 Open Journal of Gastroenterology, 2013, 3, 25-34 OJGas http://dx.doi.org/10.4236/ojgas.2013.31004 Published Online February 2013 (http://www.scirp.org/journal/ojgas/) Risk factors for internal anal sphincter dysfunction in Japanese adults Tatsuya Abe1*, Toru Kono2,3, Yoshikazu Hachiro1, Masao Kunimoto1, Hiroyuki Furukawa2 1Department of Proctology, Kunimoto Hospital, Asahikawa, Japan 2Division of Gastroenterologic and General Surgery, Department of Surgery, Asahikawa Medical University, Asahikawa, Japan 3Advanced Surgery Center, Sapporo Higashi Tokushukai Hospital, Sapporo, Japan Email: *t-abe@cf6.so-net.ne.jp Received 13 December 2012; revised 13 January 2013; accepted 20 January 2013 ABSTRACT Purpose: The internal anal sphincter provides most of the resting anal tone and is the main muscle response- ble for continence. This study was designed to esti- mate the prevalence of, and identify risk factors asso- ciated with, internal anal sphincter dysfunction in Japanese adults. Methods: Anorectal manometry was performed in 1193 women and 1124 men aged 20 years or older. The maximal resting pressure, meas- ured by a rapid pull-through technique, was defined as the highest resting pressure recorded. Internal anal sphincter dysfunction was defined as a maximal resting pressure less than 30 mmHg. Potential risk factors were assessed through self-reports, interviews, physical examinations, and medical record reviews. Multivariate logistic regression analysis was used to identify independent risk factors for internal anal sphincter dysfunction. Results: Significant differences in maximal resting pressure wer e seen bet ween w omen (58.1 ± 24.9 mmHg) and men (68.8 ± 23.5 mmHg, P < 0.001). Maximal resting pressure decreased signifi- cantly with increasing age in both sexes. The preva- lence of internal anal sphincter dysfunction was 1 0 .4% (15.5% in women, 5.1% in men). In a multivariate logistic regression model, age, mental disease, pelvic organ prolapse repair, and fecal incontinence were independently associated with a greater risk of inter- nal anal sphincter dysfunction in women and men. Conclusions: Internal anal sphincter dysfunction is a common problem for women and men. Several of the identified risk factors are preventable or modifiable, and may direct future research in fecal incontinence therapy. Keywords: Fecal Incontinence; Internal Anal Sphincter; Anorectal Manometry; Maximal Resting Pressure 1. INTRODUCTION Fecal incontinence (FI) is a common disorder with a sig- nificant impact on quality of life. The prevalence of FI in the general population increases with age in both women and men [1]. Although the cause of FI is often multifac- torial, FI is frequently caused by anal sphincter insuffi- ciency [2]. The anal sphincter consist of the circular in- ternal (IAS) and external (EAS) anal sphincter muscles together with the sling-shaped puborectalis muscle. Al- though both sphincter muscles are important for the main- tenance of continence, the IAS, composed of smooth muscle arranged in oblique bundles, provides most of the resting anal tone and is the main muscle responsible for preventing fecal leakage. The IAS contributes an esti- mated 55% - 85% to maximal resting pressure (MRP) [3,4]. Low MRP is the most important predictor of FI and correlates with the Fecal Incontinence Severity Index (FISI) [5]. Despite a number of studies on FI in western countries [6-8], there has been a paucity of research about risk factors associated with IAS dysfunction (IASD). Identi- fying preventable or modifiable risk factors for IASD may guide future research for the prevention or treatment of FI. The objective of this study was to provide a compre- hensive description of IASD in Japanese adults and to describe demographic and other risk factors associated with IASD after multivariate adjustments. 2. MATERIALS AND METHODS Between January 2006 and December 2008, 3190 con- secutive subjects aged 20 years or older referred to our hospital were enrolled. Factors potentially associated with IASD or FI were assessed by questionnaires, interviews, medical record reviews, and physical examinations. Participants filled in a structured questionnaire, which included questions about demographic characteristics (age, sex, body mass *Corresponding author. OPEN ACCESS  T. Abe et al. / Open Journal of Gastroenterology 3 (2013) 25-34 26 index (BMI), employment), stool consistency, bowel movements, presence of selected medical conditions, previous anorectal surgeries, previous cholecystectomy, current habits, laxative use, FI, and parity in women. All medical conditions and medication use were determined according to self-reports as well as in-person interviews during which written responses were clarified. Patient weight and height were measured, and BMI was catego- rized according to the classification proposed for Japa- nese adults. The validated Bristol Stool Scale was used to describe the participant’s usual stool consistency [9]. This scale consists of 7 types of stool and includes pic- tures of each stool type to aid participants in classifying their stools. We combined stool type 1 and 2 (hard and lumpy), 3 - 5 (normal consistencies), and 6 and 7 (mushy and watery). Smoking status was categorized as non- smoker and current smoker (≥1 cigarettes per day). Al- cohol intake was categorized as nondrinker and current drinker (more than once a week). For this study, FI was defined as any involuntary loss of mucus, liquid, or solid stool during the last 30 days; this definition of FI did not include gas. Anorectal manometry (ARM) was performed by a sin- gle experienced examiner using a 5 mm diameter, 1- channel solid-state catheter with a microtipped trans- ducer ARM system (P-31, Star Medical Co., Tokyo, Ja- pan). All subjects were examined in the left lateral posi- tion with the hips flexed to 90˚. The lubricated catheter was introduced into the rectum. The MRP measured by means of a rapid pull-through technique was defined as the highest resting pressure recorded. IASD was defined as “low MRP” in cases where MRP was less than 30 mmHg [10]. Statistical analysis was performed using JMP version 9.0.2 (SAS Institute Inc, Cary, NC). MRP values were expressed as means ± standard deviation. The Mann- Whitney U test was used to analyze for sex differences in MRP, and regression analysis was used to search for as- sociations with age. The chi-square test was used to test the association between low MRP and each risk factor individually. All risk factors found to be significantly associated with low MRP in univariate analyses were combined into separate multivariate logistic models for women and men. Adjusted odds ratios (OR) and 95% confidence intervals (CI) were used to describe associa- tions between risk factors and low MRP in univariate analyses and in multivariate analyses. P values < 0.05 were considered to indicate statistical significance for all analyses. This study was a retrospective review of existing clinical data prospectively collected on a hospital anal physiology unit computer database. This study was ap- proved by the research and ethics committee of Kuni- moto Hospital and informed consent for ARM and future use of their data was provided by all participants in the study. 3. RESULTS Data from 2317 subjects (1193 women and 1124 men) who gave complete responses were analyzed. Mean age was 56.0 ± 17.9 years in women and 53.2 ± 16.1 years in men. All participants were stratified into 10-year age groups. The general characteristics of the participants are shown in Ta bl e 1 . The most common medical condition was hypertension (26.8%). FI was reported by 15.1% of women and 7.7% of men. Significant differences in MRP were seen between women (58.1 ± 24.9 mmHg) and men (68.8 ± 23.5 mmHg; P < 0.001). MRP decreased significantly with increasing age in both sexes (Figure 1). The prevalence of low MRP was 10.4% (15.5% in women, 5.1% in men). Among women, prevalence in- creased from 1.7% for 20 - 29 year-olds up to 52.4% for 80 years or older. For women and men combined, the most common medical conditions associated with low MRP were cognitive impairment and pelvic organ prolapse repair (Tables 2 and 3). In univariate analyses, variables associated with low MRP in women and men were age, employment status, hypertension, stroke, cognitive impairment, mental dis- ease, ischemic heart disease, any history of cancer, pelvic organ prolapse repair, laxative use, and FI. In addition, parity, dyslipidemia, spinal cord injury, anal fistula sur- gery, and hysterectomy were associated with low MRP in women but not in men, while BMI, usual stool consis- tency, diabetes mellitus, hemorrhoidectomy, and rectal surgery were associated with low MRP in men alone. In a multivariate logistic regression model, age, men tal disease, pelvic organ prolapse repair, and FI were independently associated with a greater risk of low MRP in women and men. In addition, ischemic heart disease was associated with low MRP in women alone. By con- trast, smoking or alcohol intake and diabetes mellitus were significantly negative associated with low MRP in women (Tables 4 and 5). 4. DISCUSSION The present study investigated the influence of age, sex, and other possible risk factors for low MRP in a large number of Japanese adults spanning a wide age range. The main results are the following: 1) women had sig- nificantly lower MRP than men; 2) MRP decreased with increasing age in both sexes; 3) independent risk factors for low MRP in both sexes include advancing age, FI, mental disease, and pelvic organ prolapse repair. FI is defined as either the involuntary passage of or the inability to control the discharge of fecal matter through Copyright © 2013 SciRes. OPEN ACCESS  T. Abe et al. / Open Journal of Gastroenterology 3 (2013) 25-34 Copyright © 2013 SciRes. 27 OPEN ACCESS Table 1. General characteristics of study subjects. Characteristic Overall n = 2317 Women n = 1193 Men n = 1124 Age (year) 20 - 29 201 (8.7) 117 (9.8) 84 (7.5) 30 - 39 348 (15.0) 149 (12.5) 199 (17.7) 40 - 49 339 (14.6) 157 (13.2) 182 (16.2) 50 - 59 470 (20.3) 216 (18.1) 254 (22.6) 60 - 69 421 (18.2) 231 (19.4) 190 (16.9) 70 - 79 391 (16.9) 220 (18.4) 171 (15.2) ≥80 147 (6.3) 103 (8.6) 44 (3.9) BMI Underweight (<18.5) 140 (6.0) 87 (7.3) 53 (4.7) Normal (18.5 - 24.9) 1628 (70.3) 898 (75.3) 730 (64.9) Overweight (≥25) 549 (23.7) 208 (17.4) 341 (30.3) Parity At least one vaginal 924 (77.4) Employment status Employed/student 1281 (55.3) 470 (39.4) 811 (72.2) Unemployed/other 1036 (44.7) 723 (60.6) 313 (27.8) Usual stool consistency Normal stools 1403 (60.6) 635 (53.2) 768 (68.3) Hard, lumpy stools 688 (29.7) 466 (39.1) 222 (19.8) Loose, watery stools 150 (6.5) 47 (3.9) 103 (9.2) Other 76 (3.3) 45 (3.8) 31 (2.8) Frequency of bowel movements >21 BM/week 113 (4.9) 49 (4.1) 64 (5.7) 3 - 21 BM/week 2001 (86.4) 1001 (83.9) 1000 (89.0) <3 BM/week 203 (8.8) 143 (12.0) 60 (5.3) Comorbid conditions Hypertension 620 (26.8) 317 (26.6) 303 (27.0) Diabetes mellitus 155 (6.7) 75 (6.3) 80 (7.1) Dyslipidemia 257 (11.1) 154 (12.9) 103 (9.2) Stroke 93 (4.0) 44 (3.7) 49 (4.4) Cognitive impairment 29 (1.3) 17 (1.4) 12 (1.1) Mental disease 102 (4.4) 60 (5.0) 42 (3.7) Ischemic heart disease 64 (2.9) 27 (2.3) 37 (3.3) Any history of cancer 108 (4.7) 58 (4.9) 50 (4.4) Bronchial asthma 98 (4.2) 57 (4.8) 41 (3.6) Spinal cord injury 46 (2.0) 22 (1.8) 24 (2.1) Previous surgeries Hemorrhoidectomy 314 (13.6) 156 (13.1) 158 (14.1) Anal fissure surgery 28 (1.2) 16 (1.3) 12 (1.1) Anal fistula surgery 82 (3.5) 16 (1.3) 66 (5.9) Hysterectomy 97 (8.1) Pelvic organ prolapse repair 51 (2.2) 43 (3.6) 8 (0.7) Rectal surgery 16 (0.7) 7 (0.6) 9 (0.8) Cholecystectomy 43 (1.9) 17 (1.4) 26 (2.3) Current habits Smoking 572 (24.7) 194 (16.3) 378 (33.6) Alcohol intake 979 (42.3) 293 (24.6) 686 (61.0) Laxative use 471 (20.3) 347 (29.1) 124 (11.0) Fecal incontinence 266 (11.5) 180 (15.1) 86 (7.7) BMI = body mass index. BM = bowel movements. Data are numbers of subjects with percentages in parentheses. Percentages may not sum to 100 because of rounding. 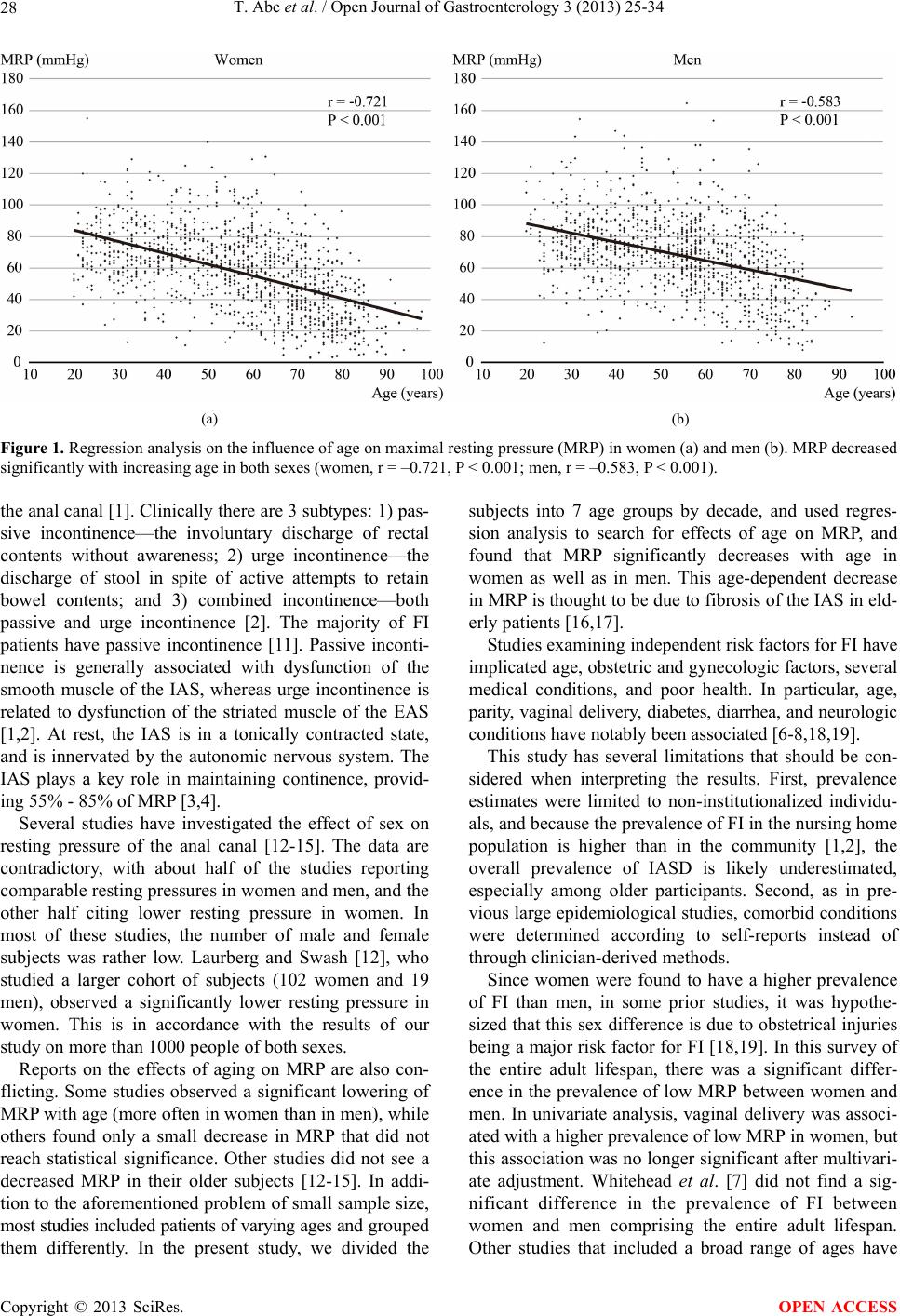 T. Abe et al. / Open Journal of Gastroenterology 3 (2013) 25-34 28 (a) (b) Figure 1. Regression analysis on the influence of age on maximal resting pressure (MRP) in women (a) and men (b). MRP decreased significantly with increasing age in both sexes (women, r = –0.721, P < 0.001; men, r = –0.583, P < 0.001). the anal canal [1]. Clinically there are 3 subtypes: 1) pas- sive incontinence—the involuntary discharge of rectal contents without awareness; 2) urge incontinence—the discharge of stool in spite of active attempts to retain bowel contents; and 3) combined incontinence—both passive and urge incontinence [2]. The majority of FI patients have passive incontinence [11]. Passive inconti- nence is generally associated with dysfunction of the smooth muscle of the IAS, whereas urge incontinence is related to dysfunction of the striated muscle of the EAS [1,2]. At rest, the IAS is in a tonically contracted state, and is innervated by the autonomic nervous system. The IAS plays a key role in maintaining continence, provid- ing 55% - 85% of MRP [3,4]. Several studies have investigated the effect of sex on resting pressure of the anal canal [12-15]. The data are contradictory, with about half of the studies reporting comparable resting pressures in women and men, and the other half citing lower resting pressure in women. In most of these studies, the number of male and female subjects was rather low. Laurberg and Swash [12], who studied a larger cohort of subjects (102 women and 19 men), observed a significantly lower resting pressure in women. This is in accordance with the results of our study on more than 1000 people of both sexes. Reports on the effects of aging on MRP are also con- flicting. Some studies observed a significant lowering of MRP with age (more often in women than in men), while others found only a small decrease in MRP that did not reach statistical significance. Other studies did not see a decreased MRP in their older subjects [12-15]. In addi- tion to the aforementioned problem of small sample size, most studies included patients of varying ages and grouped them differently. In the present study, we divided the subjects into 7 age groups by decade, and used regres- sion analysis to search for effects of age on MRP, and found that MRP significantly decreases with age in women as well as in men. This age-dependent decrease in MRP is thought to be due to fibrosis of the IAS in eld- erly patients [16,17]. Studies examining independent risk factors for FI have implicated age, obstetric and gynecologic factors, several medical conditions, and poor health. In particular, age, parity, vaginal delivery, diabetes, diarrhea, and neurologic conditions have notably been associated [6-8,18,19]. This study has several limitations that should be con- sidered when interpreting the results. First, prevalence estimates were limited to non-institutionalized individu- als, and because the prevalence of FI in the nursing home population is higher than in the community [1,2], the overall prevalence of IASD is likely underestimated, especially among older participants. Second, as in pre- vious large epidemiological studies, comorbid conditions were determined according to self-reports instead of through clinician-derived methods. Since women were found to have a higher prevalence of FI than men, in some prior studies, it was hypothe- sized that this sex difference is due to obstetrical injuries being a major risk factor for FI [18,19]. In this survey of the entire adult lifespan, there was a significant differ- ence in the prevalence of low MRP between women and men. In univariate analysis, vaginal delivery was associ- ated with a higher prevalence of low MRP in women, but this association was no longer significant after multivari- ate adjustment. Whitehead et al. [7] did not find a sig- nificant difference in the prevalence of FI between women and men comprising the entire adult lifespan. Other studies that included a broad range of ages have Copyright © 2013 SciRes. OPEN ACCESS 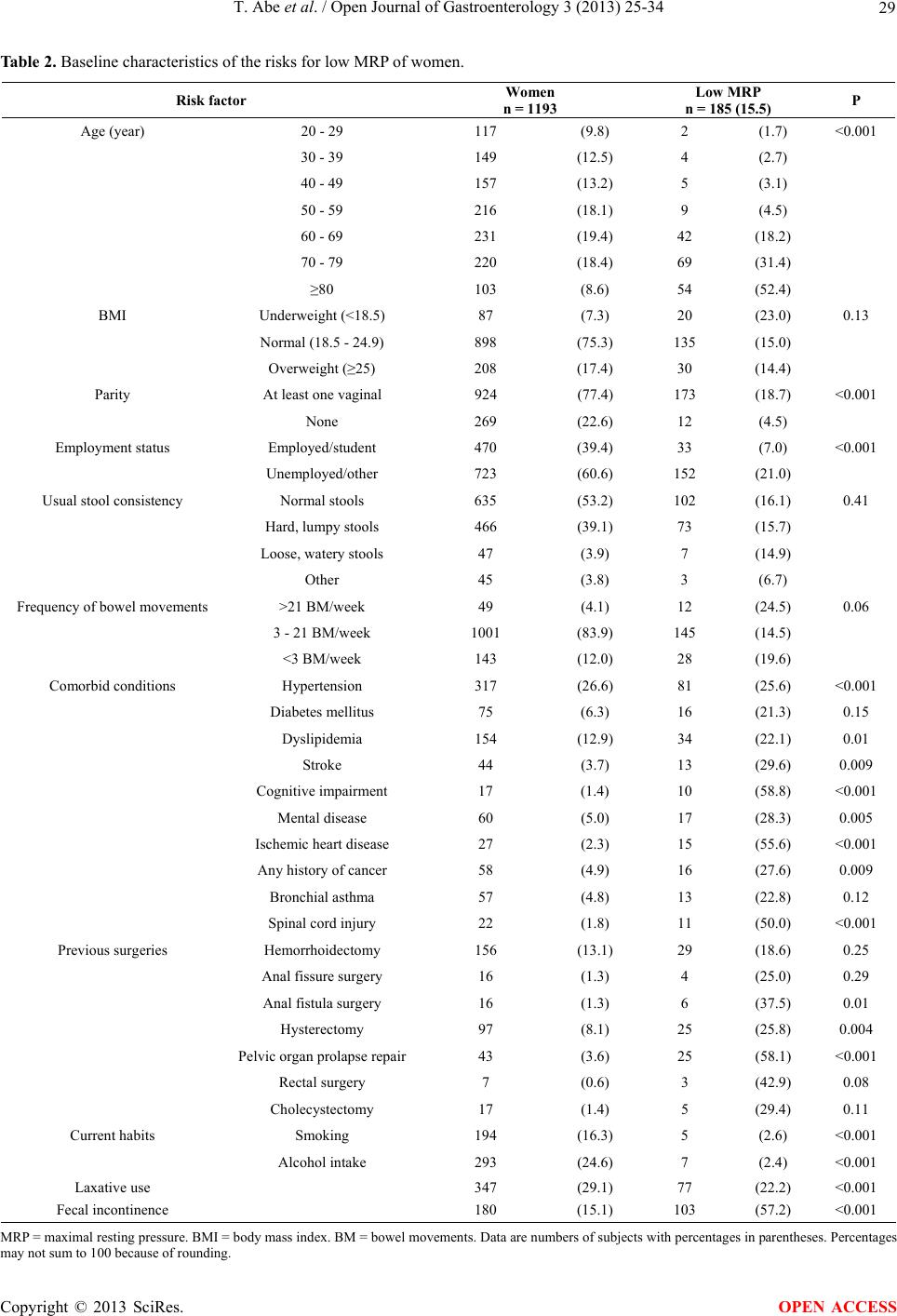 T. Abe et al. / Open Journal of Gastroenterology 3 (2013) 25-34 29 Table 2. Baseline characteristics of the risks for low MRP of women. Risk factor Women n = 1193 Low MRP n = 185 (15.5) P Age (year) 20 - 29 117 (9.8) 2 (1.7) <0.001 30 - 39 149 (12.5) 4 (2.7) 40 - 49 157 (13.2) 5 (3.1) 50 - 59 216 (18.1) 9 (4.5) 60 - 69 231 (19.4) 42 (18.2) 70 - 79 220 (18.4) 69 (31.4) ≥80 103 (8.6) 54 (52.4) BMI Underweight (<18.5) 87 (7.3) 20 (23.0) 0.13 Normal (18.5 - 24.9) 898 (75.3) 135 (15.0) Overweight (≥25) 208 (17.4) 30 (14.4) Parity At least one vaginal 924 (77.4) 173 (18.7) <0.001 None 269 (22.6) 12 (4.5) Employment status Employed/student 470 (39.4) 33 (7.0) <0.001 Unemployed/other 723 (60.6) 152 (21.0) Usual stool consistency Normal stools 635 (53.2) 102 (16.1) 0.41 Hard, lumpy stools 466 (39.1) 73 (15.7) Loose, watery stools 47 (3.9) 7 (14.9) Other 45 (3.8) 3 (6.7) Frequency of bowel movements >21 BM/week 49 (4.1) 12 (24.5) 0.06 3 - 21 BM/week 1001 (83.9) 145 (14.5) <3 BM/week 143 (12.0) 28 (19.6) Comorbid conditions Hypertension 317 (26.6) 81 (25.6) <0.001 Diabetes mellitus 75 (6.3) 16 (21.3) 0.15 Dyslipidemia 154 (12.9) 34 (22.1) 0.01 Stroke 44 (3.7) 13 (29.6) 0.009 Cognitive impairment 17 (1.4) 10 (58.8) <0.001 Mental disease 60 (5.0) 17 (28.3) 0.005 Ischemic heart disease 27 (2.3) 15 (55.6) <0.001 Any history of cancer 58 (4.9) 16 (27.6) 0.009 Bronchial asthma 57 (4.8) 13 (22.8) 0.12 Spinal cord injury 22 (1.8) 11 (50.0) <0.001 Previous surgeries Hemorrhoidectomy 156 (13.1) 29 (18.6) 0.25 Anal fissure surgery 16 (1.3) 4 (25.0) 0.29 Anal fistula surgery 16 (1.3) 6 (37.5) 0.01 Hysterectomy 97 (8.1) 25 (25.8) 0.004 Pelvic organ prolapse repair 43 (3.6) 25 (58.1) <0.001 Rectal surgery 7 (0.6) 3 (42.9) 0.08 Cholecystectomy 17 (1.4) 5 (29.4) 0.11 Current habits Smoking 194 (16.3) 5 (2.6) <0.001 Alcohol intake 293 (24.6) 7 (2.4) <0.001 Laxative use 347 (29.1) 77 (22.2) <0.001 Fecal incontinence 180 (15.1) 103 (57.2) <0.001 MRP = maximal resting pressure. BMI = body mass index. BM = bowel movements. Data are numbers of subjects with percentages in parentheses. Percentages may not sum to 100 because of rounding. Copyright © 2013 SciRes. OPEN ACCESS 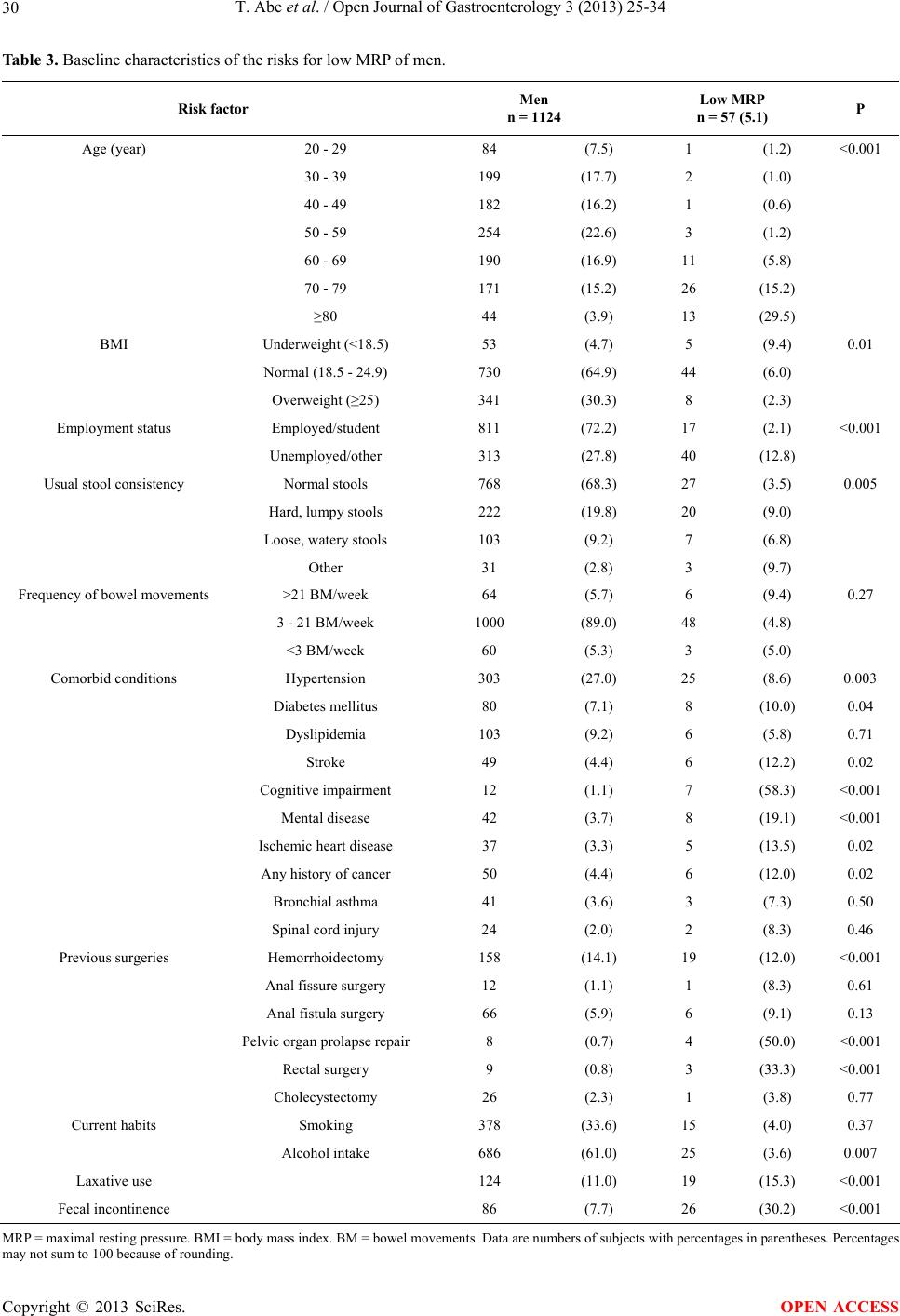 T. Abe et al. / Open Journal of Gastroenterology 3 (2013) 25-34 30 Table 3. Baseline characteristics of the risks for low MRP of men. Risk factor Men n = 1124 Low MRP n = 57 (5.1) P Age (year) 20 - 29 84 (7.5) 1 (1.2) <0.001 30 - 39 199 (17.7) 2 (1.0) 40 - 49 182 (16.2) 1 (0.6) 50 - 59 254 (22.6) 3 (1.2) 60 - 69 190 (16.9) 11 (5.8) 70 - 79 171 (15.2) 26 (15.2) ≥80 44 (3.9) 13 (29.5) BMI Underweight (<18.5) 53 (4.7) 5 (9.4) 0.01 Normal (18.5 - 24.9) 730 (64.9) 44 (6.0) Overweight (≥25) 341 (30.3) 8 (2.3) Employment status Employed/student 811 (72.2) 17 (2.1) <0.001 Unemployed/other 313 (27.8) 40 (12.8) Usual stool consistency Normal stools 768 (68.3) 27 (3.5) 0.005 Hard, lumpy stools 222 (19.8) 20 (9.0) Loose, watery stools 103 (9.2) 7 (6.8) Other 31 (2.8) 3 (9.7) Frequency of bowel movements >21 BM/week 64 (5.7) 6 (9.4) 0.27 3 - 21 BM/week 1000 (89.0) 48 (4.8) <3 BM/week 60 (5.3) 3 (5.0) Comorbid conditions Hypertension 303 (27.0) 25 (8.6) 0.003 Diabetes mellitus 80 (7.1) 8 (10.0) 0.04 Dyslipidemia 103 (9.2) 6 (5.8) 0.71 Stroke 49 (4.4) 6 (12.2) 0.02 Cognitive impairment 12 (1.1) 7 (58.3) <0.001 Mental disease 42 (3.7) 8 (19.1) <0.001 Ischemic heart disease 37 (3.3) 5 (13.5) 0.02 Any history of cancer 50 (4.4) 6 (12.0) 0.02 Bronchial asthma 41 (3.6) 3 (7.3) 0.50 Spinal cord injury 24 (2.0) 2 (8.3) 0.46 Previous surgeries Hemorrhoidectomy 158 (14.1) 19 (12.0) <0.001 Anal fissure surgery 12 (1.1) 1 (8.3) 0.61 Anal fistula surgery 66 (5.9) 6 (9.1) 0.13 Pelvic organ prolapse repair 8 (0.7) 4 (50.0) <0.001 Rectal surgery 9 (0.8) 3 (33.3) <0.001 Cholecystectomy 26 (2.3) 1 (3.8) 0.77 Current habits Smoking 378 (33.6) 15 (4.0) 0.37 Alcohol intake 686 (61.0) 25 (3.6) 0.007 Laxative use 124 (11.0) 19 (15.3) <0.001 Fecal incontinence 86 (7.7) 26 (30.2) <0.001 MRP = maximal resting pressure. BMI = body mass index. BM = bowel movements. Data are numbers of subjects with percentages in parentheses. Percentages may not sum to 100 because of rounding. Copyright © 2013 SciRes. OPEN ACCESS  T. Abe et al. / Open Journal of Gastroenterology 3 (2013) 25-34 Copyright © 2013 SciRes. 31 OPEN ACCESS Table 4. Odds ratios for variables associated with low MRP of women. Risk factor Univariate analysis OR (95% CI) P Multivariate analysis OR (95% CI) P Age (10-year interval) 2.34 (2.04, 2.71) <0.0011.74 (1.44, 2.12) <0.001 BMI (vs. normal) Underweight 1.69 (0.97, 2.82) 0.06 2.05 (0.92, 4.42) 0.08 Overweight 0.95 (0.61, 1.44) 0.82 0.70 (0.41, 1.16) 0.17 Parity (vs. none) 4.93 (2.82, 9.49) <0.0011.83 (0.87, 4.17) 0.11 Employment status (vs. employed/student) Unemployed/other 3.53 (2.40, 5.32) <0.0011.49 (0.86, 2.56) 0.15 Usual stool consistency (vs. normal stools) Hard, lumpy stools 0.97 (0.70, 1.34) 0.86 0.75 (0.46, 1,23) 0.26 Loose, watery stools 0.91 (0.37, 1.98) 0.83 0.93 (0.33, 2.83) 0.89 Other 0.37 (0.09, 1.05) 0.06 0.81 (0.17, 2.92) 0.77 Comorbid condition Hypertension 2.55 (1.84, 3.53) <0.0010.74 (0.46, 1.16) 0.19 Diabetes mellitus 1.52 (0.85, 2.71) 0.15 0.40 (0.18, 0.82) 0.01 Dyslipidemia 1.67 (1.10, 2.53) 0.01 1.41 (0.82, 2.38) 0.21 Stroke 2.38 (1.22, 4.64) 0.009 0.47 (0.19, 1.12) 0.09 Cognitive impairment 8.17 (3.07, 21.8) <0.0010.83 (0.24, 3.07) 0.78 Mental disease 2.27 (1.27, 4.08) 0.005 2.23 (1.00, 4.84) 0.049 Ischemic heart disease 7.32 (3.37, 15.9) <0.0013.14 (1.23, 8.22) 0.02 Any history of cancer 2.18 (1.20, 3.96) 0.009 1.11 (0.47, 2.50) 0.81 Spinal cord injury 5.73 (2.45, 13.4) <0.0012.51 (0.82, 7.58) 0.10 Previous surgeries Hemorrhoidectomy 1.29 (0.83, 2.00) 0.25 0.97 (0.55, 1.65) 0.91 Anal fistula surgery 3.35 (1.20, 9.32) 0.01 1.95 (0.48, 7.04) 0.33 Hysterectomy 2.03 (1.25, 3.30) 0.004 1.57 (0.82, 2.95) 0.17 Pelvic organ prolapse repair 8.59 (4.58, 16.1) <0.0012.48 (1.07, 5.84) 0.03 Rectal surgery 4.14 (0.80, 18.9) 0.08 1.11 (0.17, 6.75) 0.91 Current habits Smoking 0.12 (0.04, 0.27) <0.0010.25 (0.10, 0.56) <0.001 Alcohol intake 0.10 (0.04, 0.20) <0.0010.27 (0.08, 0.72) 0.007 Laxative use 1.95 (1.41, 2.69) <0.0011.29 (0.81, 2.05) 0.29 Fecal incontinence 15.17 (10.5, 22.1) <0.0018.16 (5.20, 12.9) <0.001 also failed to find a sex difference in FI prevalence or an effect of obstetrical injury [6,8,20]. This suggests that obstetrical injuries are not the most common cause of FI in women. Perineal tears are classified into 4 degrees. A fourth-degree tear is defined as an injury that extends through the fascia and musculature of the perineal body and involves the anal sphincter complex (EAS and IAS) and anal epithelium. Third-degree tears involve some or all of the fibers of the EAS, but do not necessarily in- volve the IAS. Faltin et al. [21] reported that clinically undetected tears of the anal sphincter were diagnosed by anal endosonography in 42 of 150 women (28%). EAS injury alone was observed in 30 women (20%), IAS in- jury alone in 2 (1.3%), and both in 10 (7%). In a study of 62 women with FI related to obstetrical procedures, anal endosonography revealed EAS defects in 90% and IAS defects in 65% of the subjects [22]. Our data also sug- gests that EAS injury is the predominant cause of FI after vaginal delivery. Previous studies have found diabetes mellitus to in- crease the risk of FI [8,23]. Ward and Tunuguntla [23] reported that 4% of diabetic patients suffered from FI. 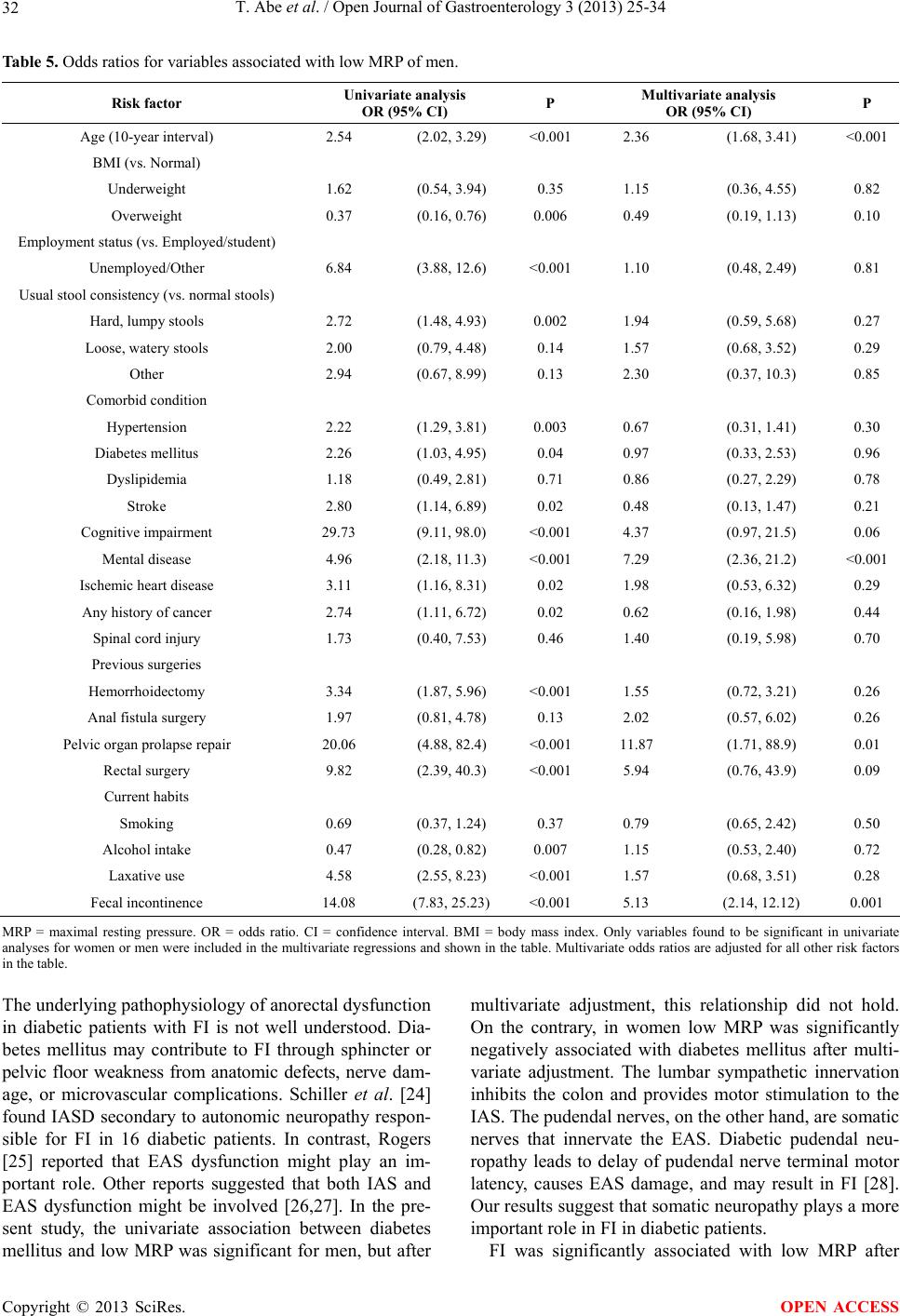 T. Abe et al. / Open Journal of Gastroenterology 3 (2013) 25-34 32 Table 5. Odds ratios for variables associated with low MRP of men. Risk factor Univariate analysis OR (95% CI) P Multivariate analysis OR (95% CI) P Age (10-year interval) 2.54 (2.02, 3.29) <0.0012.36 (1.68, 3.41) <0.001 BMI (vs. Normal) Underweight 1.62 (0.54, 3.94) 0.35 1.15 (0.36, 4.55) 0.82 Overweight 0.37 (0.16, 0.76) 0.006 0.49 (0.19, 1.13) 0.10 Employment status (vs. Employed/student) Unemployed/Other 6.84 (3.88, 12.6) <0.0011.10 (0.48, 2.49) 0.81 Usual stool consistency (vs. normal stools) Hard, lumpy stools 2.72 (1.48, 4.93) 0.002 1.94 (0.59, 5.68) 0.27 Loose, watery stools 2.00 (0.79, 4.48) 0.14 1.57 (0.68, 3.52) 0.29 Other 2.94 (0.67, 8.99) 0.13 2.30 (0.37, 10.3) 0.85 Comorbid condition Hypertension 2.22 (1.29, 3.81) 0.003 0.67 (0.31, 1.41) 0.30 Diabetes mellitus 2.26 (1.03, 4.95) 0.04 0.97 (0.33, 2.53) 0.96 Dyslipidemia 1.18 (0.49, 2.81) 0.71 0.86 (0.27, 2.29) 0.78 Stroke 2.80 (1.14, 6.89) 0.02 0.48 (0.13, 1.47) 0.21 Cognitive impairment 29.73 (9.11, 98.0) <0.0014.37 (0.97, 21.5) 0.06 Mental disease 4.96 (2.18, 11.3) <0.0017.29 (2.36, 21.2) <0.001 Ischemic heart disease 3.11 (1.16, 8.31) 0.02 1.98 (0.53, 6.32) 0.29 Any history of cancer 2.74 (1.11, 6.72) 0.02 0.62 (0.16, 1.98) 0.44 Spinal cord injury 1.73 (0.40, 7.53) 0.46 1.40 (0.19, 5.98) 0.70 Previous surgeries Hemorrhoidectomy 3.34 (1.87, 5.96) <0.0011.55 (0.72, 3.21) 0.26 Anal fistula surgery 1.97 (0.81, 4.78) 0.13 2.02 (0.57, 6.02) 0.26 Pelvic organ prolapse repair 20.06 (4.88, 82.4) <0.00111.87 (1.71, 88.9) 0.01 Rectal surgery 9.82 (2.39, 40.3) <0.0015.94 (0.76, 43.9) 0.09 Current habits Smoking 0.69 (0.37, 1.24) 0.37 0.79 (0.65, 2.42) 0.50 Alcohol intake 0.47 (0.28, 0.82) 0.007 1.15 (0.53, 2.40) 0.72 Laxative use 4.58 (2.55, 8.23) <0.0011.57 (0.68, 3.51) 0.28 Fecal incontinence 14.08 (7.83, 25.23) <0.0015.13 (2.14, 12.12) 0.001 MRP = maximal resting pressure. OR = odds ratio. CI = confidence interval. BMI = body mass index. Only variables found to be significant in univariate analyses for women or men were included in the multivariate regressions and shown in the table. Multivariate odds ratios are adjusted for all other risk factors in the table. The underlying pathophysiology of anorectal dysfunction in diabetic patients with FI is not well understood. Dia- betes mellitus may contribute to FI through sphincter or pelvic floor weakness from anatomic defects, nerve dam- age, or microvascular complications. Schiller et al. [24] found IASD secondary to autonomic neuropathy respon- sible for FI in 16 diabetic patients. In contrast, Rogers [25] reported that EAS dysfunction might play an im- portant role. Other reports suggested that both IAS and EAS dysfunction might be involved [26,27]. In the pre- sent study, the univariate association between diabetes mellitus and low MRP was significant for men, but after multivariate adjustment, this relationship did not hold. On the contrary, in women low MRP was significantly negatively associated with diabetes mellitus after multi- variate adjustment. The lumbar sympathetic innervation inhibits the colon and provides motor stimulation to the IAS. The pudendal nerves, on the other hand, are somatic nerves that innervate the EAS. Diabetic pudendal neu- ropathy leads to delay of pudendal nerve terminal motor latency, causes EAS damage, and may result in FI [28]. Our results suggest that somatic neuropathy plays a more important role in FI in diabetic patients. FI was significantly associated with low MRP after Copyright © 2013 SciRes. OPEN ACCESS  T. Abe et al. / Open Journal of Gastroenterology 3 (2013) 25-34 33 multivariate adjustment in both sexes. More treatment options for FI makes a proper pretreatment evaluation increasingly important, whether by means of subjective severity questionnaires, such as the FISI, or by objective measurements, such as ARM, anal endosonography, electromyography, and defecography [1]. Bordeianou et al. [5] reported MRP was the only objective measure- ment that seems to correlate with both FISI and the presence of sphincteric defects on anal endosonography, while maximal squeeze pressure (MSP) generated by the EAS was not correlated. Our results confirm a strong association between low MRP and FI. Mental disease was found to be independently associ- ated with low MRP. The IAS has spontaneous myogenic tone, but it also receives inputs from the sympathetic and parasympathetic nervous systems, both of which exert excitatory and inhibitory influences on the sphincteric smooth muscle [29]. Yamato and Rattan [30] demon- strated that stimulation of α1-adrenoreceptors causes an increase in the resting pressure developed by the IAS. Multi-acting receptor targeted antipsychotics (MARTA) have a high affinity for a number of receptors including the dopaminergic D2, serotonergic 5-HT2A, α1-adren- ergic, and muscarinic receptors. Two cases of FI have been reported in patients taking MARTA [31,32]. The pathophysiology is thought to be related to its central mechanism of action and inhibitory effects on the anal sphincters. Siproudhis et al. [33] reported that a single oral administration of 2 types of antidepressants (amitrip- tyline and fluoxetine) reduced pressure in the upper anal canal in 10 healthy male volunteers. Both drugs are likely to decrease the contractile activity of smooth mus- cle by inhibiting calcium channels. Pelvic organ prolapse repair was also an independent risk factor for low MRP. Most studies reported a greater than 10% prevalence of FI in women with genital prolapse, and more than a third of women and men with rectal prolapse reported FI [34]. Internal rectal prolapse is a circular infolding of the rectal wall that occurs on straining to defecate. It descends into the rectum and may reach into the anal canal. Harmston et al. [35] found a significant reduction in MRP with progressive in- creases in the grade of internal rectal prolapse without a demonstrable effect on MSP. By contrast, external rectal prolapse was associated with a significant reduction in both MRP and MSP [36]. The underlying mechanism has been postulated to result from nerve damage by stretch- ing due to perineal descent or sphincter dilatation and inappropriate stimulation of the recto-anal inhibitory re- flex by the prolapsing rectum [37]. The finding of de- creased MRP without a significant change in MSP sug- gests that the predominant effect of internal rectal prolapse is on reducing IAS tone without an effect on EAS. 5. CONCLUSION IASD has received little attention, and the results of this study indicate that it is a major problem for elderly peo- ple in Japan. In addition, the importance of IASD as a public health problem is likely to increase in the near future as the elderly population continues to grow. This cross-sectional study offers evidence that IASD is corre- lated with the presence of certain conditions such as age, sex, FI, mental disease, and pelvic organ prolapse. Sev- eral of these risk factors for IASD are potentially pre- ventable or modifiable; for example, several studies have shown a recovery in MRP after rectal prolapse repair [35]. Improving our understanding of risk factors, par- ticularly modifiable risk factors, is critical for developing future prevention guidelines and improving the specific- ity of FI treatment. REFERENCES [1] Madoff, R.D., Parker, S.C., Varma, M.G., et al. (2004) Faecal incontinence in adults. Lancet, 364, 621-632. doi:10.1016/S0140-6736(04)16856-6 [2] Rao, S.S.C. (2004) Diagnosis and management of fecal incontinence. American Journal of Gastroenterology, 99, 1585-1604. doi:10. 1111/j.1572-0241.2004.40105.x [3] Frenckner, B. and Euler, C.V. (1975) Influence of pu- dendal block on the function of the anal sphincters. Gut, 16, 482-489. doi:10.1136/gut.16.6.482 [4] Barnett, J.L., Hasler, W.L. and Camilleri, M. (1999) American gastroenterological association medical posi- tion statement on anorectal testing techniques. Gastroen- terology, 116, 732-760. doi:10.1016/S0016-5085(99)70194-0 [5] Bordeianou, L., Lee, K.Y., Rockwood, T., et al. (2008) Anal resting pressures at manometry correlate with the fecal incontinence severity index and with presence of sphincter defects on ultrasound. Disease of Colon and Rectum, 51, 1010-1014. doi:10.1007/s10350-008-9230-7 [6] Varma, M.G., Brown, J.S., Creasman, J.M., et al. (2006) Fecal incontinence in females older than aged 40 years: Who is at risk? Disease of Colon and Rectum, 49, 841- 851. doi:10.1007/s10350-006-0535-0 [7] Whitehead, W.E., Borrud, L., Goode, P.S., et al. (2009) Fecal incontinence in US adults: Epidemiology and risk factors. Gastroenterology, 137, 512-517. doi:10.1053/j.gastro.2009.04.054 [8] Joh, H.K., Seong, M.K. and Oh, S.W. (2010) Fecal in- continence in elderly Koreans. Journal of the American Geriatrics Society, 58, 116-121. doi:10.1111/j.1532-5415.2009.02613.x [9] Lewis, S.J. and Heaton, K.W. (1997) Stool form scale as a useful guide to intestinal transit time. Scandinavian Journal of Gastroenterology, 32, 920-924. doi:10.3109/00365529709011203 [10] Rao, S.S. and Patel, R.S. (1997) How useful are ma- nometric tests of anorectal function in the management of Copyright © 2013 SciRes. OPEN ACCESS  T. Abe et al. / Open Journal of Gastroenterology 3 (2013) 25-34 Copyright © 2013 SciRes. 34 OPEN ACCESS defecation disorders? American Journal of Gastroen- terology, 92, 469-475. [11] Abe, T., Kunimoto, M. and Hachiro, Y. (2008) Diagnosis and management of fecal incontinence at a specialty out- patient clinic. Journal of Japan Society of Coloproctology, 61, 247-253. doi:10.3862/jcoloproctology.61.247 [12] Laurberg, S. and Swash, M. (1989) Effects of aging on the anorectal sphincters and their innervation. Disease of Colon and Rectum, 32, 737-742. doi:10.1007/BF02562120 [13] Enck, P., Kuhlbusch, R., Lübke, H., et al. (1989) Age and sex and anorectal manometry in incontinence. Disease of Colon and Rectum, 32, 1026-1030. doi:10.1007/BF02553874 [14] Rao, S.S., Hatfield, R., Soffer, E., et al. (1999) Manomet- ric tests in anorectal function in healthy adults. American Journal of Gastroenterology, 94, 773-783. doi:10.1111/j.1572-0241.1999.00950.x [15] Gundling, F., Seidl, H., Scalercio, N., et al. (2009) Influ- ence of gender and age on anorectal function: Normal values from anorectal manometry in a large Caucasian population. Digestion, 81, 207-213. doi:10.1159/000258662 [16] Speakman, C.T.M., Hoyle, C.H.V., Kamm, M.A., et al. (1995) Abnormal internal anal sphincter fibrosis and elasticity in fecal incontinence. Disease of Colon and Rectum, 38, 407-410. doi:10.1007/BF02054231 [17] Abe, T., Sato, Y., Kunimoto, M., et al. (2008) Effect of aging and gender on internal anal sphincter thickness. Anti-Aging Medicine, 5, 46-48. doi:10.3793/jaam.5.46 [18] MacLennan, A.H., Taylor, A.W., Wilson, D.H., et al. (2000) The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. British Journal of Obstetrics and Gynaecology, 107, 1460-1470. doi:10. 1111/j.1471-0528.2000.tb11669.x [19] Nelson, R., Norton, N., Cautley, E., et al. (1995) Com- munity-based prevalence of anal incontinence. Journal of the American Medical Association, 274, 559-561. doi:10.1001/jama.274.7.559 [20] Bharucha, A.E., Zinsmeister, A.R., Locke, G.R., et al. (2006) Risk factors for fecal incontinence: A population- based study in women. American Journal of Gastroen- terology, 101, 1305-1312. doi:10.1111/j.1572-0241.2006.00553.x [21] Faltin, D.L., Boulvain, M., Irion, O., et al. (2000) Diag- nosis of anal sphincter tears by postpartum endosonogra- phy to predict fecal incontinence. Obstetrics and Gyne- cology, 95, 643-647. doi:10.1016/S0029-7844(99)00631-6 [22] Burnett, S.J., Spence-Jones, C., Speakman, C.T., et al. (1991) Unsuspected sphincter damage following child- birth revealed by anal endosonography. British Journal of Radiology, 64, 225-227. doi:10.1259/0007-1285-64-759-225 [23] Ward, A. and Tunuguntla, A.K. (1984) Anorectal sen- sorimotor dysfunction in fecal incontinence and diabetes mellitus. New England Journal of Medicine, 310, 1282- 1287. doi:10.1056/NEJM198405173102003 [24] Schiller, L.R., Santa Ana, C.A., Schmulen, C., et al. (1982) Pathogenesis of fecal incontinence in diabetes mellitus: Evidence for internal anal sphincter dysfunction. New England Journal of Medicine, 307, 1666-1671. doi:10.1056/NEJM198212303072702 [25] Rogers, J., Levy, D.M., Henry, M.M., et al. (1988) Pelvic floor neuropathy: A comparative study of diabetes melli- tus and idiopathic faecal incontinence. Gut, 29, 756-761. doi:10.1136/gut.29.6.756 [26] Pintor, M.P., Zara, G.P., Falletto, E., et al. (1994) Pu- dendal neuropathy in diabetic patients with faecal incon- tinence. International Journal of Colorectal Disease, 9, 105-109. doi:10.1007/BF00699423 [27] Erckenbrecht, J.F., Winter, H.J., Cicmir, I., et al. (1988) Faecal incontinence in diabetes mellitus: Is it correlated to diabetic autonomic or peripheral neuropathy? Zeits- chrift fur Gastroenterologie, 26, 731-736. [28] Watanabe, M., Tsunoda, A., Kamiyama, G., et al. (2003) Pathophysiology in diabetic patients with fecal inconti- nence. Showa University Journal of Medical Sciences, 15, 21-26. [29] Mills, K. and Chess-Williams, R. (2009) Pharmacology of the internal anal sphincter and its relevance to faecal incontinence. Autonomic and Autacoid Pharmacology, 29, 85-95. doi:10. 1111/j.1474-8673.2009.00437.x [30] Yamato, S. and Rattan, S. (1990) Role of alpha adreno- ceptors in opossum internal anal sphincter. Journal of Clinical Investigation, 86, 424-429. doi:10.1172/JCI114728 [31] Mendhekar, D.N., Srivastav, P.K., Sarin, S.K., et al. (2003) A case report of olanzapine-induced fecal incon- tinence. Journal of Clinical Psychiatry, 61, 601-602. [32] Sagar, R., Varghese, S.T. and Balhara, Y.P. (2005) Olan- zapine-induced double incontinesnce. Indian Journal of Medical Sciences, 59, 163-164. doi:10.4103/0019-5359.16123 [33] Siproudhis, L., Dinasquet, M., Sébille, V., et al. (2004) Differential effects of two types of antidepressants, ami- trptyline and fluoxetine, on anorectal motility and vis- ceral perception. Alimentary Pharmacology and Thera- peutics, 20, 689-695. doi:10.1111/j.1365-2036.2004.02151.x [34] Shamliyan, T., Wyman, J., Bliss, D.Z., et al. (2007) Pre- vention of urinary and fecal incontinence in adults. Evi- dence Reports/Technology Assessments, 161, 1-379. [35] Harmston, C., Jones, O.M., Cunningham, C., et al. (2011) The relationship between internal rectal prolapse and in- ternal anal sphincter function. Colorectal Disease, 13, 791-795. doi:10. 1111/j.1463-1318.2010.02266.x [36] Hiltunen, K.M., Matikainen, M., Auvinen, O., et al. (1986) Clinical and manometric evaluation of anal sphinc- ter function in patients with rectal prolapse. American Journal of Surgery, 151, 489-492. doi:10.1016/0002-9610(86)90110-8 [37] Furouk, R., Duthie, G.S., MacGregor, A.B., et al. (1994) Recto-anal inhibition and incontinence in patients with rectal prolapse. British Journal of Surgery, 81, 743-746. doi:10.1002/bjs.1800810542
|