Green and Sustainable Chemistry
Vol. 2 No. 4 (2012) , Article ID: 24781 , 7 pages DOI:10.4236/gsc.2012.24020
Green Synthesis of Silver Nanoparticles Using Extracts of Ananas comosus
1Department of Botany, Patna University, Patna, India
2Department of Physics, A. N. College, Magadh University, Patna, India
Email: *seema_sharma26@yahoo.com
Received August 13, 2012; revised September 23, 2012; accepted October 6, 2012
Keywords: AgNPs; XRD; SAED; Green-Silver; Ananas comosus
ABSTRACT
The biosynthesis of nanoparticles has been proposed as a cost effective and environmental friendly alternative to chemical and physical methods. Plant mediated synthesis of nanoparticles is a green chemistry approach that intercomnects nanotechnology and plant biotechnology. In the present study, synthesis of silver nanoparticles (AgNPs) or (Green-Silver) has been demonstrated using extracts of Ananas comosus reducing aqueous silver nitrate. The AgNPs were characterized by Ultraviolet-Visible (UV-vis) Spectrometer, Energy Dispersive X-ray Analysis (EDAX), Selected Area Diffraction Pattern (SAED) and High Resolution Transmission Electron Microscopy (HRTEM). TEM micrographs showed spherical particles with an average size of 12 nm. The XRD pattern showed the characteristic Bragg peaks of (111), (200), (220) and (311) facets of the face center cubic (fcc) silver nanoparticles and confirmed that these nanoparticles are crystalline in nature. The different types of antioxidants presented in the pineapple juice synergistically reduce the Ag metal ions, as each antioxidant is unique in terms of its structure and antioxidant function. The reaction process was simple for formation of silver nanoparticles and AgNPs presented in the aqueous medium were quite stable, even up to 4 months of incubation. This work proved the capability of using biomaterial towards the synthesis of silver nanoparticle, by adopting the principles of green chemistry.
1. Introduction
Currently, sustainability initiatives that use green chemistry to improve and/or protect our global environment are focal issues in many fields of research. The development of cost efficient and ecologically benign methods of synthesis of nanomaterials still remains a scientific challenge as metal nanoparticles are of use in various catalytic applications, viz electronics, biology and biomedical applications, material science, physics, environmental remediation fields [1-8]. It is well known that the toxicity of nanomaterials essentially depends on the structural features such as size, shape, composition and the surface chemistry. To prolong the life span of metal nanoparticles it is vital to select stabilizing agents and pathways that are environmentally friendly, non toxic and easy to implement. Novel methods of ideally synthesizing NPs are thus being thought which are formed at ambient temperatures, neutral pH, low costs and environmentally friendly fashion. Keeping these goals in view nanomaterials have been synthesized using various routes. Among the biological alternatives, plants and plant extracts seem to be the best option. Plants are nature’s “chemical factories”. They are cost efficient and require little or no maintenance. A vast repertoire of secondary metabolites is found in all plants which possess redox capacity and can be exploited for biosynthesis of nanoparticles. As a wide range of metabolites are presented in the plant products/extracts, nanoparticles produced by plants are more stable and the rate of synthesis is faster in comparison to microorganisms. Thus, the advantages of using plant and plant-derived materials for biosynthesis of metal nanoparticles have instigated researchers to investigate mechanisms of metal ions uptake and bioreduction by plants, and to understand the possible mechanism of metal nanoparticle formation in and by the plants [9-18].
Ananas comosus L. belongs to Bromeliaceae family and is a subtropical fruit native to Thailand, Phillipines, China, Brazil and India. Pineapple has several beneficial properties including antioxidant activity. It contains enzymes which are a mixture of protease and which are known and sold as a nutritional supplement to “promote digestive health” and as an anti-inflammatory medication [19]. It has been applied in the anticancer activity [20] and in the immunization of influenza virus [21]. Phytochemicals, especially phenolics, in fruits and vegetables are suggested to be the major bioactive compounds for the health benefits [22]. Pineapple contains phytochemicals [23,24] which include antioxidant substances that fight against free radical cell damage and have been commonly used in traditional Chinese medicine [25,26]. Polyphenols possess outstanding antioxidant and free radical scavenging properties, suggesting a possible protective role in humans [27,28]. Their antioxidant potential is closely related to the number of hydroxyls, the higher the number, the more potent the chain breaking antioxidant action of the compound [29]. Plant phenolics are the largest class of plant secondary metabolites, which serve in plant defense mechanism to counteract reactive oxygen species (ROS) in order to survive and prevent molecular damage. In this communication we report a green method for the synthesis of silver nanoparticles at room temperature by using plant extracts of Ananas comosus as reducing/stabilizing agents and the probable mechanism for the formation of NPs.
2. Materials and Methods
2.1. Synthesis of Silver Nanoparticles
Pineapple (Ananas comosus L. var. queen) fruits were purchased from the local market. The extraction sample was prepared by extracting the juice of the pulpy fruit, sieving it and storing it for the synthesis of AgNPs. Both fresh and refrigerated juice (frozen for 24 h and 48 h) were used and they yielded similar results.
Aqueous solution of 10,000 ppm mol/L of AgNO3 was prepared. The pineapple juice was added to different flasks containing AgNO3 for bioreduction. The volume ratio of pineapple juice to aqueous AgNO3 was 1:10. Addition of the pineapple broth to aqueous AgNO3 resulted in change of colour within minutes resulting in the formation of AgNPs showing its signatory colour. The bioreduction of Ag+ ions was monitored by periodic sampling by the UV spectrophotometer.
2.2. Characterization of the Synthesized Silver Nanoparticles
The optical absorbance was recorded on UV-Vis spectrophotometer (Systronics 2202 double beam model) in 200 - 800 nm wavelength range. It was observed that upon addition of the extract into the flask containing the aqueous silver nitrate solution, the colour of the medium changed to brown within 2 min. This indicated the formation of silver nanoparticles. The solution containing the signatory colour of AgNPs (dark brown—Figure 1) was then poured out into petri-dishes and left in the oven for drying at 250˚C for 24 h. The formation and quality of compounds were checked by XRD technique. The X-ray diffraction (XRD) pattern measurements of dropcoated film of AgNPs on glass substrate were recorded in a wide range of Bragg angles θ at a scanning rate of 2˚min−1, carried out on a Philips PW 1830 instrument that was operated at a voltage of 40 kV and a current of 30 mA with CuKα radiation (1.5405 Å). High Resolution Transmission Electron Microscopy (HRTEM) was performed by TECHNAIG20-STWIN (200 KV) machine with a line resolution 2.32 (in angstrom). These images were taken by drop coating AgNPs on a carbon-coated copper grid. Energy Dispersive Absorption Spectroscopy photograph of AgNPs were carried out by the HRTEM equipment as mentioned above.
3. Results
Figure 1 shows UV-Vis spectra recorded at different time intervals from aqueous solution of silver nitrate with Ananas comosus extract. The samples display an optical absorption band peak at about 430 nm, typical of absorption for metallic Ag nanoclusters, due to the Surface Plasmon Resonance (SPR). Effect of the reaction time on AgNPs synthesis was also evaluated with UV-Visible spectra and it is noted that with an increase in time the peak becomes sharper. The increase in intensity could be due to increasing number of nanoparticles formed as a result of reduction of silver ions presented in the aqueous solution. The weak absorption peak at 200 nm (not shown here) indicates the presence of several organic compounds which are known to interact with silver ions into solution and suggests a possible mechanism for the reduction of the metal ions presented in the solution. Disturbances in the 200 nm - 320 nm were observed after 1 hour of the reaction probably indicating that the capping occurs after the reduction of the silver nanoparticles.
The exact nature of the silver particles formed can be deduced from the XRD spectrum of the sample. XRD pattern of the plant derived AgNPs (Figure 2) shows
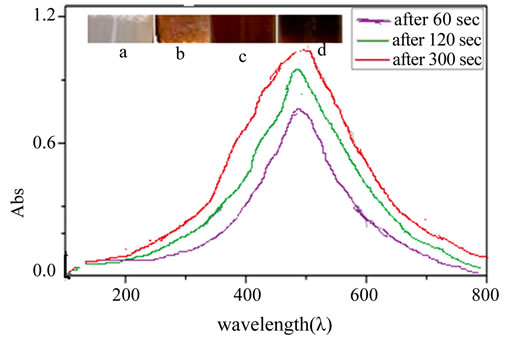
Figure 1. UV-Vis spectra recorded at different time intervals from aqueous solution of silver nitrate with Ananas comosus extract: 60 sec, 120 sec and 300 sec. Inbox showing change in colour before and after adding AgNO3, (a) Before reaction; (b) 60 sec; (c) 120 sec and (d) 300 sec respectively.

Figure 2. XRD spectrum of AgNPs synthesized from the extract of Ananas comosus.
four intense peaks in the whole spectrum of 2θ values ranging from 20˚ to 80˚. XRD spectra of pure crystalline silver structures have been published by the Joint Committee on Powder Diffraction Standards (file no. 04- 0783). A comparison of our XRD spectrum with the Standard confirmed that the silver particles formed in our experiments were in the form of nanocrystals, as evidenced by the peaks at 2θ values of 38.45˚, 44.48˚, 64.69˚ and 77.62˚, corresponding to (111), (200), (220), and (311) planes for silver, respectively. The unassigned peaks could be due to the crystallization of bioorganic phase that occurs on the surface of the nanoparticle. Two small insignificant impurity peaks observed at 68˚ and 75˚ are attributed to the presence of other organic substances in culture supernatant. The X-ray diffraction peaks were found to be broad around their bases indicating that the silver particles are in nanosizes. The peak broadening at half maximum intensity of the X-ray diffraction lines is due to a reduction in crystallite size, flattening and micro-strains within the diffracting domains.
The mean particle diameter of AgNPs was calculated from the XRD pattern, according to the line width of the maximum intensity reflection peak. The size of the nanoparticles through the Scherrer equation is given by Equation (1).
 (1)
(1)
where θ is the Bragg angle and λ is the wavelength of the X-ray used, β is the breadth of the pure diffraction profile in radians on 2θ scale and k is a constant approximately equals to unity and related both to the crystalline shape and to the way in which θ is defined. The best possible value of k has been estimated as 0.89. The Full Width at Half Maximum (FWHM) values measured for (111), (200), (220), and (311) planes of reflection were used with the Debye-Scherrer Equation (1) to calculate the size of the nanoparticles.
Further analysis of the silver particles by energy dispersive spectroscopy confirmed the presence of the signal characteristic of elemental silver. Figure 3 shows the Energy Dispersive Absorption Spectroscopy photographs of derived AgNPs. All the peaks of Ag are observed and are assigned. Peaks for Cu and C are from the grid used and the peaks for S, P and N correspond to the protein capping over the AgNPs. Silver nanocrystallites display an optical absorption band peak at approximately 3 keV, which is typical of the absorption of metallic silver nanocrystallites due to surface.
HRTEM images of AgNPs derived from the extract of pineapple are shown in Figure 4.
The morphology of the NPs was predominantly spherical. Some of the NPs were found to be oval and/or

Figure 3. EDAX spectra of AgNPs.
elliptical. Such variation in shape and size of nanoparticles synthesized by biological systems is common. It was noticeable that the edges of the particles were lighter than the centres, suggesting that biomolecules, such as proteins, capped the silver NPs. TEM analysis showed that most particles had a size of about 12 nm. The particle size distribution histogram for the AgNPs determined from the TEM image is shown in Figure 5. From this figure, it is clear that the frequency peak comes at approximately 10 nm - 15 nm, and particles, whose sizes range from 5 nm to 30 nm, account for about 75% of the total particles observed.
Figure 6 shows selected area electron diffraction pattern (SAED) of the silver nanoparticles. The silver particles are crystalline, as can be seen from the selected area diffraction pattern recorded from one of the nanoparticles in the aggregate. SAED spots that corresponded to the different crystallographic planes of face-centered cubic (fcc) structure of elemental silver are seen in Figure 6. The XRD spectrum of silver nanoparticles (Figure 2)

Figure 4. HRTEM micrograph recorded on a drop-coated film with Ananas comosus fruit extract and AgNPs aqueous suspension.

Figure 5. Particle size distribution histogram of silver nanoparticles determined from TEM image.

Figure 6. Selected area electron diffraction showing the characteristic crystal planes of elemental silver.
 (a)
(a) (b)
(b)
Figure 7. (a) Possible mechanism of reaction for the formation of silver nanoparticles; (b) Possible mechanism of reaction for the formation of silver nanoparticles.
exhibiting the characteristic peaks of the silver crystallites observed at 2θ values of 38.5˚, 44.48˚, 64.69˚ and 77.62˚ corresponding to (111), (200), (220) and (311) of the face centered cubic (fcc) silver nanoparticles, is also in agreement with SAED result.
4. Discussion
Plants contain a complex network of antioxidant metabolites and enzymes that work together to prevent oxidative damage to cellular components. Pineapple contains a number of essential nutrients, including vitamin C, manganese, and fibre. It also contains beneficial plant phytochemicals (Ferulic acid and chlorogenic acid) which have antioxidant and anti-cancer activities. These antioxidative compounds delay or inhibit the oxidation of molecules by inhibiting the initiation or propagation of oxidative chain reaction. The antioxidative activity of phenolic compounds is mainly due to their redox property, which plays an important role in absorbing and neutralizing free radicals, quenching singlet and triplet oxygen or decomposing peroxides [30]. Several reports have conclusively shown close relationship between total phenolic contents and antioxidative capacity [31-33]. The antioxidant activity is the result of a combination of different compounds having synergistic and antagonistic effect.
Ferulic acid due to its phenolic nucleus and an extended side chain conjugation readily forms a resonance stabilized phenoxy radical which accounts for its potent antioxidant potential. UV absorption by Ferulic acid catalyzes stable phenoxy radical formation. By virtue of effectively scavenging deleterious radicals and suppressing radiation-induced oxidative reactions, Ferulic acid may serve an important antioxidant function. This radical is highly resonance stabilized since the unpaired oxygen although present, may be delocalised across the whole molecule [34].
It is thus possible that Ferulic acid acts as a reducing agent and is oxidized by AgNO3, resulting in the formation of silver NPs (Figure 7). The reaction could be summarised as:

Another phenol, with high radical scavenging capacity presented in the pine apple extract is the chlorogenic acid. Hydrocinnamic acids, caeffic acids, rosemarinic acid and chlorogenic acids are more efficient scavengers of free radicals than benzoic acid derivatives [28,35,36].
The structural feature responsible for the antioxidant ability and free scavenging ability is attributed to the orthodihydroxl functionality in the catechol ring of the compound. The radical scavenging capacity has been attributed to the H− ion donating capacity of the compound [36].
Both antioxidant and antinitrosaminic properties of chlorogenic acid stem from the oxidizable ortho-diphenolic functionality, which can act as an H-atom donor toward reactive oxygen species and other biological oxidants.
Thus the different types of antioxidants presented in the pineapple juice synergistically reduce the Ag metal ions as each antioxidant is unique in terms of its structure and antioxidant function of trapping the different free radicals. In addition different antioxidants react with one another to regenerate each other forming an antioxidant network.
5. Conclusions
The rapid synthesis of stable silver nanoparticles of average size ~12 nm using pineapple juice was demonstrated. Achievement of such rapid time scales for synthesis of silver nanoparticles makes it more efficient as a biosynthetic pathway, though there still remains some scope for further decreasing the reduction time periods to make it a viable alternative to chemical synthesis methods.
Probably the biomolecules responsible for the reduction and stabilisation of AgNPs are phenols. The phenolics in pineapple exhibit excellent antioxidant activity and these phenols can react with a free radical to form the phenoxyl radicals. Therefore, the use of natural antioxidants for the synthesis of AgNPs seems to be a good alternative which can be due to its benign composition. The plant material responsible for the reduction and stabilisation of NPs needs further study including extraction and identification of the compounds presented in the extract.
REFERENCES
- A. L. González and C. Noguezm, “Influence of Morphology on the Optical Properties of Metal Nanoparticles,” Journal of Computational and Theoretical Nanoscience, Vol. 4, No. 2, 2007, pp. 231-238.
- M. Gross, M. A. Winnacker and P. J. Wellmann, “Electrical, Optical and Morphological Properties of Nanoparticle Indium-Tin-Oxide Layers,” Thin Solid Films, Vol. 515, No. 24, 2007, pp. 8567-8572. doi:10.1016/j.tsf.2007.03.136
- J. Y. Kim, M. Kim, H. M. Kim, J. Joo and J. H. Choi, “Electrical and Optical Studies of Organic Light Emitting Devices Using SWCNTs-Polymer Nanocomposites,” Optical Materials, Vol. 21, No. 1-3, 2003, pp. 147-151. doi:10.1016/S0925-3467(02)00127-1
- W. J. Parak, D. Gerion, T. Pellegrino, D. Zanchet, C. Micheel, S. C. Williams, R. Boudreau, M. A. Le Gros and C. A. Larabell and A. P. Alivisatos, “Biological Applications of Colloidal Nanocrystals,” Nanotechnology, Vol. 14, No. 7, 2003, pp. 15-27. doi:10.1088/0957-4484/14/7/201
- D. A. Schultz, “Plasmon Resonant Particles for Biological Detection,” Current Opinion Biotech, Vol. 14, No. 1, 2003, pp. 13-22. doi:10.1016/S0958-1669(02)00015-0
- A. M. Smith, H. Duan, M. N. Rhyner, G. Ruan and S. Nie, “A Systematic Examination of Surface Coatings on the Optical and Chemical Properties of Semiconductor Quantum Dots,” Physical Chemistry Chemical Physics, Vol. 8, No. 33, 2006, pp. 3895-3903. doi:10.1039/b606572b
- G. H. Wei, Z. Zhou and Z. Liu, “A Simple Method for the Preparation of Ultrahigh Sensitivity Surface Enhanced Raman Scattering (SERS) Active Substrate,” Applied Surface Science, Vol. 240, No. 1-4, 2005, pp. 260-267. doi:10.1016/j.apsusc.2004.06.116
- H. Y. Wang, Y. F. Li and C. Z. Hua, “Detection of Ferulic Acid Based on the Plasmon Resonance Light Scattering of Silver Nanoparticles, Special Issue on ChinaJapan-Korea Environmental Analysis,” Talanta, Vol. 72, No. 5, 2007, pp. 1698-1703.
- N. Ahmad, M. K. Alam, V. N. Singh and S. Sharma, “Bioprospecting AgNPs from Wild Desmodium Species,” Journal of Bionanoscience, Vol. 3, No. 2, 2009, pp. 97-104.
- N. Ahmad, S. Sharma, M. K. Alam, V. N. Singh, S. F. Shamsi, B. R. Mehta and A. Fatma, “Rapid Synthesis of Silver Nanoparticles Using Dried Medicinal Plant of Basil,” Colloids and Surfaces B: Biointerfaces, Vol. 81, No. 1, 2010, pp. 81-86. doi:10.1016/j.colsurfb.2010.06.029
- N. Ahmad and S. S. Ahmad, “Biomediated AgNPS from Some Ethnobotanical Weeds—Pyllanthus amarus,” International Journal of Green Nanotechnology, Vol. 3, No. 2, 2011, pp. 109-117. doi:10.1080/19430892.2011.574569
- B. Ankamwar, M. Chaudhary and M. Sastry, “Gold Nanotriangles Biologically Synthesized Using Tamarind Leaf Extract and Potential Application in Vapor Sensing,” Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry, Vol. 35, No. 1, 2005, pp. 19-26. doi:10.1081/SIM-200047527
- S. P. Chandran, M. Chaudhary, R. Pasricha, A. Ahmad and M. Sastry, “Synthesis of Gold Nanotriangles and Silver Nanoparticles Using Aloevera Plant Extract,” Biotechnology Progress, Vol. 22, No. 2, 2006, pp. 577-583. doi:10.1021/bp0501423
- S. P. Dubey, M. Lahtinenb, S. Heikki and M. Sillan, “Bioprospective of Sorbus aucuparia Leaf Extract in Development of Silver and Gold Nanocolloids,” Colloids and Surfaces B: Biointerfaces, Vol. 80, No. 1, 2010, pp. 26-33. doi:10.1016/j.colsurfb.2010.05.024
- A. A. Zahir, A. Bagavan, C. Kamaraj, G. Elango and A. A. Rahuman, “Efficacy of Plant-Mediated Synthesized Silver Nanoparticles against Sitophilus oryzae,” Journal of Biopesticides, Vol. 288, Suppl. 5, 2012, pp. 95-102.
- J. L. Gardea-Torresdey, J. G. Parsons, E. Gomez, J. PeraltaViddea, H. E. Troiani, P. Santiago and M. J. Yacaman, “Formation and Growth of Au Nanoparticles inside Live Alfalfa Plants,” Nano Letters, Vol. 2, No. 4, 2002, pp. 397-401. doi:10.1021/nl015673+
- V. Kumar and S. K. Yadav, “Plant-Mediated Synthesis of Silver and Gold Nanoparticles and Their Applications,” Journal of Chemical Technology and Biotechnology, Vol. 84, No. 2, 2009, pp. 151-157. doi:10.1002/jctb.2023
- S. Shankar, A. Ahmad and M. Sastry, “Geranium Leaf Assisted Biosynthesis of Silver Nanoparticles,” Biotechnology Progress, Vol. 19, No. 6, 2003, pp. 1627-1631. doi:10.1021/bp034070w
- L. P. Hale, P. K. Greer, C. T. Trinh and C. L. James, “Proteinase Activity and Stability of Natural Bromelain Preparations,” International Immunopharmacology, Vol. 5, No. 4, 2005, pp. 783-793. doi:10.1016/j.intimp.2004.12.007
- T. Harrach, F. Garbin, E. Munzig, K. Eckert and H. R. Maurer, “P182 Bromelain: An immunomodulator with Anticancer Activity,” European Journal of Pharmaceutical Sciences, Vol. 2, No. 1-2, 1994, pp. 164-167. doi:10.1016/0928-0987(94)90355-7
- E. R. Secor Jr., W. F. Carlson, M. M. Cloutier, L. A. Guernsey, C. M. Schramm, C. A. Wu and R. S. Thrall, “Bromelain Exerts Anti-Inflammatory Effects in an Ovalbumin-Induced Murine Model of Allergic Airway Disease,” Cellular Immunology, Vol. 237, No. 1, 2005, pp. 68-75. doi:10.1016/j.cellimm.2005.10.002
- J. Sun, Y. Chu, X. Wu and R. Liu, “Antioxidant and Antiproliferative Activities of Common Fruits,” Journal of Agricultural and Food Chemistry, Vol. 50, No. 25, 2002, pp. 7449-7454. doi:10.1021/jf0207530
- Y. Z. Caia, Q. Luob, M. Sunc and H. Corke, “Antioxidant Activity and Phenolic Compounds of 112 Traditional Chinese Medicinal Plants Associated with Anticancer,” Life Sciences, Vol. 74, No. 17, 2004, pp. 2157-2184. doi:10.1016/j.lfs.2003.09.047
- A. Haripyarie, K. Guneshwor and M. Damayanti, “Evaluation of Antioxidant Properties of Phenolics Extracted from Ananas comosus L.,” Notulae Scientia Biologicae, Vol. 2, No. 2, 2010, pp. 68-71.
- M. Mhatre, J. Tilak-Jain, S. De and T. P. Devasagayam, “Evaluation of the Antioxidant Activity of Non-Transformed and Transformed Pineapple: A Comparative Study,” Food and Chemical Toxicology, Vol. 47, No. 11, 2009, pp. 2696-2702. doi:10.1016/j.fct.2009.06.031
- E. Q. Zhang, “Chinese Medicinal Diet,” Publishing House of Shanghai College of Traditional Chinese Medicine, Shanghai, 1990, pp. 50-53.
- M. J. Laughton, P. J. Evans, M. A. Moroney, J. R. S. Howlt and B. Halliwell, “Inhibition of Mammalian 5- Lipoxygenase and Cyclo-Oxygenase by Flavonoids and Phenolic Dietary Additives: Relationship to Antioxidant Activity and to Iron Ion-Reducing Ability,” Biochemical Pharmacology, Vol. 42, No. 9, 1991, pp. 1673-1681. doi:10.1016/0006-2952(91)90501-U
- B. C. Scott, J. B. Healliwll and O. B. Aruoma, “Evaluation of the Antioxidant Actions of Ferulic Acid and Catechins,” Free Radical Research Communications, Vol. 19, No. 4, 1993, pp. 241-253. doi:10.3109/10715769309056512
- R. Hussain, S. J. Cillard and P. Cillard, “Hydroxyl Radical Scavenging Activity of Flavonoids,” Phytochemistry, Vol. 26, No. 9, 1989, pp. 2489-2491. doi:10.1016/S0031-9422(00)83860-1
- S. Panchawat and S. S. Sisodia, “In Vitro Antioxidant activity of Saraca Asoca Roxb. De Wilde Stem Bark Extracts from Various Extraction Processes,” Asian Journal of Pharmaceutical and Clinical Research, Vol. 3, No. 3, 2010, pp. 231-233.
- N. Deighton, R. Brennan, C. Finn and H. V. Davis, “Antioxidant Properties of Domesticated and Wild Rubus Species,” Journal of Science of Food and Agriculture, Vol. 80, No. 9, 2000, pp. 1307-1313. doi:10.1002/1097-0010(200007)80:9<1307::AID-JSFA638>3.0.CO;2-P
- M. A. Hossain and S. M. N. Rehman, “Total Phenolics, Flavonoids and Antioxidant Activity of Tropical Fruit Pineapple,” Food Research International, Vol. 44, No. 3, 2011, pp. 672-676. doi:10.1016/j.foodres.2010.11.036
- E. S. Yapo, H. T. Kouakou, L. K. Kouakou, J. Y. Kouadio, P. Kouaméand and J. M. Mérillon, “Phenolic Profiles of Pineapple Fruits (Ananas comosus L. Merrill) Influence of the Origin of Suckers,” The Australian Journal of Basic and Applied Sciences, Vol. 5, No. 6, 2011, pp. 1372- 1378.
- G. Ernst, “Antioxidant Potential of Ferulic Acid,” Free Radical Biology and Medicine, Vol. 13, No. 4, 1999, pp. 435-448.
- J. H. Chen and C. T. Ho, “Antioxidant Activities of Caffeic Acid and Its Related Hydroxycinnamic Acid Compounds,” Journal of Agricultural and Food Chemistry, Vol. 45, No. 7, 1997, pp. 2374-2378. doi:10.1021/jf970055t
- L. Panzella, A. Napolitano and M. d’Ischia, “Oxidative Conjugation of Chlorogenic Acid with Glutathione: Structural Characterization of Addition Products and a New Nitrite-Promoted Pathway,” Bioorganic & Medicinal Chemistry, Vol. 11, No. 22, 2003, pp. 4797-4805. doi:10.1016/S0968-0896(03)00460-7
NOTES
*Corresponding author.

