Journal of Water Resource and Protection
Vol. 2 No. 9 (2010) , Article ID: 2713 , 4 pages DOI:10.4236/jwarp.2010.29092
Phenol and Benzoic Acid Degradation by Pseudomonas aeruginosa
1 Laboratory of materials and catalysis, Faculty of Sciences, Djilali Liabes University, Sidi Bel-Abbès, Algeria
2Laboratory of Environmental Chemistry, Faculty of Science, University Ibn Khaldoun, Tiaret, Algeria
E-mail: Hibatelrahmane@yahoo.fr
Received June 11, 2010; revised June 22, 2010; accepted June 28, 2010
Keywords: Acid Benzoic, Degradation, Pseudomonas Aeruginosa, Phenol.
ABSTRACT
The present work attempts to solve pollution problems in watery surroundings by aromatic compounds such as the phenol and the benzoic acid. Several ways of elimination of these compounds were the object of different research among which is the use of bacteria. In this framework, Pseudomonas aeruginosa bacterium is used to eliminate phenol and the benzoic acid. This made it possible to isolate the Pseudomonas aeruginosa bacterium directly on the nourishing environment containing phenol and benzoic acid as source of energy then the bacteria is incubated at 37˚C during a minimal duration of four days. Furthermore, we studied the influence of the Pseudomonas aeruginosa bacterium on the deterioration of an area exposed to a phenol and the benzoic acid concentration. Results obtained at the time of the different experimentations clearly show that phenol and the benzoic acid were eliminated by the Pseudomonas aeruginosa bacterium. However, it was noted that during the various investigations the bacterium Pseudomonas aeruginosa develops better in a phenol milieu and therefore degrades phenol more than benzoic acid.
1. Introduction
E Phenol and phenolic compounds are important for many industries, which are involved in pesticide, tincture, bakelite, and medicine extensively. Therefore, a large amount of phenol and phenolic compounds are effused into stream and soil, and have badly polluted the environment [1]. Phenol is toxic to most microorganisms and potentially carcinogenic to humans [2]. Phenols are present in wastewater of various industries, such as refineries (6-500 mg/l), coking operations (28-3900 mg/l), coal processing (9-6800 mg/l), and manufacture of petrochemicals (2.8-1220 mg/l) [3].
Benzoic acid is a common additive for preserving foods, fats, fruit juices, alkaloid solutions, and curing tobacco. A major source of benzoic acid and its derivatives released into the aquatic environments are from the effluents of coal refining, paper and pulp mills, and in agricultural runoff [4]. Moreover, the wastewater discharged from processes of manufacture and application can pollute water, soil and atmosphere. The concentration of benzoic acid was found to range from 10 to 27500 μg/L in the groundwater [5].
Different treatment methods are available for reduction of phenol content in wastewater. The technologies for the treatment of wastewater containing phenol include chlorination, ozonation, adsorption, solvent extraction, membrane process, coagulation, flocculation and biological treatment [6] and compared with physicochemical methods, the biodegradation methods of phenol reduction is universally preferred, because of lower costs and the possibility of complete mineralization [7] The use of microorganisms to degrade aromatic pollutants is an interesting low-cost approach for the treatment of industrial sewage disposal, contaminated sediments, soil and groundwater. Typically, biodegradation is practiced by providing favorable environmental conditions in order to stimulate the removal of pollutants [8].
Many aerobic phenol-degrading microorganisms were isolated and the pathways for the aerobic degradation of phenol are now definitely well established. The first step consists of oxygenation of phenol by phenol hydroxylase enzymes to form catechol, followed by ring cleavage adjacent to, or in between, the two hydroxyl groups of catechol. Phenol hydroxylases ranging from simple flavoprotein monooxygenases to multi component hydroxylases, as well as the genes coding for these enzymes, were described for few aerobic phenol-degrading microorganisms [9].
In this study, we report the biodegradation of benzoic acid and phenol at high initial concentrations. The biodegradation of these compounds was investigated individually using Pseudomonas aeroginosa. One of the purposes of this study was to biodegrade benzoic acid and phenol in short times and to obtain more information on interactions among aromatics during the biodegradation at high initial concentrations.
2. Materials and Methods
2.1. Microorganism and Growth Medium
Isolation has been realized directly on the King A and King B medium from urban wastewaters. Incubation is done at 37˚C during 4 days.
After blue colony apparition in the King A medium and the red colony In the King B medium, Pseudomonas aeruginosa bacterium were added to the polluted water that contains the necessary mineral salts to the growth of the bacterium: KH2PO4 = 1.7 g/l, K2HPO4 = 4.3 g/l, (NH4)2SO4 = 2.69 g/l, CaCl2 = 0.03 g/l, MgSO4 = 0.2 g/l (Table 1), phenol and the benzoic acid have been used as the only source of carbon and energy. The solution sterilized to the autoclave and the incubation is done at 37˚C, the reading is done after 24 h.
2.2. Cell Density Measurement
Bacterial growth was measured spectrophotometrically by optical density at 600 nm (OD600) [11].The transformation of DO into UFC / ml is made by the relation:
DO = 0.7 ![]() 108 UFC/ml [12].
108 UFC/ml [12].
2.3. Chemicals
Benzoic acid and phenol were used as the carbon sources, and all the solutions/media were made in double distilled

Table 1. Composition of the synthetic medium [10].
water.
2.4. Effect of pH on Phenol and Benzoic Acid Biodegradation
To value the effect of the pH on the biodegradation of the two pollutants by Pseudomonas aeruginosa we prepared phenol and benzoic acid of the same 20 mg/l concentration in addition to necessary mineral salts to the growth of Pseudomonas aeruginosa. The pH was fixed to the different values 5.6, 7 and 8, and every solution was put in contact with a number of Pseudomonas aeruginosa of 0.047.108 UFC/ml and submitted to agitation then incubated to 37˚C.
After 24 hours, the mixture of every solution was centrifuged at 4000 g for 15 min; the degraded quantity of the compound was determined by the equation obtained by standardization curves.
2.5. The Effect of Initial Concentration on Phenol and Benzoic Acid Biodegradation
To study the effect of the concentration of the two compounds on the biodegradation by Pseudomonas aeruginosa, we prepared phenol solutions and benzoic acid to different concentrations of 10, 50, 70, 80 mg/l. After that, volume of every solution was 50 ml, which we mixed with 0.047.108 UFC/ml of Pseudomonas aeruginosa and submitted to agitation and incubation for 24 h.
3. Results and Discussions
After the biodegradation of 10, 50, 70 and 80 mg/l phenol 10, 50, 70 and 80 mg/l benzoic acid were conducted, the results obtained for phenol and benzoic acid biodegradation experiments are shown in Figures 1,2 respectively. These substrates were completely biodegraded except 80 mg/l (87.22%) phenol and 80 mg/l (81.25%) benzoic acid. Growth was apparent after a lag period and was exactly mirrored by phenol or benzoic acid consumption. The results showed that the lag period, the biodegradation time, and the number of microorganism increased on increasing the substrate concentrations. Also, the biodegradation time of benzoic acid was lower than the biodegradation time of phenol. It became obvious that microorganism used phenol as sole carbon and energy source better than benzoic acid.
The biodegradation times were first increased then decreased by increasing the concentration of phenol and benzoic acid. At low substrate concentrations, the biodegradation times of these compounds were, for example, 10 and 50 mg/l phenol were biodegraded in 96 h, 120 h

Figure 1. The biodegradation of 10 (■), 50 (▲), 70 (●) and 80 (♦) mg/l phenol, and the bacteria number for 10 (), 50 (∆), 70 (○) and 80 (◊) mg/l phenol.
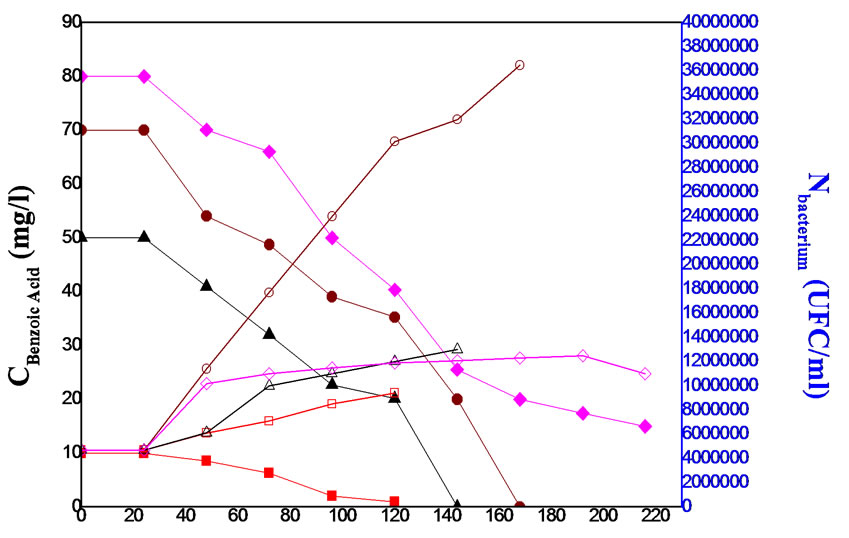
Figure 2. The biodegradation of 10 (■), 50 (▲), 70 (●) and 80 (♦) mg/l benzoic acid and the bacteria number for 10 (), 50 (∆), 70 (○) and 80 (◊) mg/l benzoic acid.
respectively and 10, 50 mg/l benzoic acid were biodegraded in 120 h, 140 h respectively.
In addition, the microorganism number obtained increased by increasing the concentration of the compounds, but there was no important increase in the microorganism number at the high substrate concentration of phenol and benzoic acid. For example 0.11.108 UFC/ml as obtained from the biodegradation of 80 mg/l phenol and 0.9.108 UFC/ml from the biodegradation of 10 mg/l (Figure 1) and because of that, the biodegradation was decreased at the high concentrations of these compounds. These results show that the microorganism was inhibited by the presence of the high substrate concentrations.
The results for effect of pH on phenol and benzoic acid biodegradation experiments are shown in Figures 3,4 respectively.
The degradation time of phenol for pH 5.6, 8 and 7 was 96 h, 120 h, 72 h respectively.
Results show that phenol degradation is faster at pH 7 because it is the appropriate pH for the growth of Pseudomonas aeruginosa.

Figure 3. The effect of pH on phenol biodegradation.

Figure 4. The effect of pH on benzoic acid biodegradation.
Finally, the degradation time for benzoic acid for pH 5.6, 8 and 7 was 120 h, 96 h, and 120 h respectively. We notice that Pseudomonas aeruginosa totally destroyed the phenol and benzoic acid quantity in a short degradation time at PH = 7.
4. Conclusions
Pseudomonas aeruginosa bacterium developed better in a phenolic medium and therefore damaged the phenol more than the benzoic acid did because the presence of COOH grouping functional on the benzene cycle had an influence on the bacterium degradation capacity.
Phenol and benzoic acid degradation is faster at PH = 7 because it is the appropriate pH for the growth of the Pseudomonas aeruginosa bacterium.
However, Pseudomonas aeroginosa isolated in this study could not degrade very high concentrations of phenol and benzoic acid completely (> 80 mg/l).
REFERENCES
- G. Wei, J. Yu, Y. Zhu, W. Chen and L. Wang, “Characterization of Phenol Degradation by Rhizobium Sp. CCNWTB 701 Isolated from Astragalus Chrysopteru in Mining Tailing Region,” Journal of Hazardous Materials Vol. 151, No. 1, 2008, pp. 111-117.
- K. L. Ho, B. Lin, Y. Y. Chen and D. J. Lee, “Biodegradation of Phenol Using Corynebacterium Sp. DJ1 Aerobic Granules,” Bioresource Technology, Vol. 100, No. 21, 2009, pp. 5051-5055.
- G. Buscaa, S. Berardinelli, C. Resini and L. Arrighib Rodrigues, “Technologies for the Removal of Phenol from Fluid Streams: A Short Review of Recentdevlopement,” Journal of Hazardous Materials, Vol. 160, No. 2- 3, 2008, pp. 265-288.
- P. Y. Lee and C. Y. Chen, “Toxicity and Quantitative Structure-Activity Relationships, of Benzoic Acids to Pseudokirchneriella Subcapitata,” Journal of Hazardous Materials, Vol. 165, No. 1-3, 2009, pp. 156-161.
- D. F. Goerlitz, D. E. Troutman and E. M. Godsy, “Migration of Wood-Preserving Chemicals in Contaminated Groundwater in a Sand Aquifer at Pensacola,” Science &.Technology, Vol. 15, No. 3-4, 1985, pp. 955-961.
- V. Arutchelvan, V. Kanakasabai, S. Nagarajan and V. Muralikrishnan, “Kinetics of High Strength Phenol Degradation Using Bacillus Brevis,” Journal of Hazardous Materials, Vol. 129, No. 1-3, 2006, pp. 216-222.
- G. Wei, J. Yu, Y. Zhu, W. Chen and L. Wang, “Characterization of Phenol Degradation by Rhizobium Sp CCNWTB 701 Isolated from Astragalus Chrysopteru in Mining Tailing Region,” Journal of Hazardous Materials, Vol. 151, No. 1, 2008, pp. 111-117.
- A. Papazi and K. Barbieri, “Inductive and Resonance Effects of Substituents Adjust the Microalgal Biodegradation of Toxical Phenolic Compounds,” Journal of Biotechnology, Vol. 135, No. 4, 2008, pp. 366-373.
- M. Afzal, S. Iqbal, S. Rauf and Z. M. Khalid, “Characteristics of Phenol Biodegradation in Saline Solutions by Monocultures of Pseudomonas Aeruginosa and Pseudomonas Pseudomallei,” Journal of Hazardous Materials, Vol. 149, No. 1, 2007, pp. 60-66.
- Á. M. G. Monteiro, R. A. R. Boaventura and A. E. Rodrigues, “Phenol Biodegradation by Pseudomonas Putida DSM 548 in a Batch Reactor,” Biochemical Engineering Journal, Vol. 6, No. 1, 2000, pp. 45-49.
- Y. Zheng, D. Liu, H. Xu, Y. Zhong and Y. Yuan, “Biodegradation of P-Nitrophenol by Pseudomonas Aeruginosa HS-D38 and Analysis of Metabolites with HPLC– ESI/MS,” International Biodeterioration & Biodegradation, Vol. 63, No. 8, 2009, pp. 1125-1129.
- F. Hamadi, H. Latrache, E. Mliji and B. mallouki, “Adhésion de staphylococus aureus au verre et au teflon,” Rcv. Microbiol, Ind , San et Environ, Vol. 3, No. 1, 2009, pp. 1-16.

