Journal of Sensor Technology
Vol.2 No.1(2012), Article ID:17918,6 pages DOI:10.4236/jst.2012.21003
Structural Characterization of Nanocrystalline La1−xSrxCrO3 Thick Films for H2S Gas Sensors
1Prof. Ram Meghe College of Engineering and Management, Amravati, India
2Nano Technology Research Laboratory, Department of Chemistry, Shri Shivaji Science College, Amravati, India
Email: {*ashishkadu26, nano.d}@rediffmail.com
Received November 14, 2011; revised December 6, 2011; accepted January 5, 2012
Keywords: La1−xSrxCrO3; H2S Gas; Selectivity; Response & Recovery Time
ABSTRACT
The nanocrystalline of La1−xSrxCrO3 (x = 0.0, 0.1, 0.2, 0.3 & 0.4) were prepared by sol-gel method and their crystal structures & morphology were characterized by X-ray diffraction (XRD) and Transmission Electron Microscopy (TEM). XRD patterns indicate that the average particle size of the nanocrystalline La0.7Sr0.3CrO3 in the range of 30 - 35 nm. The gas sensing properties were studied towards reducing gases like Ammonia gas (NH3), liquefied petroleum gas (LPG), hydrogen sulphide (H2S) and H2 gas and it is observed that undoped LaCrO3 shows response to H2S gas at relatively high operating temperature 300˚C. The La1−xSrxCrO3 based sensor with x = 0.3 shows better sensitivity towards H2S gas at an operating temperature 210˚C. The effect of Sr doping on sensitivity, response time and recovery time of the sensor in the presence of H2S and other reducing gases were studied and discussed.
1. Introduction
The sensors are required basically for measurement of physical quantities and for use of controlling some systems. Presently, the atmospheric pollution has become a global issue. Gases from auto and industrial exhausts are polluting the environment. In order to detect, measure and control these gases, one should know the amount and type of gases present in the ambient. Thus, the need to monitor and control these gases has led to the research and development of a wide variety of sensors using different materials and technologies. Gas sensitive resistors based on semiconducting oxides are simple and robust devices which owe their response to changes in chargecarrier concentration within a depletion layer at the solidgas interface, in turn caused by a change in the surface density of electron trap states [1,2]. They raise interesting questions of surface chemistry: the effects are considered due either to change in the surface coverage of the adsorbed oxygen species, caused by a reaction with the gas, or to adsorption of a gas species generating a new surface trap state.
H2S is a colourless, toxic, flammable and malodorous gas as sources from gasoline, natural gases, city sewage, volcanic gases and hot springs with smells like rotten eggs. It can also be produced from bacterial breakdown of organic matter or wastes produced by human and animal. Other sources are craft paper mills, tanneries and petroleum refineries. H2S gas is badly harmful to human body and the environment. Meanwhile the type of oil and natural gas is correlative with the concentration of H2S. The oil and natural gases mines can be found depending on the concentration of H2S. Therefore, the detection and monitoring of H2S are of high importance for both resource exploitation and human health. In the recent researchers, a number of semiconductor sensors have been found to be sensitive to H2S including WO3, In2O3, ZnO and a few pervoskite type materials [3-7].
The pervoskite oxides (ABO3) were used as gas sensor materials for their stability in thermal and chemical atmospheres. Modifications in microstructure, processing parameters and also concentration of acceptor/donor dopant can vary the temperature coefficient of the resistance and conductivity of ABO3 oxides. Sensors based on ABO3-type complex oxide material, of rare earth elements have an outstanding merit of its high sensitive and selective characteristics. These characteristics can be controlled by selecting suitable A and B atoms or chemically doping A′ and B′ elements equivalent respectively to A and B into ABO3 to obtain  compound [8,9].
compound [8,9].
In the recent years, a number of semiconductor sensors have been found to be suitable for H2S gas e.g. SnO2, WO3, In2O3, ZnO2 and a few perovskite-type materials like NdFeO3 and NiFeO4 [10-13]. LaCrO3 based compounds are usually synthesized by the traditional solid state reaction method, urea combustion method [14,15]. Sol-gel approach, as a classical method, has a lot of inherent merits with better homogeneity, higher purity, and lower temperature of preparation.
In this present paper, synthesis of nanocrystalline La1−xSrxCrO3 powder by a simple chemical route has been reported. The gas sensing properties of nanocrystalline La1−xSrxCrO3 (x = 0.0, 0.1, 0.2, 0.3 & 0.4) are reported and the gas sensor based on the nanocrystalline La0.7Sr0.3CrO3 material shows good sensitivity and selectivity to the H2S gas.
2. Experimental Details
2.1. Material Synthesis
The La1−xSrxCrO3 (x = 0.0, 0.1, 0.2, 0.3 & 0.4) were synthesized by sol-gel method using poly ethylene glycol as a solvent. La(NO3)3·6H2O, Sr(NO3)2, Cr(NO3)3·9H2O and citrate acid (all analytically pure) were firstly dissolved in ion free water at 80˚C for 2 h. Then ethylene glycol was added under constant stirring to obtain a homogeneous and stable sol. The solution was further heated in pressure vessel at about 140˚C for 14 h. During this reaction transparent solution was transform into a gel state with very high viscosity. The material was then heated in a furnace at 350˚C for 3 h and a violent combustion was occurs which spontaneously propagates until all the gel was burnt out to form a loose powder. The powder was then calcined at 800˚C for 6 h in order to improve the crystallinity of materials.
2.2. Characterization of Samples
The synthesized samples were characterized by powder XRD using a Siemens D 5000 diffractometer .The XRD data were recorded by using Cu Kα radiation (1.5406 Å). The intensity data were collected over a 2θ range of 10˚ - 70˚. The average crystallite size of the samples was estimated with the help of Scherrer equation using the diffraction intensity of all prominent lines. TEM examination of the synthesized powder was performed using an H-800 electron microscope.
2.3. Measurement of Sensing Characteristics
For gas sensing properties, the calcined powder was then mixed with 2% PVA (polyvinyl alcohol) as a binder and 5% ethanol as a solvent; the resulting paste was coated onto an Al2O3 tube provided with platinum wires as electrodes at each end. The Al2O3 tube was about 8 mm in length, 2 mm in external diameter and 1.6 mm in internal diameter. A small Ni-Cr alloy coil was placed inside the tube to serve as a heater, which provided operating temperatures from 50˚C - 350˚C. The temperature was controlled by adjusting the heating power. Finally, the sensor was sintered at 600˚C for 1 h so that the PVA decomposes and strength of the element markedly increases. The different test gases are injected into the specimen chamber through an inlet. The sensing performance of the sensors was examined using the “flow gas sensing system”.
The gas sensing studies were carried out on a static gas sensing system under normal laboratory conditions. The electrical resistance of thick films in air (Ra) and in the presence of H2S (Rg) was measured to evaluate the Sensitivity (S) and is given by the relation,
 (1)
(1)
where Ra is the resistance of the La1−xSrxCrO3 thick films in air and Rg is the resistance of the La1−xSrxCrO3 thick films in H2S atmosphere. The response of the sensor for H2S was tested in the presence of other gases so that the selectivity can be determined. The response and recovery time of the sensor was measured.
3. Results and Discussion
3.1. X-Ray Diffraction Study
The XRD patterns of the precursor powders LaCrO3 and La0.7Sr0.3CrO3 calcined at 800˚C for 6 h are shown in Figure 1. Figure 1(a), shows the XRD pattern of LaCrO3, which is in good agreement with XRD results previously reported in the literature [16]. All the diffraction peaks of the phases are indexed as perovskite-type with tetragonal structure. The diffraction data is good agreement with JCPD card of LaCrO3 (JCPDS No.24-1016). Figure 1(b), shows the XRD pattern of La0.7Sr0.3CrO3 nanomaterial, which indicates that ions Sr2+ partially substitute for ions La3+ in the LaCrO3 crystal lattice. The ionic radii of Sr2+ (1.21 Å) are very close to that of La3+ and Sr is incorporated into the LaCrO3 lattice at the La site. The mean crystallite sizes (D) of La0.7Sr0.3CrO3 powder was deduced from half height width of XRD peaks based on the Scherer’s equation, t = 0.9λ/βcosθ, where t is the average size of the particles, λ is wavelength of X-ray radiation, β the full width at half maximum of the diffracted peak and θ is the angle of diffraction [17]. Extremely broad reflections are observed indicating nanosized particle nature of the material obtained. The average particle size of the nanocrystalline La0.7Sr0.3CrO3 according to the scherrer formula was in the range of 30 - 35 nm.
3.2. Surface Morphology
The TEM specimens were prepared by placing microdrops of colloid solutions on a carbon film supported by

Figure 1. XRD spectra of (a) LaCrO3; (b) La1−xSrxCrO3 calcined at 800˚C.
a copper grid. The TEM images of the nanocrystalline La0.7Sr0.3CrO3 calcinated at 800˚C is shown in Figure 2. It indicates the presence of La0.7Sr0.3CrO3 nanoparticles with 30 - 50 nm size which form spherical type of oriental aggregation, agglomeration and polymeric linkage throughout the region.
3.3. Gas Sensing Characteristics
Electrical characterization of samples La1−xSrxCrO3 (x = 0.1, 0.2, 0.3 & 0.4) synthesized by sol-gel method and calcinied at 800˚C has been carried out in a gas test chamber. The resistance of the films was measured by using Equation (1). The resistance of the films decreased upon exposure to H2S gas. Figure 3 shows gas sensing response of H2S gas, at different operating temperatures (50˚C - 400˚C) for thick films of pure LaCrO3. It is observed that response for pure LaCrO3 is obtained at high operating temperature 350˚C, than decreases as increase in operating temperature. At a low operating temperature, the low response can be expected because the gas molecules do not have enough thermal energy to react with the surface adsorbed oxygen species. As the temperature increases, the thermal energy obtained was high enough to overcome the potential barrier, and a significant increase in electron concentration resulted from the sensing reaction. The response of semiconductor oxide gas sensor to the presence of a given gas depends on the speed of the chemical reaction on the surface of the grains and the speed of diffusion of the gas molecules to that surface which are activation processes, and the activation energy of the chemical reaction is higher. In this case, at low temperatures the sensor response is restricted by the speed of the chemical reaction, and at higher temperatures (above 350˚C) it is restricted by the speed of diffusion of gas molecules. At some intermediate temperature (at 350˚C), the speed, values of the two processes be-

Figure 2. TEM micrograph of La0.7Sr0.3CrO3 calcined at 800˚C.
 Operating Temperature (˚C)
Operating Temperature (˚C)
Figure 3. H2S gas response vs operating temperatures for LaCrO3 calcined at 800˚C.
come equal, and at that point the sensor response reaches its maximum [18].
Thus, the pure LaCrO3 shows response towards H2S gas at high operating temperature 350˚C, which is rather high and practically inconvenient in view of commercial standards. Therefore, efforts were made to modify the LaCrO3 based sensors by doping with Sr, so as to be operated at lower operating temperatures with high sensitivity and selectivity. Figure 4 shows gas sensing response of H2S gas, for thick films of pure LaCrO3 doped with different concentrations of Sr. From the figure it is clear that the response values of every doped sample apparently increased with increasing the operating temperature. In this figure, it is observed that the La0.7Sr0.3CrO3 thick film had the largest sensing response at an operating temperature 210˚C. It is indicating that Sr doping can greatly improve the sensitivity of LaCrO3 based sensor towards H2S gas. We know that the semiconductor oxide gas sensors can detect different gases by changing the conductivity of their surface due to the reaction of the reducing gases with adsorbed oxygen. Sr doping in La-
 Operating Temperature (˚C)
Operating Temperature (˚C)
Figure 4.H2S gas response vs operating temperatures for (A) La0.9Sr0.1CrO3, (B) La0.8Sr0.2CrO3; (C) La0.7Sr0.3CrO3 & (D) La0.6Sr0.4CrO3 calcined at 800˚C.
CrO3 promotes the deviation from stoichiometry and enhances the surface defect. A large surface defect concentration creates more active surface states for adsorbed oxygen [19,20].
Selectivity or specificity is defined as the ability of a sensor to respond to certain gas in the presence of other gases. Figure 5 shows the selectivity of La0.7Sr0.3CrO3 for different gases. It is clear from the figure that La0.7Sr0.3CrO3 sample is more selective to H2S gas at 210˚C against all other tested gases viz: NH3, LPG and H2. As easily seen, the La0.7Sr0.3CrO3 was most selective to H2S among the gases tested, while it was least selective to NH3.
The sensor selects a particular gas at a particular temperature. Thus by setting the temperature, one can use the sensor for particular gas detection. The same sensor could be used for the detection of different gases by operating it at particular temperature for a typical gas. This can be attributed to different chemical reactivity’s of different gases on the sensor surface. Different gases have different energies for adsorption, desorption, and reaction on the metal oxide surface, and therefore the response of the sensor at different temperatures would depend on the gas being sensed. The amount of oxygen adsorbed (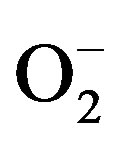 , O–, O2–) on the sensor surface goes on increasing with an increase in temperature, reaches to the maximum and then decreases with a further increase in operating temperature. The response to the gas to be detected follows the same behavior. When a reducing gas comes in contact with the sensor surface, it gets oxidized. The rate of oxidation would be the function of the amount of adsorbed oxygen on the surface and the type of gas to be detected. The larger the rate of oxidation, the larger would be the number of electrons released, and in turn the larger would be the gas response. At higher temperatures, the amount of oxygen adsorbed would be smaller, leading to a slower rate of reduction of a target gas and, therefore, the smaller gas response.
, O–, O2–) on the sensor surface goes on increasing with an increase in temperature, reaches to the maximum and then decreases with a further increase in operating temperature. The response to the gas to be detected follows the same behavior. When a reducing gas comes in contact with the sensor surface, it gets oxidized. The rate of oxidation would be the function of the amount of adsorbed oxygen on the surface and the type of gas to be detected. The larger the rate of oxidation, the larger would be the number of electrons released, and in turn the larger would be the gas response. At higher temperatures, the amount of oxygen adsorbed would be smaller, leading to a slower rate of reduction of a target gas and, therefore, the smaller gas response.
 Operating Temperature (˚C)
Operating Temperature (˚C)
Figure 5. Cross sensitivity vs operating temperatures for different reducing gases for La0.7Sr0.3CrO3
Figure 6 depicts the variation of sensitivity of La0.7Sr0.3CrO3 sample with H2S gas concentrations at 210˚C temperature. It is clear from the figure that the gas response goes on increasing linearly with gas concentration up to 200 ppm and saturated beyond it. The rate of increase in gas response was relatively larger up to 200 ppm. It is observed that as the concentration of H2S gas increases, the average sensitivity increases linearly in the beginning and later it becomes saturated. The linear relationship between sensitivity and gas concentration may be attributed to the availability of sufficient number of sensing sites. The low concentration implies a lower surface coverage of gas molecules, resulting in a lower surface reaction between the surface adsorbed oxygen species and the gas molecules. The increase in H2S gas concentration increases the surface reaction due to a large surface coverage. Further on increasing the H2S gas concentration, the surface reaction does not increase and eventually saturation takes place. Thus, the maximum sensitivity was obtained at higher concentration of H2S gas, i.e., 200 ppm.
The response and recovery time is an important parameter, used for characterizing sensors. It is defined as the time required to reach 90% of the final change in voltage or resistance, when the gas is turned on or off, respectively. The response and recovery times of La0.7Sr0.3CrO3 sample is represented in Figure 7. The response was quick (~20 s) to 200 ppm of H2S while the recovery was moderate (~60 s). The quick response may be due to faster oxidation of gas. Its high volatility explains its quick response .The large recovery time would be due to lower operating temperature. At lower temperature O2- species is more prominently adsorbed on the surface and thus it is less reactive as compared to other species of oxygen, O– and O2.
The long-term stability is another important factor for gas sensors. The stability of the La0.7Sr0.3CrO3 nanocrystalline based sensor was studied in the presence of 200 ppm H2S gas for fifty day at 210˚C. Repeated experi-

Figure 6. Gas response of La0.7Sr0.3CrO3 to H2S gas of different concentration at an operating temperature 210˚C.
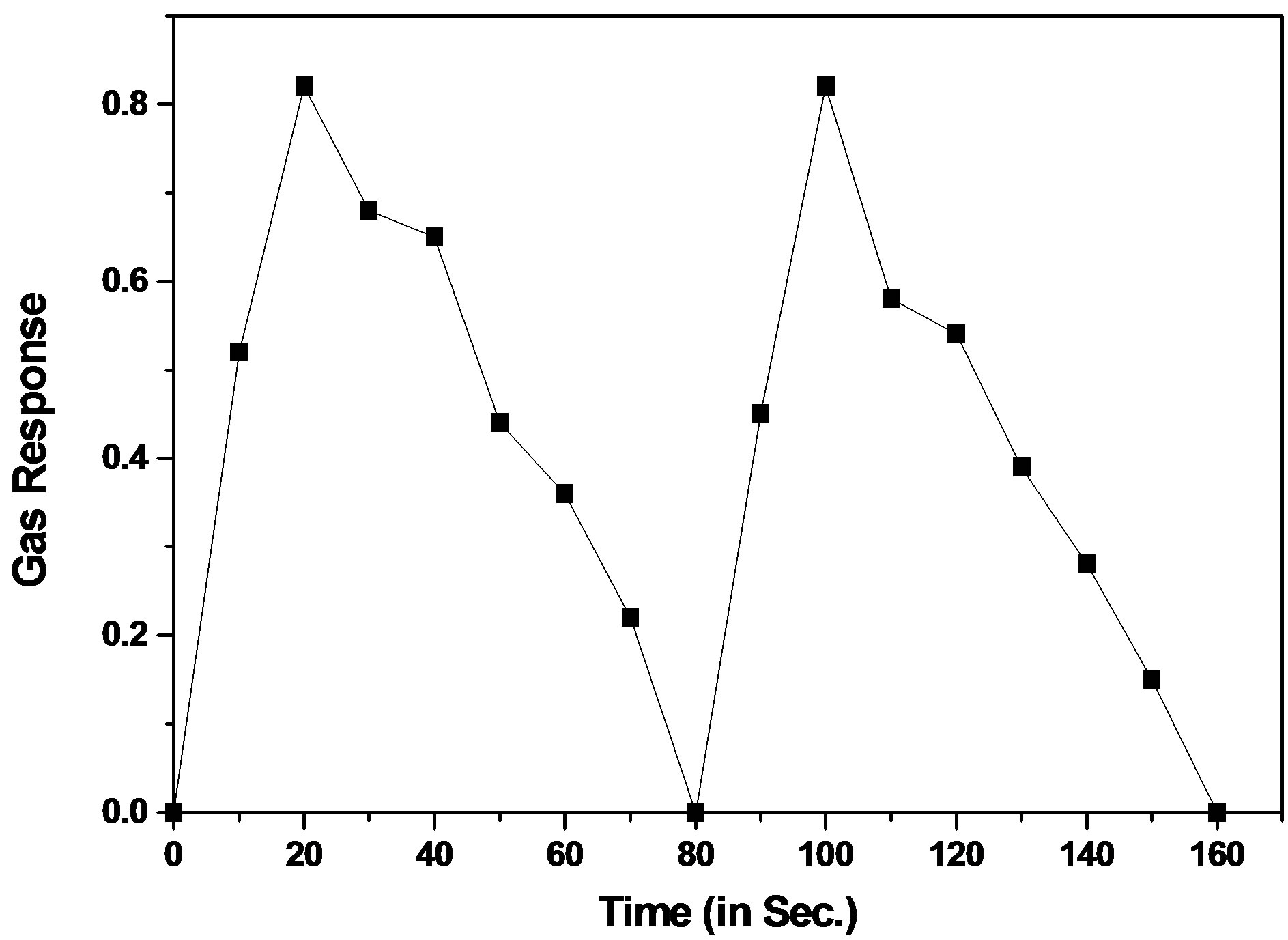
Figure 7. The response and recovery characteristics to 300 ppm H2S gas of La0.7Sr0.3CrO3.
ments were carried out and it was observed that both La0.7Sr0.3CrO3 sensor showed good response performance even after 50 days (shown in Figure 8). It is observed that its resistance is stable except for a small variation for first ten days. This indicates that the present nanocrystalline La0.7Sr0.3CrO3 sensor can be effectively used as H2S gas sensor.
4. Conclusion
In this work the La0.7Sr0.3CrO3 have been presented as suitable semiconductor materials for selective H2S detection. The XRD pattern of La0.7Sr0.3CrO3 shows perovskite-type with tetragonal structure. The results revealed that the particle size is in the range of 30 - 35 nm for La0.7Sr0.3CrO3 with good crystallinity. From the results obtained, pure LaCrO3 showed low response to H2S gas. Sr doped LaCrO3 thick films were found to be high sensitive for H2S gas. Among all other synthesized samples La0.7Sr0.3CrO3 thick film was found to be optimum and showed highest response to H2S gas at 210˚C. The sensor shows high degree of selectivity towards H2S gas than

Figure 8. The stability characteristics of La0.7Sr0.3CrO3 sensor towards H2S gas.
other reducing gases. Also this sensor showed very rapid response and recovery to H2S gas. Over long exposure it was observed that sensor exhibited a good stability and repeatability as gas sensor with consistent pattern and response magnitude.
5. Acknowledgements
The authors are also indebted to Principal, Dr.M.S Ali, Principal, Prof Ram Meghe College of Engineering & Management, Badnera-Amravati, India for his kind cooperation during this research work. The authors are thankful to University Grants Commission (UGC), New Delhi for providing financial support for this work.
REFERENCES
- D. E. Williams, “Semiconducting Oxides as Gas-Sensitive Resistors,” Sensors and Actuators B, Vol. 57, No. 1-3, 1999, pp. 1-16. doi:10.1016/S0925-4005(99)00133-1
- N. Barsan, M. Schweizer-Berberich and W. Göpel, “Fundamental & Practical Aspects in the Design of Nanoscaled SnO2 Gas Sensors,” Fresenius’ Journal of Analytical Chemistry, Vol. 365, No. 4, 1999, pp. 287-304. doi:10.1007/s002160051490
- G. Sberveglieri, S. Groppelli, P. Nelli, C. Perego, G. Valdre and A. Camanzi, “Detection of Sub-ppm H2S Concentrations by SnO2 (pt) Thin Films Grown by the RGTO Technique,” Sensors and Actuators B, Vol. 55, 1998, pp. 86-89.
- D. J. Smith, J. F. Velelina, R. S. Falconer and E. L. Wittman, “Stability Sensitivity and Selectivity of Tungsten Trioxide Films for Sensing Applications,” Sensors and Actuators B, Vol. 13, No. 1-3, 1993, pp. 264-268. doi:10.1016/0925-4005(93)85377-M
- W. H. Tao and C. H. Tsai, “H2S Sensing Properties of Noble Metal Doped WO3 Thin Film Sensor Fabricated by Micromachining,” Sensors and Actuators B, Vol. 81, No. 2-3, 2002, pp. 237-247. doi:10.1016/S0925-4005(01)00958-3
- J. Q. Xu, X. H. Wang and J. N. Shen, “Hydrothermal Synthesis of In2O3 for Detecting H2S,” Sensors and Actuators B, Vol. 115, No. 2, 2006, pp. 642-646. doi:10.1016/j.snb.2005.10.038
- Y. L. Liu, H. Wang, Y. Yang, Z. M. Liu, H. F. Yang, G. L. Shen and R. Q. Yu, “Hydrogen Sulphide Sensing Properties of NiFeO4 Nanopowder Doped with Noble Metal,” Sensors and Actuators B, Vol. 102, No. 1, 2004, pp. 148- 154. doi:10.1016/j.snb.2004.04.014
- W. Yan, L. Sun, M. Lui and W. Li, “Study of Sensing Characteristics of Rare Earth Pervoskites for Alcohol,” Acta Scientiarium Naturalium Universitaties Jilinesis, Vol. 2, 1991, pp. 52-56.
- L. Kong and Y. Shen, “Gas Sensing Property of and Mechanism of CaxLa1-xFeO3 Ceramics,” Sensors and Actuators B, Vol. 30, No. 1, 1996, pp. 217-221. doi:10.1016/0925-4005(96)80052-9
- W. H. Tao and C. H. Tasi, “H2S Sensing Properties of Noble Metal Doped WO3 Thin Film Sensor Fabricated by Micromachining,” Sensors and Actuators B, Vol. 81, No. 2-3, 2002, pp. 237-247. doi:10.1016/S0925-4005(01)00958-3
- J. Q. Xu, X. H. Wang and J. N. Shen, “Hydrothermal Synthesis of In2O3 for Detecting H2S,” Sensors and Actuators B, Vol. 115, No. 2, 2006, pp. 642-646. doi:10.1016/j.snb.2005.10.038
- C. H. Wang, X. F. Chu and M. M. Wu, “Detection of H2S Down to ppb Levels at Room Temperature Using Sensors Based on ZnO Nanorods,” Sensors and Actuators B, Vol. 113, No. 1, 2006, pp. 320-323. doi:10.1016/j.snb.2005.03.011
- Y. L. Liu, H. Wang, Y. Yang, Z. M. Liu, H. F. Yang, G. L. Shen and R. Q. Yu, “Hydrogen Sulfide Sensing Properties of NiFeO4 Nanopowder Doped with Noble Metal,” Sensors and Actuators B, Vol. 102, No. 1, 2004, pp. 155-161. doi:10.1016/j.snb.2004.04.014
- X. F. Zhu, Q. Zhong, X. J. Zhao and H. Yan, “Synthesis and Performance of Y-Doped La0.7Sr0.3CrO3 as A Potential Anode Material for Solid Oxygen Fuel Cells,” Applied Surface Science, Vol. 257, No. 6, 2011, pp. 1967- 1971. doi:10.1016/j.apsusc.2010.09.036
- A. Martinelli, M. Ferretti and M. R. Cimberle, “The Crystal & Magnetic Structure of Ti-Substituted LaCrO3,” Materials Research Bulletin, Vol. 46, No. 2, 2011, pp. 190- 193. doi:10.1016/j.materresbull.2010.11.016
- X. Ding, Y. Liu, L. Gao and L. Guo, “Synthesis and Characterization of Doped LaCrO3 Perovskite Prepared by EDTA-Citrate Complexing Method,” Journal of Alloys and Compounds, Vol. 458, No. 1-2, 2008, pp. 346- 350. doi:10.1016/j.jallcom.2007.03.110
- B. D. Cullity, “Elements of X-Ray Diffraction,” 2nd Edition, Addison Wesley, New York, 1978, pp. 155-165.
- T. G. Nenov and S. P. Yordanov, “Ceramic Sensors, Technology and Applications,” Technomic Publishers, Lanscaster, 1996, pp. 138-144.
- V. E. Henrich and P. A. Cox, “The Surface Science of Metal Oxides,” Cambridge University Press, Cambridge, 1994, pp. 257-264.
- X. Liu, B. Cheng, J. F. Hu, H. W. Qin and M. H. Jiang, “Preparation, Structure, Resistance and Methane-Gas Sensing Properties of Nominal La1−xMgxFeO3,” Sensors and Actuators B, Vol. 133, No. 1, 2008, pp. 340-344. doi:10.1016/j.snb.2008.02.033
NOTES
*Corresponding author.

