Open Journal of Inorganic Non-metallic Materials
Vol. 2 No. 4 (2012) , Article ID: 23427 , 6 pages DOI:10.4236/ojinm.2012.24007
Synthesis, Characterization and Antimicrobial Screening Mixed-Ligand Cu(II) and Zn(II) Complexes: DNA Binding Studies on Cu(II) Complex
Chemistry Department, Education College for Women, Al-Anbar University, Ramadi, Iraq
Email: dromaralobaidi@yahoo.com
Received July 1, 2012; revised August 9, 2012; accepted August 30, 2012
Keywords: Mixed-Ligand Complexes; Thiourea; Thioacetamide; DNA Binding
ABSTRACT
Several mixed ligand Cu(II), Zn(II) complexes using (benzylidenethiourea) (obtained by the condensation of benzaldehyde and thiourea) as the primary ligand and (acetamide or thioacetamide) as an additional ligand were synthesized and characterized analytically and spectroscopically, magnetic susceptibility and molar conductance measurements ,as well as by UV-Vis and IR spectroscopy. The interaction of the complexes with calf thymus (CT) DNA was studied using absorption spectra, while the concentration of DNA in gel electrophoresis remained constant at 10 µl. They exhibit absorption hypochromicity increased during the binding of the complexes to calf thymus DNA. The complexes show enhanced antimicrobial activities complexes with the free ligand. A theoretical treatment of the formation of complexes in the gas phase was studied. This was done by using the HYPERCHEM-6 program for the Molecular mechanics and Semi-empirical calculations.
1. Introduction
The therapeutic and diagnostic properties of transition metal complexes have attracted considerable attention leading to their application in many areas of modern medicine [1]. Many coordination compounds of transition metal ions accomplish nucleolytic cleavage [2]. In this regard, mixed-ligand metal complexes were found to be particularly useful because of their potential to bind DNA via a multitude of interactions and to cleave the duplex by virtue of their intrinsic chemical, electrochemical, and photochemical reactivities [3-9]. A singular advantage in the use of these metallo—intercalators for such studies is that the ligands or the metal ion in them can be varied in an easily controlled manner to facilitate individual applications [10,11]. although DNA interactions of number of mixedligand complexes previously appeared in the literature, there is still scope to design as study Schiff base with metal salt as new chemical nucleases. Bearing these facts in mind, the nuclease activity of mixed ligand complexes of Cu(II) and Zn(II) containing Schiff base is reported in here. DNA binding was also searched. Hence, the present study is very valuable in understanding the mode of complex binding to DNA, as well as laying the foundation for the rational design of DNA structure and antitumor drugs.
2. Experimental
2.1. Instrumentation
A Fisher-100 infrared spectrophotometer was used to recorded the ir spectra as KBr and CsI disc. UV/VIS spectra were measured by a BIOTECH 80D spectrophotometer, Elemental Analysis (C.H.N) founded on (Carlo Erloa microanalyizer type 1106), determination of all metals percentage by atomic absorption spectrophotometry on AA-680G (Shimadzu). Electrical conductance was measured on conductivity CDC304 (Jenway4070). Melting points determined by an electric heated block apparatus (Gallen Kamp), and were uncorrected. Room temperature magnetic susceptibility measurements were carried out on a B.M 6 BRUKER type magnets, balance, Diamagnetic correction was done using pascal constants [12].
2.2. Materials
All chemicals used in the present work and “CuCl2·6H2O, ZnCl2.6H2O” were of analytical reagent grade ( produced by Merk, Germany). Commercial solvents were distilled and then used for the preparation of ligand and its complexes. Calf-Thymus (CT) DNA and pUC19 DNA were obtained from Sigma. All other common chemicals and solvents were procured from locally available sources. All solvents were purified before use as per standard procedures [13], deionized, triply distilled water used for preparing various buffers.
2.3. Preparation of the Ligands
The ligands L1 and L2 were prepared by the reaction of (benzaldehyde) with (thiourea), according to the literature [14]. The physical properties of these compounds (L1) are yellow color, yield: 77%, M.P: 166˚C - 168˚C, Elemental analysis: (%cal.) %found C: (53.31) 53.25, H: (4.25) 4.53, N: (15.85) 15.36. The characters ir bands and Uv-Vis spectrum in DMSO are shown in Table 1. The preparation of the Schiff base is schematically presented in Figure 1.
2.4. Synthesis of the [Cu(L1)2 (L2)]Cl2 and [Zn(L1)2(L3)]Cl2 Complexes
The complexes were prepared by mixing the appropriate molar quantities of the ligand and the metal salts using the following procedure. A methanolic solution of (L1) Schiff base (0.003 mol) was stirred with a methanolic solution (5 mL) of the required anhydrous metal (II) chloride (0.003 mol) for ca 1 hr. To the above mixture, a methanolic solution (5 mL) of (L2)/(L3) (0.006 mol) was added in a 1:2:1 molar ratio and the stirring was continued for ca. 1 hr. The obtained solid product was filtered and washed with methanol. The physical properties of prepared complexes are listed in Table 2.
2.5. DNA-Binding Experiments
The interactions between the metal complexes and DNA were studied using electronic absorption method. The disodium salt of calf thymus was stored at 4˚C. A solution of DNA in the buffer 50 mmol/L NaCl—5 mmol/L tris-HCl (pH 7.2) in water gave a ratio 1.9 of the UV absorbance at 260 and 280 nm, A260/A280, indicating that the DNA was sufficiently free from protein [15]. The concentration of DNA was measured using its extinction coefficient at 260 nm (6600 mol–1·L·cm–1) after a 1:100 dilution. Stock solutions were stored at 4˚C and used no more than 4 days. Doubly distilled water was used to prepare the solutions. Concentrated stock solutions of the complexes were prepared by dissolving the complexes in DMSO and diluting suitably with the corresponding buffer to the required concentration for all the experiments.
2.6. pUC19 DNA Cleavage Study
The cleavage of pUC19 DNA was determined by agarose gel electrophoresis. The gel electrophoresis experiments were performed by incubation of the samples containing 30 µmol/L pUC19 DNA, 50 µmol/L copper complex in tris-HCl/NaCl buffer (pH 7.2) at 37˚C for 2 hr. After incubation, the samples were electrophoresed for 2 h at 50 V on 1% agarose gel using Tris-acetic acid-EDTA buffer (pH 7.2). The gel was than stained using 1 µg·cm–3 ethidium bromide (EB) and photographed under 360 nm ultraviolet light. All experiments were performed at room temperature unless otherwise stated.

Figure 1. Synthesis of the schiff base.

Table 1. Infrared and UV-Visible spectra data of the prepared compounds.

Table 2. Physical characterization, analytical and molar conductance data of the compounds.
2.7. Antibacterial Activity Studies
The in vitro antibacterial activity of the ligand and its complexes were tested against the bacteria Staphylococcus aureus and Escherichia coli by the paper disc method using nutrient agar as the medium. The extract loaded discs inoculated with microorganisms were incubated at 30˚C for 24 h for the bacteria. During the incubation period, the test solution diffused and the growth of the inoculated microorganisms was affected.
3. Result and Discussion
The ligands L1 and L2/L3 and the mixed ligand complexes were stable in air. The higher molar conductance values of the complexes in DMSO show their electrolytic nature. All the data are given in Table 2. The structural formulas of the complexes are shown in Figure 2. Thus, the spectral data reinforced the conclusion drawn from the analytical and conductance values.
3.1. IR Spectra [16-21]
The IR spectrum of the free ligand (L1) showed abroad band around 3257 cm–1, which can be attributed to N-H stretching vibration of the Schiff base moiety. The position of this band remained at nearly the same frequency in spectra of the metal complexes, suggesting the uncoordination of this group. The band at 1240 cm–1 in the spectrum of the free ligand, assigned to υC=S of Schiff base moiety, shifted towards lower values in the com- plexes, indicating the coordination of the sulpher atom of the ligand L1 residue. The C=N stretching frequency of the Schiff base liagnd appears in the region 1613 - 1608 cm–1, which was shifted towards lower values in all the complexes, indicating the involvement of the -C=Nnitrogen in coordination to the central metal ion. The band at 1620 cm–1 in the spectrum of the free ligand L2, assigned to υC=O, shifted towards lower values in the complexes, indicating the coordination of the carbonyl oxygen atom of the ligand L2 residue. The appearance of three new bands in the regions 480 - 470 and 540 - 530 and 370 - 400 cm–1 in the spectra of the complexes, due to υM-N, υM-O and υM-S stretching vibrations, respec-
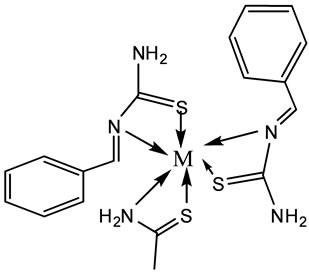

Figure 2. The structural formulas of the complexes [M (L1)2(L2)]Cl2 and [M(L1)2(L3)]Cl2, M=Cu(II) or Zn (II).
tively, also confirmed the formation of metal complexes. The characteristic IR data are presented in Table 2.
3.2. Electronic Absorption Spectra [22-25]
The UV-Vis Spectra of the complexes were recorded in DMSO solution. In addition, the complexes exhibited intense bands in the 215 - 265 nm region, which are attributed to charge transfer transitions. The intense higher energy bands at around 215 nm can be attributed to intra-ligand π-π* transitions. The copper complexes showed magnetic moment values in the range of 1.85 - 1.88 µB which indicate the monomeric nature of the complexes and characteristics for a distorted octahedral structure. The zinc (II) complexes showed bands at 215 and 260 nm, assigned to intra-ligand charge transfer transitions. They are diamagnetic.
Based on the magntic moment, electronic and IR data, the proposed structures of the complexes are given in Figure 2.
3.3. Theoretical Study
The ball and cylinders and some of selected structural parameters (bond length and angles) of the optimized geometries are shown in Figure 3 and Table 3.
As shown in this figure, there is no obvious trend for the variation of these parameters. The values of the bond length and angles of the optimized geometries are quite similar to the experimental results of the corresponding compounds.
3.4. CT-DNA Binding Studies
Electronic absorption titration. The binding interaction of the metal complexes with DNA was monitored by UVVis Spectroscopy. The absorption spectra of the complexes in the presence or without DNA were mutually compared , which is shown in Figure 4. In the UV region

Figure 3. The optimized structural geometry of Cu(II) complexes.


Table 3. Structural parameters, bond length (˚A) and angles (˚) of the [Ni2(L2)2Cl4] complexe.
of the spectra, all the copper complexes exhibited an intense absorption around 265 nm, which is due to n-π* transitions with increasing concentration of DNA, both the copper complexes showed hyperchromicity and a redshifted charge transfer peak maxima in the absorption spectra.
The hypochromicity values of the complexes [Cu (L1)2L2]Cl2, observed in the presence of DNA. The change in the absorbance values with increasing amount of DNA which are given in Figure 4.
The change in hyperchromicity may be attributed to the nature of the binding of the mixed ligand complexes with DNA, which is significant due to π-stacking or hydrophobic interactions of the aromatic phenol ring. However, the metal ions play crucial role in DNA binding by these complexes.
3.5. Chemical Nuclease Activity
The cleavage efficiency of the complexes compared to that of the control is due to their efficient DNA – binding ability. DNA cleavage was controlled by relaxation of the supercoiled from of pUC19 DNA into the nicked circular from and linear form. When pUC19 DNA is subjected to electrophoresis, the fastest migration will be observed for the super coiled form (form I). If one stand

Figure 4. Absorption spectrum of [Cu(L1)2(L2)]Cl2 in the absence (top curve) and presence (subsequent curve) of increasing concentrations of CT DNA(0 - 400 μM). A graph within a graph is an “inset”, not an “insert”. The word alternatively is preferred to the word “alternately” (unless you really mean something that alternates).

Figure 5. Changes in the agarose gel electrophoretic pattern of pUC19 DNA and [Cu(L1)2(L2)]Cl2 complexes. Lane 1, DNA alone; Lane 12 Ligand alone; Lanes 2,3 and 4: pUC19 DNA + [Cu(L1)2(L2)]Cl2 (10, 20, 30) μM respectively. Lanes 5, 6 and 7: pUC19 DNA + [Cu(L1)2(L2)]Cl2 (40, 50, 60 ) μM respectively. Lanes 8, 9 and 10: pUC19 DNA + [Cu(L1)2(L2)]Cl2 (70, 80, 90) μM respectively. Lanes 11, 13, 15 and 16: pUC19 DNA + [Cu(L1)2(L2)]Cl2 (100, 110, 120) μM respectively.
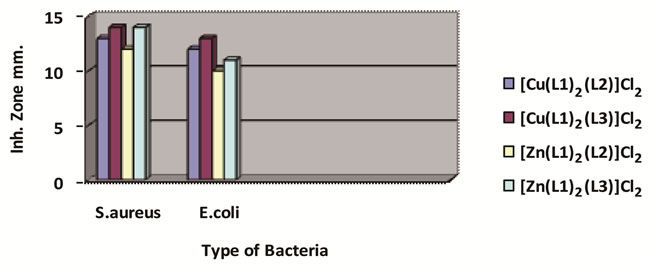
Figure 6. The effect of ligands and their metal complexes toward bacteria.
is cleavage, the supercoils relax to produce the slowermoving open circular form (form II).
DNA cleavage was analyzed by monitoring the conversion of supercoiled DNA (form I) to nocked DNA (form II). The electrophoresis results are shown in Figure 5.
3.6. Antimicrobial Screening
The synthesized ligand and its complexes were tested for their in vitro antimicrobial activity. They were tested against the bacterial Staphylococcus aureus and Escherichia coli. A comparison of the value of L with these of the complexes indicates that the metal complexes exhibited higher antibacterial activity then ligands. Such increased activity of the complexes can be explained based on Overtones concept [26] and the tweedy chelation theory [27]. Morover, the copper complexes were more active than the zinc complexes against the tested microorganisms as shown in Figure 6.
4. Conclusion
Four mixed ligand Cu(II) and Zn(II) complexes of benzaldehyde and Thiourea Schiff base (L1) and acetamide, Thioacetamide are synthesized and characterized by various physicochemical studies such as elemental analysis, molar conductivity, magnetic susceptibility, electronic and FT-IR, UV-Vis spectra studies confirmed the bonding features of the studied mixed ligand complexes as an octahedral geometry proposed for all of these complexes as shown in Figure 2. The results of cleavage studies using pUC19 DNA shoued that the complexes had higher nuclease activities than the ligand.
REFERENCES
- C. B. Spillane, M. N. F. Dabo, N. C. F. Fletcher, J. L. Morgan, R. Keen, I. Haq and N. J. Buurma, “The Dichotomy in the DNA-Binding Behaviour of Ruthenium(II) Complexes Bearing Benzoxazole and Benzothiazole Groups,” Journal of Biological Inorganic Chemistry, Vol. 102, No. 4, 2008, p. 673.
- M. S. S. Babu, P. G. Krishna, K. Hussain Reddy, G. H. Philip, “Synthesis, Characterization and DNA Cleavage Activity of Nickel(II) Adducts with Aromatic Heterocyclic Bases,” Journal of the Serbian Chemical Society, Vol. 75, No. 1, 2010, p. 61.
- D. S. Sigma, A. Mazumder and D. M. Perrin, “Chemical Nucleases,” Chemical Reviews, Vol. 93, No. 6, 1993, pp. 2295-2316. doi:10.1021/cr00022a011
- T. Ghosh, B. G. Maiya and A. Samanta, “Mixed-Ligand Complexes of Ruthenium(II) Containing New Photoactive or Electroactive Ligands: Synthesis, Spectral Characterization and DNA Interactions,” Journal of Biological Inorganic Chemistry, Vol. 10, No. 5, 2005, pp. 496-508. doi:10.1007/s00775-005-0660-6
- N. J. Turro, J. K. Barton and D. A. Tomalia, “Molecular Recognition and Chemistry in Restricted Reaction Spaces. Photophysics and Photoinduced Electron Transfer on the Surfaces of Micelles, Dendrimers, and DNA,” Accounts of Chemical Research, Vol. 24, No. 11, 1991, pp. 332-340. doi:10.1021/ar00011a003
- F. Q. Liu, Q. X. Wang, K. Jiao, F. F. Jian, J. Y. Liu and R. X. Li, “Synthesis, Crystal Structure, and DNA-Binding Properties of a New Copper (II) Complex Containing Mixed-Ligands of 2,2’-Bipyridine and p-Methylbenzoate,” Inorganica Chimica Acta, Vol. 395, No. 5, 2006, pp. 1524-1530. doi:10.1016/j.ica.2005.12.035
- T. F. Tullis, “Metal-DNA Chemistry,” ACS Symposium Series No. 402, ACS, Washington DC, 10 August 1989.
- J. K. Barton, “Metals and DNA: Molecular Left-Handed Complements,” Science, Vol. 233, No. 4765, 1986, p. 727.
- S. P. Singh, S. K. Shukla and L. P. Awasthi, “Synthesis of Some 3-(4'-Nitrobenzoylhydrazone)-2-indolinones as Potential Antiviral Agents,” Current Science, Vol. 52, 1983, p. 766.
- S. Arounaguiri and B. G. Maiya, “Electro-Photo Switch” and “Molecular Light Switch” Devices Based on Ruthenium(II) Complexes of Modified Dipyridophenazine Ligands: Modulation of the Photochemical Function through Ligand Design,” Inorganic Chemistry, Vol. 38, No. 5, 1999, pp. 842-843. doi:10.1021/ic981109z
- R. Pulimamidi, R. Nomula and R. Karnativ, “The Effect of Oven-Heat Flux on Powder-Coating Issues of Sheet Molding Compound Panels,” Industrial & Engineering Chemistry Research, Vol. 48, No. 3, 2009, pp. 1638-1649. doi:10.1021/ie801130g
- M. J. M. Campbell, D. W. Card and R. Grzeskowiak, “Cobalt(II) and Nickel(II) Halide Complexes of 2-Aminobenzothiazole,” Inorganic and Nuclear Chemistry Letters, Vol. 5, No. 1, 1969, pp. 39-43. doi:10.1016/0020-1650(69)80234-5
- D. D. Perrin, W. L. F. Armarego, D. R. Perrin, “Purification of Laboratory Chemicals,” 2nd Edition, Pergamone Press, New York, 1980.
- A. Quiroga, J. Perez, E. Montero, et al., “Palladated and Platinated Complexes Derived from Phenylacetaldehyde Thiosemicarbazone with Cytotoxic Activity in cis-DDP Resistant Tumor Cells. Formation of DNA Interstrand Cross-Links by These Complexes,” Journal of Inorganic Biochemistry, Vol. 70, No. 2, 1998, pp. 117-123. doi:10.1016/S0162-0134(98)10007-7
- J. A. Marmur, “A Procedure for the Isolation of Deoxyribonucleic Acid from Micro-Organisms,” Journal of Molecular Biology, Vol. 3, No. 2, 1961, pp. 208-218. doi:10.1016/S0022-2836(61)80047-8
- N. Ramman, Y. P. Raja and A. Kulandaisory, “Synthesis and Characterization of Cu(II) , Ni(II), Mn(II), Zn(II) and VO(II) Schiff Base Complexes Derived from Acetoacetalnilililde and O-Phenylenediamine,” India Academy of Science, Vol. 113, No. 3, 2001, pp. 183-189.
- D. H. Williamsand and I. Fleming, “Spectroscopic Methods in Organic Chemistry,” Mc. Graw Hill, London. 1989.
- O. Bostoup and C. K. Jørgensen, “The Decrease of FkIntegrals, Tetragonal Perturbations, and Effects of Average Environment in the Reflection Spectra of Nickel(II) Complexes,” Acta Chemica Scandinavica, Vol. 11, 1957, pp. 1223-1231. doi:10.3891/acta.chem.scand.11-1223
- A. B. P. Lever, “The Electronic Spectra of Tetragonal Metal Complexes Analysis and Significance,” Coordination Chemistry Reviews, Vol. 3, No. 2, 1968, pp. 119-140. doi:10.1016/S0010-8545(00)80107-1
- M. M. Mostava, A. M. Shallaby and A. A. El. Asmy, “Transition Metal Complexes of 4-Phenylthiosemicarbazide,” Journal of Inorganic and Nuclear Chemistry, Vol. 43, No. 11, 1981, pp. 2992-2995.
- K. Nakamoto, “Infrared and Raman Spectra of Inorganic and Coordination Compoundes,” Wiley-Interscience, New York, 1977.
- M. A. Pujar, B. S. Hadimani, S. Meonakumari, S. M. Gaddad and Y. F. Neelgund, “Azo and Schiff Bases and Their Metal Complexes as Antibacterial Compounds,” Current Science, Vol. 55, No. 7, 1986, p. 353.
- A. B. R. Lever, “Crystal Field Spectra: Inorganic Electronic Spectroscopy,” Elsevier, Amsterdam, 1968, pp. 249-360.
- P. Fleteher, “Practical Methods of Optimization,” Wiley, New York, 1990.
- F. A. Cotton and G. Wilkinson, “Advanced Inorganic Chemistry,” 5th Edition, Wiley, New York, 1988.
- Y. Anjaneyalu and R. P. Rao, “Preparation, Characterization and Antimicrobial Activity Studies on Some Ternary Complexes of Cu(II) with Acetyl Acetone and Various Salicylic Acids,” Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry, Vol. 16, No. 3, 1986, pp. 257-272.
- L. Mishra, A. K. Pandey and U. C. Agarwala, “Co(II), Ni(II), Cu(ll) and Zn(II) Complexes of Benzimidazole Derivatives and Dimeric Adduct of Co(II) with 2-(-thiomethyl)-2’-benzimidazolyl) Benzimidazole,” Indian Journal of Chemistry, Vol. 32A, 1993, p. 446.

