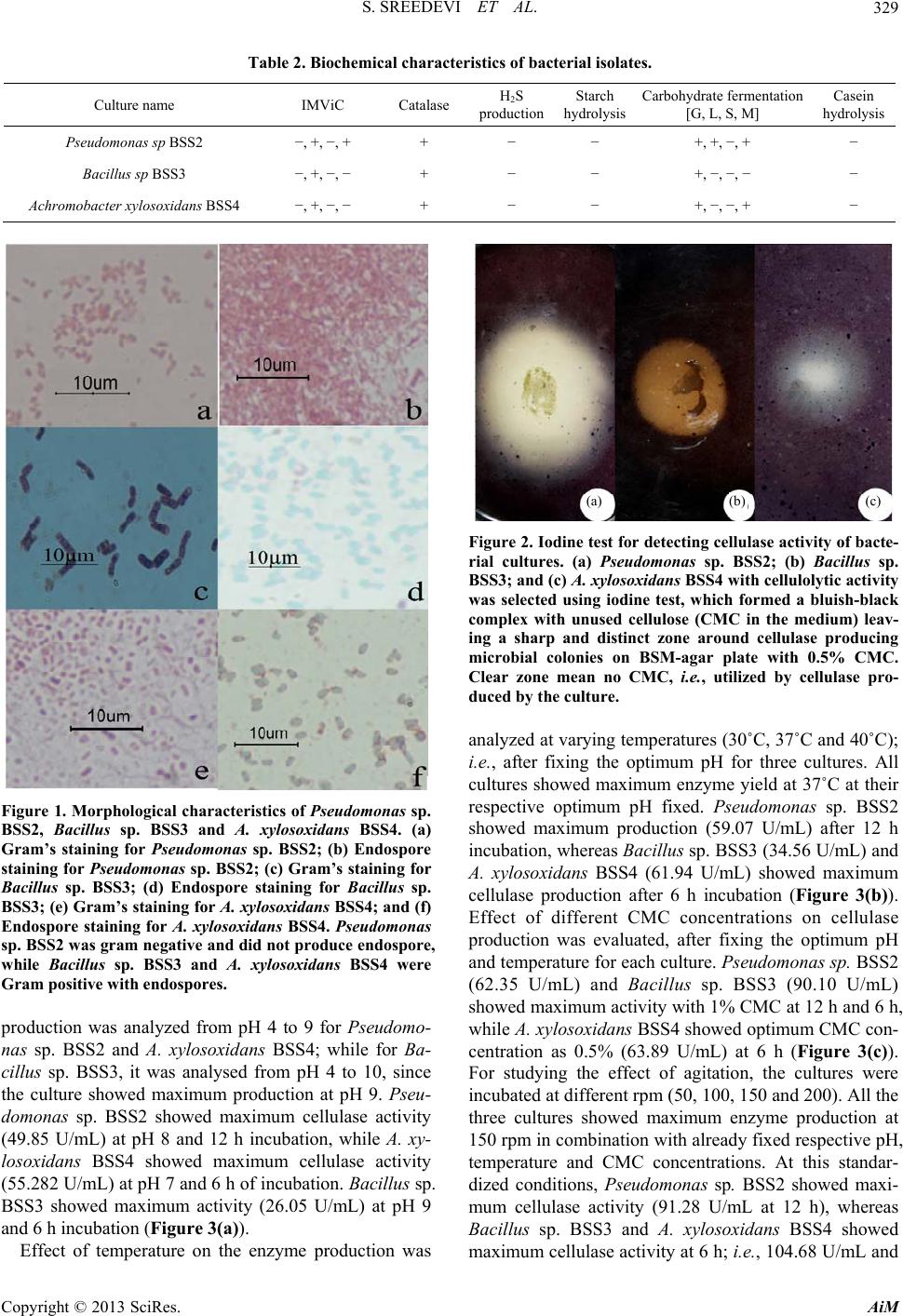 S. SREEDEVI ET AL. AiM 329 Table 2. Biochemical characteristics of bacterial isolates. Culture name IMViC Catalase H2S production Starch hydrolysis Carbohydrate fermentation [G, L, S, M] Casein hydrolysis Pseudomonas sp BSS2 −, +, −, + + − − +, +, −, + − Bacillus sp BSS3 −, +, −, − + − − +, −, −, − − Achromobacter xylosoxidans BSS4 −, +, −, − + − − +, −, −, + − Copyright © 2013 SciRes. Figure 1. Morphological characteristics of Pseudomonas sp. BSS2, Bacillus sp. BSS3 and A. xylosoxidans BSS4. (a) Gram’s staining for Pseudomonas sp. BSS2; (b) Endospore staining for Pseudomonas sp. BSS2; (c) Gram’s staining for Bacillus sp. BSS3; (d) Endospore staining for Bacillus sp. BSS3; (e) Gram’s staining for A. xylosoxidans BSS4; and (f) Endospore staining for A. xylosoxidans BSS4. Pseudomonas sp. BSS2 was gram negative and did not produce endospore , while Bacillus sp. BSS3 and A. xylosoxidans BSS4 were Gram positive with endospores. production was analyzed from pH 4 to 9 for Pseudomo- nas sp. BSS2 and A. xylosoxidans BSS4; while for Ba- cillus sp. BSS3, it was analysed from pH 4 to 10, since the culture showed maximum production at pH 9. Pseu- domonas sp. BSS2 showed maximum cellulase activity (49.85 U/mL) at pH 8 and 12 h incubation, while A. xy- losoxidans BSS4 showed maximum cellulase activity (55.282 U/mL) at pH 7 and 6 h of incubation. Bacillus sp. BSS3 showed maximum activity (26.05 U/mL) at pH 9 and 6 h incubation (Figure 3 (a)). Effect of temperature on the enzyme production was (b) (a) (c) Figure 2. Iodine test for detecting cellulase activity of bacte- rial cultures. (a) Pseudomonas sp. BSS2; (b) Bacillus sp. BSS3; and (c) A. xylosoxidans BSS4 with cellulolytic activity was selected using iodine test, which formed a bluish-black complex with unused cellulose (CMC in the medium) leav- ing a sharp and distinct zone around cellulase producing microbial colonies on BSM-agar plate with 0.5% CMC. Clear zone mean no CMC, i.e., utilized by cellulase pro- duced by the culture. analyzed at varying temperatures (30˚C, 37˚C and 40˚C); i.e., after fixing the optimum pH for three cultures. All cultures showed maximum enzyme yield at 37˚C at their respective optimum pH fixed. Pseudomonas sp. BSS2 showed maximum production (59.07 U/mL) after 12 h incubation, whereas Bacillus sp. BSS3 (34.56 U/mL) and A. xylosoxidans BSS4 (61.94 U/mL) showed maximum cellulase production after 6 h incubation (Figure 3(b)). Effect of different CMC concentrations on cellulase production was evaluated, after fixing the optimum pH and temperature for each culture. Pseudomonas sp. BSS2 (62.35 U/mL) and Bacillus sp. BSS3 (90.10 U/mL) showed maximum activity with 1% CMC at 12 h and 6 h, while A. xylosoxidans BSS4 showed optimum CMC con- centration as 0.5% (63.89 U/mL) at 6 h (Figure 3(c)). For studying the effect of agitation, the cultures were incubated at different rpm (50, 100, 150 and 200). All the three cultures showed maximum enzyme production at 150 rpm in combination with already fixed respective pH, temperature and CMC concentrations. At this standar- dized conditions, Pseudomonas sp. BSS2 showed maxi- mum cellulase activity (91.28 U/mL at 12 h), whereas Bacillus sp. BSS3 and A. xylosoxidans BSS4 showed maximum cellulase activity at 6 h; i.e., 104.68 U/mL and 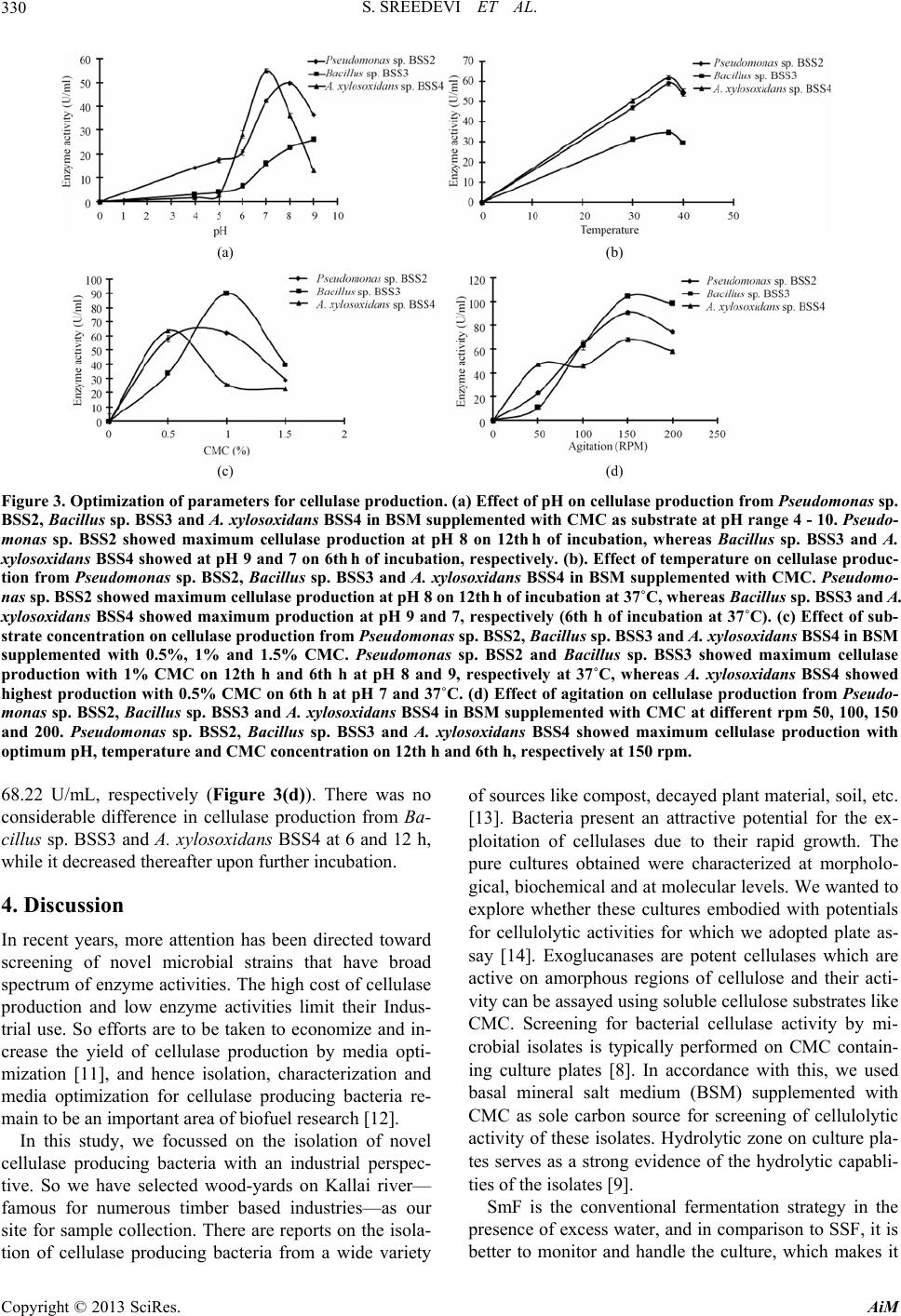 S. SREEDEVI ET AL. 330 (a) (b) (c) (d) Figure 3. Optimization of parameters for cellulase production. (a) Effect of pH on cellulase production from Pseudomonas sp. BSS2, Bacillus sp. BSS3 and A. xylosoxidans BSS4 in BSM supplemented with CM C as substrate at pH range 4 - 10. Pseudo- monas sp. BSS2 showed maximum cellulase production at pH 8 on 12th h of incubation, whereas Bacillus sp. BSS3 and A. xylosoxidans BSS4 showed at pH 9 and 7 on 6th h of incubation, respectively. (b). Effect of temperature on cellulase produc- tion from Pseudomonas sp. BSS2, Bacillus sp. BSS3 and A. xylosoxidans BSS4 in BSM supplemented with CMC. Pseudomo- nas sp. BSS2 showed maximum cellulase production at pH 8 on 12th h of incubation at 37˚C, whereas Bacillus sp. BSS3 and A. xylosoxidans BSS4 showed maximum production at pH 9 and 7, respectively (6th h of incubation at 37˚C). (c) Effect of sub- strate concentration on cellulase production from Pseudomonas sp. BSS2, Bacillus sp. BSS3 and A. xylosoxidans BSS4 in BSM supplemented with 0.5%, 1% and 1.5% CMC. Pseudomonas sp. BSS2 and Bacillus sp. BSS3 showed maximum cellulase production with 1% CMC on 12th h and 6th h at pH 8 and 9, respectively at 37˚C, whereas A. xylosoxidans BSS4 showed highest production with 0.5% CMC on 6th h at pH 7 and 37 ˚C. (d) Effect of agitation on cellulase production from Pseudo- monas sp. BSS2, Bacillus sp. BSS3 and A. xylosoxidans BSS4 in BSM supplemented with CMC at different rpm 50, 100, 150 and 200. Pseudomonas sp. BSS2, Bacillus sp. BSS3 and A. xylosoxidans BSS4 showed maximum cellulase production with optimum pH, temperature and CMC concentration on 12th h and 6th h, respectively at 150 rpm. 68.22 U/mL, respectively (Figure 3(d)). There was no considerable difference in cellulase production from Ba- cillus sp. BSS3 and A. xylosoxidans BSS4 at 6 and 12 h, while it decreased thereafter upon further incubation. 4. Discussion In recent years, more attention has been directed toward screening of novel microbial strains that have broad spectrum of enzyme activities. The high cost of cellulase production and low enzyme activities limit their Indus- trial use. So efforts are to be taken to economize and in- crease the yield of cellulase production by media opti- mization [11], and hence isolation, characterization and media optimization for cellulase producing bacteria re- main to be an important area of biofuel research [12]. In this study, we focussed on the isolation of novel cellulase producing bacteria with an industrial perspec- tive. So we have selected wood-yards on Kallai river— famous for numerous timber based industries—as our site for sample collection. There are reports on the isola- tion of cellulase producing bacteria from a wide variety of sources like compost, decayed plant material, soil, etc. [13]. Bacteria present an attractive potential for the ex- ploitation of cellulases due to their rapid growth. The pure cultures obtained were characterized at morpholo- gical, biochemical and at molecular levels. We wanted to explore whether these cultures embodied with potentials for cellulolytic activities for which we adopted plate as- say [14]. Exoglucanases are potent cellulases which are active on amorphous regions of cellulose and their acti- vity can be assayed using soluble cellulose substrates like CMC. Screening for bacterial cellulase activity by mi- crobial isolates is typically performed on CMC contain- ing culture plates [8]. In accordance with this, we used basal mineral salt medium (BSM) supplemented with CMC as sole carbon source for screening of cellulolytic activity of these isolates. Hydrolytic zone on culture pla- tes serves as a strong evidence of the hydrolytic capabli- ties of the isolates [9]. SmF is the conventional fermentation strategy in the presence of excess water, and in comparison to SSF, it is better to monitor and handle the culture, which makes it Copyright © 2013 SciRes. AiM  S. SREEDEVI ET AL. 331 suitable for large scale industrial production of microbial enzymes. SmF helps in the production of cellulase and other enzymes [15]. So we selected SmF for the produc- tion of cellulase from these isolates. Cellulase production is highly influenced by various process parameters like pH, temperature, substrate concentration, agitation, etc.; and large scale production requires understanding and proper controlling of growth parameters to increase the enzyme production [16]. Cellulase production appears to depend on pH value. Results illustrated in Figure 3 clearly show that cellulase production gradually increa- sed as the pH values increased from 4 to 8 and dropped at pH 9 for Pseudomonas sp. and A. xylosoxidans, whereas Bacillus sp. showed maximum production at pH 9. Yang, et al. [17] reported that cellulase production was high between 7 and 9 for Bacillus sp. with a yield of 49 U/mL at pH 9. Acharya and Chaudhary [18] studied on the cellulase activity of two novel strains isolated from hot springs, i.e., B. licheniformis WBS1 and Bacillus WBS3, which showed maximum cellulase activity of 0.388 and 0.342 IU/mL at pH 8 and 9, respectively. Like pH, temperature is also an important factor which influ- ences cellulase yield. It is obvious from Figure 3 that cellulase production increased with increasing temperature from 30˚C to 37˚C, but decreased at 40˚C. This was in contrast to the reports of Immanuel [19], and they recorded maximum endo- glucanase activity for Cellulomonas, Ba cillus and Mi- crococcus at 40˚C and at neutral pH with an enzyme yield of 0.0336, 0.0196 and 0.0152 U/mL, respectively. Fagade and Bamigboye [20] observed highest cellulase activity for B. licheniforms I and II at 40˚C with a value of 0.52 mg/mL and 0.44 mg/mL reducing sugar. We tried CMC as supplement at different concentrations, i.e., 0.5%, 1% and 1.5%. For Pseudomonas sp. and Bacillus sp. optimum CMC concentration was 1%, while A. xylo- soxidans showed maximum cellulase activity at 0.5% CMC concentration. Lin, et al. [21] reported that B. thur- ingiensis produced maximum relative cellulase activity of 110 U/mL at 1% CMC and 40˚C. Agitation increases aeration in the medium, and thus helped in improving contact between substrate and mi- croorganism, which ultimately favours better enzyme yield. In accordance with that, we obtained better en- zyme yield as agitation (rpm) was increased gradually from 50 to 150 though 100 rpm, while at 200 rpm, the enzyme yield was decreased. Hence, 150 rpm was found suitable for the isolates described herein for better pro- duction of cellulase. Taleb, et al. [22] reported that strains of B. alcalophilus and B. amyloliquefaciens showed ma- ximum cellulase activity (2.32 and 2.97 IU/mL, respec- tively) at 1% CMC and 150 rpm. Ray, et al. [23] reported that cellulase yield was higher in B. subtilis (26 U) and B. circulans (20.2 U) upon SSF at an optimum pH range of 7.0 to 7.5 and 40˚C temperature. Shankar, et al. [24] re- ported that B. pumilus EWBCM1 isolated from the gut of earthworm showed maximum cellulase activity (0.585 IU/mL) at pH 6 and 37˚C. Satheesh, et al. [25] studied the cellulase production by a newly isolated strain of Bacillus sp. using cellulose powder, rice husk and filter paper as substrates and found that rice husk was the most suitable substrate for cellulase production. Otajevwo, et al. [26] reported that isolates such as Bacillus, Clostrid- ium, Pseudomonas and Erwinia showed optimum cellu- lase production at 40˚C and pH 6. In conclusion, we obtained three strains, viz., Pseudo- monas sp., Bacillus sp. and A. xylosoxidans with cellu- lolytic activity, which more promising than the reported cultures of related genera. Of these, Bacillus sp. showed reasonably high cellulolytic activity. Several microor- ganisms capable of converting cellulose into simple car- bohydrates had been discovered for decades. However, demands from the enzyme industry for newly isolated cellulolytic microbes which can better convert cellulose in to value added products still active and there lies the importance of this study. 5. Acknowledgements The authors are thankful to the Kerala State Council for Science, Technology and Environment for a research grant, No. (T) 422/SRS/2009/CSTE. REFERENCES [1] G. Coral, B. Arikan, M. N. Unaldi and H. Gunvenmes, “Some Properties of Crude Carboxymethyl Cellulase of Aspergillus niger Z10 Wild Type Strain,” Turkish Jour- nal of Biology, Vol. 26, No. 4, 2002, pp. 209-221. [2] Y. H. P. Zhang, D. J. Schell and J. D. Mc Millan, “Meth- odological Analysis for Determination of Enzymatic Di- gestibility of Cellulosic Materials,” Biotechnology and Bioengineering, Vol. 96, No. 1, 2007, pp. 188-194. doi:10.1002/bit.21178 [3] R. K. Sukumaran, R. R. Singhania and A. Pandey, “Mi- crobial Cellulases—Production Applications and Challen- ges,” Journal of Scientific and Industrial Research, Vol. 64, No. 11, 2005, pp. 832-844. [4] A. E. Humphrey, “The Hydrolysis of Cellulosic Materials to Useful Products,” In: R. D. Brown, Jr. and L. Jurasek, Eds., Hydrolysis of Cellulose: Mechanisms of Enzymatic and Acid Catalysis, University of Pennyslvania, Phila- delphia, 1979, pp. 25-53. [5] T. P. Noyola and M. D. Torre, “Regulation of Cellulases and Xylanases from a Derepressed Mutant of Cellulomo- nas flavigena Growing on Sugarcane Bagasse in Conti- nous Culture,” Bioresouce Technology, Vol. 78, No. 3, 2001, pp. 285-291. doi:10.1016/S0960-8524(00)00181-4 [6] M. Maki, K. T. Lenng and Q. Wensheng, “The Prospects of Cellulase Producing Bacteria for the Bioconversion of Copyright © 2013 SciRes. AiM  S. SREEDEVI ET AL. Copyright © 2013 SciRes. AiM 332 Lignocellulosic Biomass,” Journal of Biological Science, Vol. 5, No. 5, 2009, pp. 500-516. [7] J. S. Tolan and B. Foody, “Cellulase from Submerged Fermentation,” In: G. T. Tsao, Ed., Recent Progress in Bioconversion of Lignocellulosics, 65th Edition, Springer Publishing, Berlin, 1999, pp. 41-67. [8] L. Hankin and S. Anagnostakis, “Solid Media Containing CMC to Detect CM Cellulase Activity of Microorgan- isms,” Journal of General Microbiology, Vol. 98, No. 1, 1977, pp. 109-115. doi:10.1099/00221287-98-1-109 [9] R. C. Kasana, R. Salwan, H. Dhar, S. Dutt and A. Gulati, “A Rapid and Easy Method for the Detection of Micro- bial Cellulases on Agar Plates Using Gram’s Iodine,” Current Microbiology, Vol. 57, No. 5, 2008, pp. 503-507. doi:10.1007/s00284-008-9276-8 [10] G. L. Miller, “Use of Dinitrosalicyclic Acid Reagent for Determination of Reducing Sugar,” Analytical Chemistry, Vol. 31, No. 3, pp. 426-428. doi:10.1021/ac60147a030 [11] K. L. Kalra, G. Kocher and G. Banta, “Optimization of Cellulase Production by Submerged Fermentation of Rice Straw by Trichoderma harzianum RUT-C 8280,” The In- ternet Journal of Microbiology, Vol. 5, No. 2, 2008, pp. 1-7. [12] K. Hirasawa, K. Uchimura, M. Kashima, W. D. Grant, S. Ito, T. Kobayashi and K. Horikoshi, “Salt Activated En- doglucanase of a Strain of Alkaliphilic Bacillus agarad- haerens,” Antonie Van Leuvenhoek, Vol. 89, No. 2, 2006, pp. 211-219. doi:10.1007/s10482-005-9023-0 [13] R. H. Doi, “Cellulases of Mesophilic Microorganisms: Cellulosome and Non Cellulosome Producers,” Annals of New York Academy of Sciences, Vol. 1125, No. 1, 2008, pp. 267-279. doi:10.1196/annals.1419.002 [14] M. Rubeena, K. Neethu, S. Sajith, S. Sreedevi, P. Praka- san, K. N. Unni, M. K. Sarath Josh, V. N. Jisha, S. Pradeep and S. Benjamin, “Lignocellulolytic Activities of a Novel Strain of Trichoderma harzianum,” Advances in Bioscience and Biotechnology, Vol. 4, No. 2, 2013, pp. 214-221. doi:10.4236/abb.2013.42030 [15] S. Y. Kim, S. W. Kang and J. S. Lee, “Cellulase and Xy- lanase Production by Aspergillus niger KKS in Various Bioreactors,” Bioresource Technology, Vol. 59, No. 1, 1997, pp. 63-67. doi:10.1016/S0960-8524(96)00127-7 [16] K. Neethu, M. Rubeena, S. Sajith, S. Sreedevi, P. Priji, K. N. Unni, M. K. Sarath Josh, V. N. Jisha, S. Pradeep and S. Benjamin, “A Novel Strain of Trichoderma viride Shows Complete Lignocellulolytic Activities,” Advances in Bio- science and Biotechnology, Vol. 3, No. 8, pp. 1160-1166. [17] V. W. Yang, Z. Zhang, G. Elegir and T. W. Jeffries, “Al- kaline Active Xylanase Produced by an Alkaliphilic Ba- cillus Species Isolated from Kraft Pulp,” Journal of In- dustrial microbiology, Vol. 15, No. 5, 1995, pp. 434-441. doi:10.1007/BF01569971 [18] S. Acharya and A. Chaudary, “Effect of Nutritional and Environmental Factors on Cellulases Activity by Ther- mophilic Bacteria Isolated from Hot Spring,” Journal of Scientific and Industrial Research, Vol. 70, No. 2, 2011, pp. 142-148. [19] G. Immanuel, R. Dhanusa, P. Prema and A. Palavesam, “Effect of Different Growth Parameters on Endogluca- nase Enzyme Activity by Bacteria Isolated from Coir Retting Effluents of Estuarine Environment,” Interna- tional Journal of Environment Science and Technology, Vol. 3, No. 1, 2006, pp. 25-34. [20] O. E. Fagade and O. O. Bamigboye, “Effect of Cultural Conditions on the Cellulase Activity of Bacteria Species Isolated from Degrading Corn Cob,” Archives of Applied Science Research, Vol. 4, No. 6, 2012, pp. 2540-2545. [21] L. Lin, X. Kan, H. Yan and D. Wang, “Characterization of Extracellular Cellulose-Degrading Enzymes from Ba- cillus thuringiensis Strains,” Electronic Journal of Bio- technology, Vol. 15, No. 3, 2012, pp. 1-7. doi:10.2225/vol15-issue3-fulltext-1 [22] A. Taleb, A. A. Khadiga, W. A. Mashhoor, A. N. Sohair, M. S. Sharaf and H. M. Hoda, “Nutritional and Envi- ronmental Factors Affecting Cellulase Production by Two Strains of Cellulolytic Bacilli”, Australian Journal of Ba- sic and Applied Sciences, Vol. 3, No. 3, 2009, pp. 2429- 2436. [23] A. Ray, A. Bairagi, K. S. Ghosh and S. K. Sen, “Optimi- zation of Fermentation Conditions for Cellulase Produc- tion by Bacillus subtilis CY5 and Bacillus Circulans TP3 Isolated From Fish Gut,” Acta Ichthyologica Et Piscato- ria, Vol. 37, No. 1, 2007, pp. 47-53. [24] T. Shankar and L. Isaiarasu, “Cellulase Production by Bacillus pumilus EWBCM1 under Varying Cultural Con- ditions,” Middle East Journal of Scientific Research, Vol. 8, No. 1, 2011, pp. 40-45. [25] G. S. Kumar, M. S. Chandra, M. Sumanth, A. Vishnu- priya, B. R. Reddy and Y. L. Choi, “Cellulolytic Enzymes Production from Submerged Fermentation of Different Substrates by Newly Isolated Bacillus spp. FME,” Jour- nal of Korean Society Applied Biological Chemistry, Vol. 52, No. 1, 2009, pp. 17-21. doi:10.3839/jksabc.2009.003 [26] F. D. Otajevwo and H. S. A. Aluyi, “Cultural Conditons Necessary for Optimal Cellulase Yield by Cellulolytic Bacterial Organisms as They Relate to Residual Sugars Released in Broth Medium,” Modern Applied Science, Vol. 5, No. 3, 2011, pp. 141-151.
|