Paper Menu >>
Journal Menu >>
 Open Journal of Forestry 2013. Vol.3, No.1, 41-48 Published Online January 2013 in SciRes (http://www.scirp.org/journal/ojf) http://dx.doi.org/10.4236/ojf.2013.31007 Copyright © 2013 SciRes. 41 The Effective Ecological Factors and Vegetation at Koh Chang Island, Trat Province, Thailand Nathsuda Pumijumnong, Paramate Payomrat Faculty of Environment and Resource Studies, Mahidol University, Nakhon Pathom, Thailand Email: nathsuda@gmail.com, paramate.ohm@gmail.com Received October 3rd, 2012; revised November 7th, 2012; accepted November 20th, 2012 This study aims to characterize the tropical rain forest present in the Chang Island, Trat Province, Thailand, and to analyze the environmental factors to determine its composition and structure. Thirty one plots were sampled, plant cover was measured in 20 × 40 m2 plots, and the importance value index was calculated. A total of 78 spe- cies belonging to 32 families were identified.Twenty soil samples were analyzed, and cluster analysis was em- ployed to classify the vegetation communities. Floristic and environmental data were evaluated and ordered us- ing canonical correspondence analysis. The results showed that the vegetation communities could be divided into 4 types and were significantly (p < 0.05) controlled by a secondary distribution according to elevation and the topographic wetness index (TWI). Mixed plant communities were more likely to distribute in regions with moderate to low levels of TWI, which were divided by levels of elevation into lowland multi-aged stands (Type 1) or a Calophyllum thorelii Pierre community (Type 2). The Dipterocarpus (Hopea pierrei Heim) community (Type 3) was more likely to occur in regions with moderate to high levels of TWI, but the result from cluster analysis showed that some of the plot samples from the Dipterocarpus community were separated by character- istic importance value index (IVI) values. There was also evidence that the area was impacted by an old distur- bance created by a rubber plantation. This impact was referred to as a secondary succession community (Type 4). Keywords: Chang Island; Vegetation Community; Canonical Correspondence Analysis; Ecological Factors Introduction Chang Island is one of only a few islands where a tropical rain forest is distributed over 70% of the inland area, which has been preserved in its present form (Environmental Research Institute, 2007). The island covers an area of 212.947 sq km and is a major island of the Mu Koh Chang National park in the Gulf of Thailand, which became Thailand’s 45th National Park in 1982. During the Pleistocene epoch (Tougard, 2001; Essel- styn et al., 2009), large sea level fluctuations caused the sea level to fall by 50 - 150 m, creating land bridge connections. After this period, the sea level rose and the low-lying regions were covered with water forming many islands separated from the mainland. Various fossils have confirmed these events dur- ing this period (Bird et al., 2005). The research of Chamchum- roon and Puff provides evidence to support this theory. It is remarkable that although a large proportion of rubiaceous taxa show disjunction between Koh Chang and peninsular Thailand, 20% of all rubiaceae taxa recorded from Koh Chang have been found to be distributed in peninsular Thailand (Chamchum- roon et al., 2003). This indicates that biological species found on the mainland may also be found on the Chang islands with a greater diversity. The tropical rain forest of Chang island has important roles in storing bioelements and water for local com- munities and acts as a carbon bank that impacts a large global area. Since 1902, the records of Schmidt (1900) have shown that all of the hills of Koh Chang are entirely covered with the densest jungle. In addition, the Siamese and Chinese popula- tions scattered along the coasts where the river debouches have had little influence on the forest cover of the hills. Over the past century, tropical rain forest areas around the world and in Thai- land now face loss due to human activities (Royal Forest De- partment, 1997, 2005). Chang Island is also facing the same problem as other forest resources and is in danger of losing forest cover by people focused on acquiring forest land for their settlements, farms, and plantations. Recently, land use change caused by tourism had led to construction on the island, in- cluding business buildings, luxury accommodations, roads, and harbors. This type of land use changes the ecological system of Chang Island. Although the Mu Ko Chang National Park was established at the end of 1982, negative human impact on the vegetation had already occurred in the more accessible coastal areas because the settled area of the old communities before the establishment of the National Park were not included in the pre- servation. Therefore, some species have become extinct, such as Ixoradoli chophylla, Lasianthuso ligoneuron and L. schmid- tii, which according to the reports of Schmidt and Kerr (Cham- chumroon et al., 2003; Schmidt, 1900), come from the Klong Nonsi area, a populated area near the Koh Chang District Of- fice that has no traces of natural vegetation left. None of these species have ever been identified anywhere else in the Island. The effect of climate change is another problem because the area, size, and isolation of Chang Island make it less resilient to such change. This study aimed to classify the vegetation communities on Chang Island and determine environmental factors that influ- ence their composition and structure. Knowledge of these rela- tionships may be critically important for planning appropriate adaptations to climate changes and disturbances due to human activities. 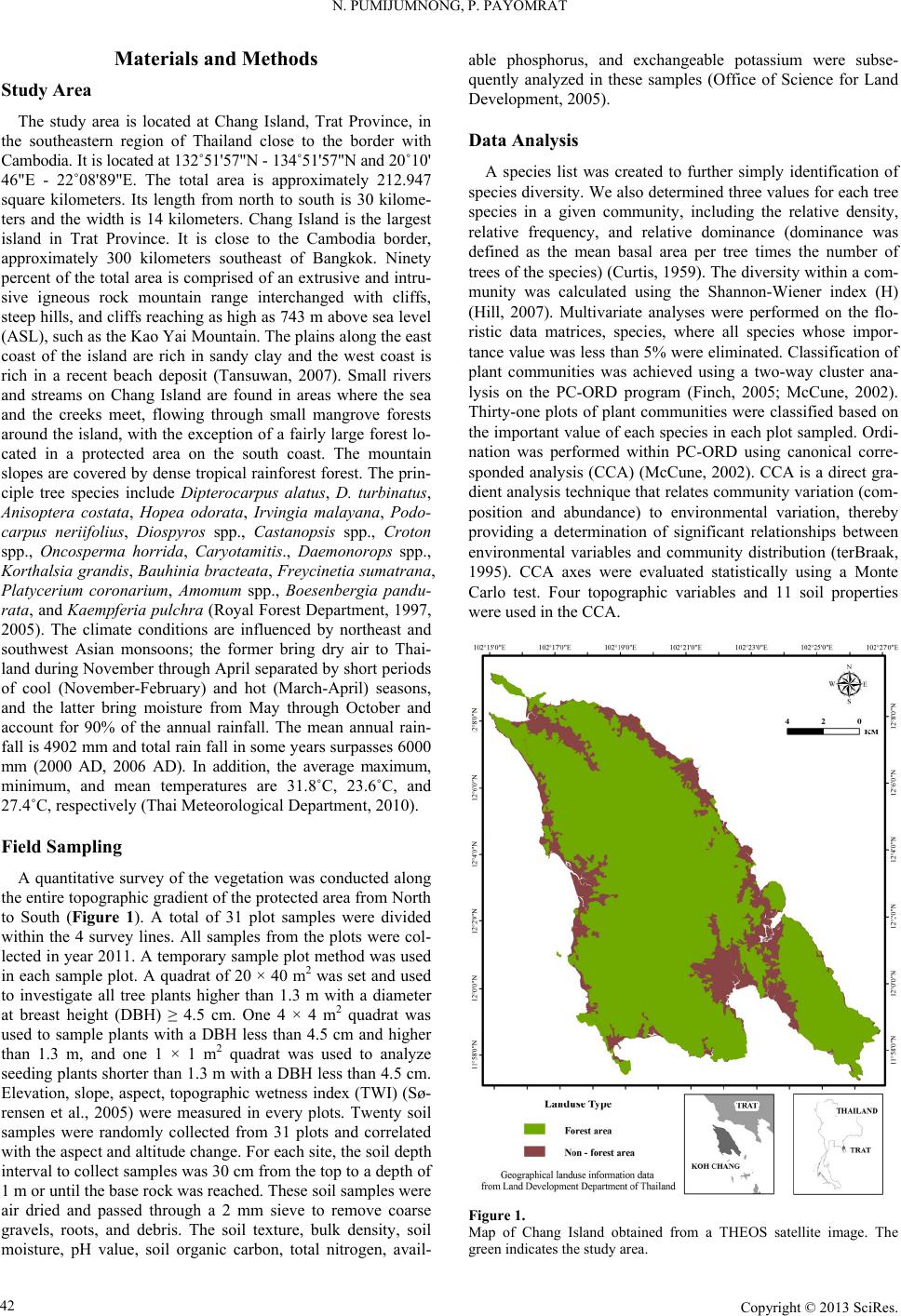 N. PUMIJUMNONG, P. PAYOMRAT Materials and Methods Study Area The study area is located at Chang Island, Trat Province, in the southeastern region of Thailand close to the border with Cambodia. It is located at 132˚51'57"N - 134˚51'57"N and 20˚10' 46"E - 22˚08'89"E. The total area is approximately 212.947 square kilometers. Its length from north to south is 30 kilome- ters and the width is 14 kilometers. Chang Island is the largest island in Trat Province. It is close to the Cambodia border, approximately 300 kilometers southeast of Bangkok. Ninety percent of the total area is comprised of an extrusive and intru- sive igneous rock mountain range interchanged with cliffs, steep hills, and cliffs reaching as high as 743 m above sea level (ASL), such as the Kao Yai Mountain. The plains along the east coast of the island are rich in sandy clay and the west coast is rich in a recent beach deposit (Tansuwan, 2007). Small rivers and streams on Chang Island are found in areas where the sea and the creeks meet, flowing through small mangrove forests around the island, with the exception of a fairly large forest lo- cated in a protected area on the south coast. The mountain slopes are covered by dense tropical rainforest forest. The prin- ciple tree species include Dipterocarpus alatus, D. turbinatus, Anisoptera costata, Hopea odorata, Irvingia malayana, Podo- carpus neriifolius, Diospyros spp., Castanopsis spp., Croton spp., Oncosperma horrida, Caryotamitis., Daemonorops spp., Korthalsia grandis, Bauhinia bracteata, Freycinetia sumatrana, Platycerium coronarium, Amomum spp., Boesenbergia pandu- rata, and Kaempferia pulchra (Royal Forest Department, 1997, 2005). The climate conditions are influenced by northeast and southwest Asian monsoons; the former bring dry air to Thai- land during November through April separated by short periods of cool (November-February) and hot (March-April) seasons, and the latter bring moisture from May through October and account for 90% of the annual rainfall. The mean annual rain- fall is 4902 mm and total rain fall in some years surpasses 6000 mm (2000 AD, 2006 AD). In addition, the average maximum, minimum, and mean temperatures are 31.8˚C, 23.6˚C, and 27.4˚C, respectively (Thai Meteorological Department, 2010). Field Sampling A quantitative survey of the vegetation was conducted along the entire topographic gradient of the protected area from North to South (Figure 1). A total of 31 plot samples were divided within the 4 survey lines. All samples from the plots were col- lected in year 2011. A temporary sample plot method was used in each sample plot. A quadrat of 20 × 40 m2 was set and used to investigate all tree plants higher than 1.3 m with a diameter at breast height (DBH) ≥ 4.5 cm. One 4 × 4 m2 quadrat was used to sample plants with a DBH less than 4.5 cm and higher than 1.3 m, and one 1 × 1 m2 quadrat was used to analyze seeding plants shorter than 1.3 m with a DBH less than 4.5 cm. Elevation, slope, aspect, topographic wetness index (TWI) (Sø- rensen et al., 2005) were measured in every plots. Twenty soil samples were randomly collected from 31 plots and correlated with the aspect and altitude change. For each site, the soil depth interval to collect samples was 30 cm from the top to a depth of 1 m or until the base rock was reached. These soil samples were air dried and passed through a 2 mm sieve to remove coarse gravels, roots, and debris. The soil texture, bulk density, soil moisture, pH value, soil organic carbon, total nitrogen, avail- able phosphorus, and exchangeable potassium were subse- quently analyzed in these samples (Office of Science for Land Development, 2005). Data Analysis A species list was created to further simply identification of species diversity. We also determined three values for each tree species in a given community, including the relative density, relative frequency, and relative dominance (dominance was defined as the mean basal area per tree times the number of trees of the species) (Curtis, 1959). The diversity within a com- munity was calculated using the Shannon-Wiener index (H) (Hill, 2007). Multivariate analyses were performed on the flo- ristic data matrices, species, where all species whose impor- tance value was less than 5% were eliminated. Classification of plant communities was achieved using a two-way cluster ana- lysis on the PC-ORD program (Finch, 2005; McCune, 2002). Thirty-one plots of plant communities were classified based on the important value of each species in each plot sampled. Ordi- nation was performed within PC-ORD using canonical corre- sponded analysis (CCA) (McCune, 2002). CCA is a direct gra- dient analysis technique that relates community variation (com- position and abundance) to environmental variation, thereby providing a determination of significant relationships between environmental variables and community distribution (terBraak, 1995). CCA axes were evaluated statistically using a Monte Carlo test. Four topographic variables and 11 soil properties were used in the CCA. Figure 1. Map of Chang Island obtained from a THEOS satellite image. The green indicates the study area. Copyright © 2013 SciRes. 42 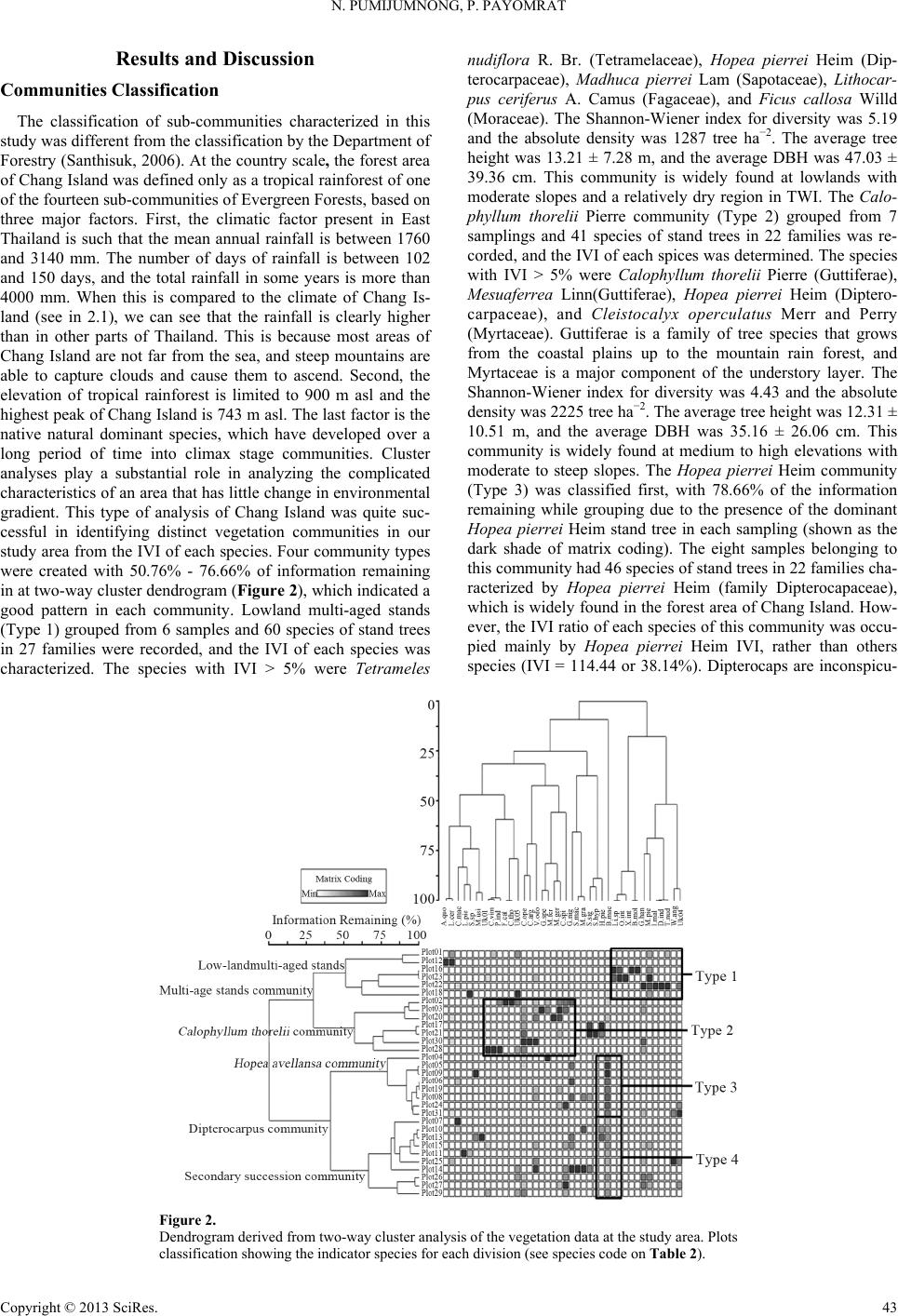 N. PUMIJUMNONG, P. PAYOMRAT Copyright © 2013 SciRes. 43 Results and Discussion Communities C lass ifi cation The classification of sub-communities characterized in this study was different from the classification by the Department of Forestry (Santhisuk, 2006). At the country scale, the forest area of Chang Island was defined only as a tropical rainforest of one of the fourteen sub-communities of Evergreen Forests, based on three major factors. First, the climatic factor present in East Thailand is such that the mean annual rainfall is between 1760 and 3140 mm. The number of days of rainfall is between 102 and 150 days, and the total rainfall in some years is more than 4000 mm. When this is compared to the climate of Chang Is- land (see in 2.1), we can see that the rainfall is clearly higher than in other parts of Thailand. This is because most areas of Chang Island are not far from the sea, and steep mountains are able to capture clouds and cause them to ascend. Second, the elevation of tropical rainforest is limited to 900 m asl and the highest peak of Chang Island is 743 m asl. The last factor is the native natural dominant species, which have developed over a long period of time into climax stage communities. Cluster analyses play a substantial role in analyzing the complicated characteristics of an area that has little change in environmental gradient. This type of analysis of Chang Island was quite suc- cessful in identifying distinct vegetation communities in our study area from the IVI of each species. Four community types were created with 50.76% - 76.66% of information remaining in at two-way cluster dendrogram (Figure 2), which indicated a good pattern in each community. Lowland multi-aged stands (Type 1) grouped from 6 samples and 60 species of stand trees in 27 families were recorded, and the IVI of each species was characterized. The species with IVI > 5% were Tetrameles nudiflora R. Br. (Tetramelaceae), Hopea pierrei Heim (Dip- terocarpaceae), Madhuca pierrei Lam (Sapotaceae), Lithocar- pus ceriferus A. Camus (Fagaceae), and Ficus callosa Willd (Moraceae). The Shannon-Wiener index for diversity was 5.19 and the absolute density was 1287 tree ha−2. The average tree height was 13.21 ± 7.28 m, and the average DBH was 47.03 ± 39.36 cm. This community is widely found at lowlands with moderate slopes and a relatively dry region in TWI. The Calo- phyllum thorelii Pierre community (Type 2) grouped from 7 samplings and 41 species of stand trees in 22 families was re- corded, and the IVI of each spices was determined. The species with IVI > 5% were Calophyllum thorelii Pierre (Guttiferae), Mesuaferrea Linn(Guttiferae), Hopea pierrei Heim (Diptero- carpaceae), and Cleistocalyx operculatus Merr and Perry (Myrtaceae). Guttiferae is a family of tree species that grows from the coastal plains up to the mountain rain forest, and Myrtaceae is a major component of the understory layer. The Shannon-Wiener index for diversity was 4.43 and the absolute density was 2225 tree ha−2. The average tree height was 12.31 ± 10.51 m, and the average DBH was 35.16 ± 26.06 cm. This community is widely found at medium to high elevations with moderate to steep slopes. The Hopea pierrei Heim community (Type 3) was classified first, with 78.66% of the information remaining while grouping due to the presence of the dominant Hopea pierrei Heim stand tree in each sampling (shown as the dark shade of matrix coding). The eight samples belonging to this community had 46 species of stand trees in 22 families cha- racterized by Hopea pierrei Heim (family Dipterocapaceae), which is widely found in the forest area of Chang Island. How- ever, the IVI ratio of each species of this community was occu- pied mainly by Hopea pierrei Heim IVI, rather than others species (IVI = 114.44 or 38.14%). Dipterocaps are inconspicu- Figure 2. Dendrogram derived from two-way cluster analysis of the vegetation data at the study area. Plots classification showing the indicator species for each division (see species code on Table 2). 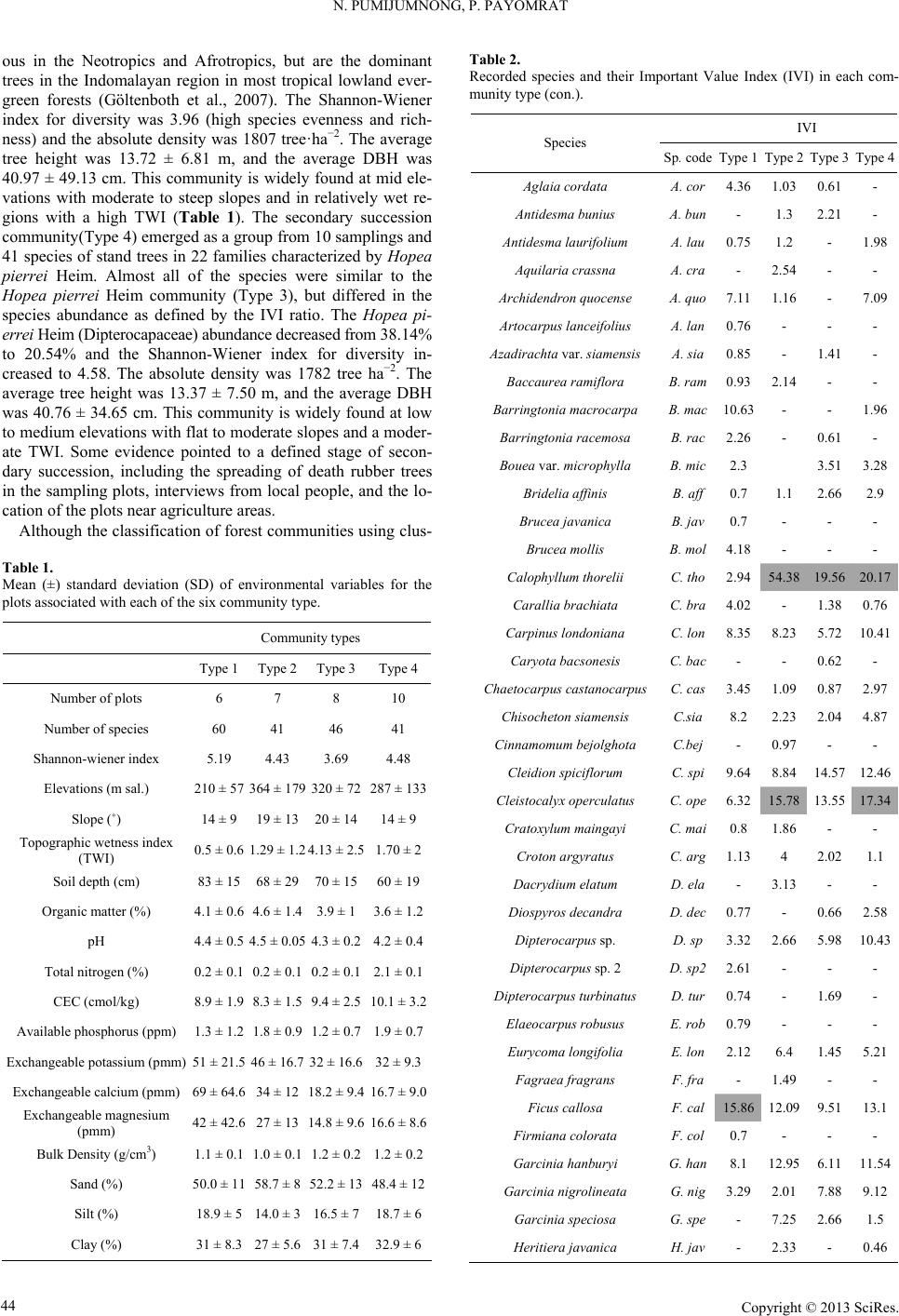 N. PUMIJUMNONG, P. PAYOMRAT us in the Neotropics mmunities using clus- a tandard deviation (SD) of environmental variables for the types oand Afrotropics, but are the dominant trees in the Indomalayan region in most tropical lowland ever- green forests (Göltenboth et al., 2007). The Shannon-Wiener index for diversity was 3.96 (high species evenness and rich- ness) and the absolute density was 1807 tree·ha−2. The average tree height was 13.72 ± 6.81 m, and the average DBH was 40.97 ± 49.13 cm. This community is widely found at mid ele- vations with moderate to steep slopes and in relatively wet re- gions with a high TWI (Table 1). The secondary succession community(Type 4) emerged as a group from 10 samplings and 41 species of stand trees in 22 families characterized by Hopea pierrei Heim. Almost all of the species were similar to the Hopea pierrei Heim community (Type 3), but differed in the species abundance as defined by the IVI ratio. The Hopea pi- errei Heim (Dipterocapaceae) abundance decreased from 38.14% to 20.54% and the Shannon-Wiener index for diversity in- creased to 4.58. The absolute density was 1782 tree ha−2. The average tree height was 13.37 ± 7.50 m, and the average DBH was 40.76 ± 34.65 cm. This community is widely found at low to medium elevations with flat to moderate slopes and a moder- ate TWI. Some evidence pointed to a defined stage of secon- dary succession, including the spreading of death rubber trees in the sampling plots, interviews from local people, and the lo- cation of the plots near agriculture areas. Although the classification of forest co Tble 1. Mean (±) s plots associated with each of the six community type. Community Type 1Type 4Type 2 Type 3 Numbef plots r o6 7 8 10 Number of species 60 41 46 41 Table 2. Recorded species and their Important Value Index (IVI) in each com- munity type (con.). IVI Species Sp. code Type 1 Type 2 Type 3Type 4 Aglaiata A. r 4.36 1.0361- a cordco 0. Antidesma bu niu s Anm 1. Ars 0.76 - A 1. 2 1. 0. 3. Ci A. bun - 1.3 2.21- tidesma laurifoliuA. lau 0.75 1.2 - 98 Aquilaria crassna A. cra - 2.54 - - Archidendron quocense A. quo 7.11 1.16 - 7.09 tocarpus lanceifoliuA. lan - - zadirachta var. siamensisA. sia 0.85 - 41 - Baccaurea rami flora B. ram 0.93 .14- - Barringtonia macrocarpa B. mac 10.63 - - 96 Barringtonia racemosa B. rac 2.26 - 61 - Bouea var. microphylla B. mic 2.3 513.28 Bridelia affinis B. aff 0.7 1.12.662.9 Brucea javanica B. jav 0.7 - - - Brucea mollis B. mol 4.18 - - - alophyllum thoreliC. tho 2.94 58 4.319.5627 C 1. 0. Ch s C 8 2 4. 6. 0.1 arallia brachiat aC. bra 4.02 - 3876 Carpinus londoniana C. lon 8.35 8.23 5.7210.41 Caryota bacso ne sis C. bac - - 0.62- aetocarpus castanocarpuC. cas 3.45 1.09 0.87 2.97 hisocheton siamensisC.sia .2 .232.04 87 Cinnamomum bejolghot a C.bej - 0.97 - - Cleidion spiciflo rum C. spi 9.64 8.84 14.5712.46 Cleistocalyx operculatus C. ope 32 15.78 S 21 369 32283 Topograss index 0 14 Soim) O Total nen (%) Availappm) E Bulk D/cm) hannon-wiener index 5.19 4.43 3.69 4.48 Elevations (m sal.) 0 ± 574 ± 170 ± 727 ± 13 Slope (˚) 14 ± 919 ± 13 20 ± 1414 ± 9 phic wetne (TWI) l depth (c .5 ± 0.6 .29 ± 1.2 .13 ± 2.51.70 ± 2 83 ± 15 68 ± 29 70 ± 1560 ± 19 rganic matter (%)4.1 ± 0.6 4.6 ± 1.4 3.9 ± 13.6 ± 1.2 pH 4.4 ± 0.5 4.5 ± 0.05 4.3 ± 0.24.2 ± 0.4 itrog0.2 ± 0.1 0.2 ± 0.1 0.2 ± 0.12.1 ± 0.1 CEC (cmol/kg) 8.9 ± 1.9 8.3 ± 1.5 9.4 ± 2.510.1 ± 3.2 ble phosphorus (1.3 ± 1.2 1.8 ± 0.9 1.2 ± 0.71.9 ± 0.7 xchangeable potassium (pmm) 51 ± 21.5 46 ± 16.7 32 ± 16.632 ± 9.3 Exchangeable calcium (pmm) 69 ± 64.6 34 ± 12 18.2 ± 9.416.7 ± 9.0 Exchangeable magnesium (pmm) ensity (g3 42 ± 42.6 27 ± 13 14.8 ± 9.616.6 ± 8.6 1.1 ± 0.1 1.0 ± 0.1 1.2 ± 0.21.2 ± 0.2 Sand (%) 50.0 ± 11 58.7 ± 8 52.2 ± 1348.4 ± 12 Silt (%) 18.9 ± 5 14.0 ± 3 16.5 ± 718.7 ± 6 Clay (%) 31 ± 8.3 27 ± 5.6 31 ± 7.432.9 ± 6 13.55 1 4 D 3. 5.13 Dipus 5. 1 7.3 Cratoxylum maingayi C. mai 0.8 1.86 - - Croton argyratus C. a rg 1.13 4 2.021.1 Dacrydium elatum D. ela - 3.13 - - iospyros decandra D. dec 0.77 - 0.662.58 Dipterocarpus sp. D. sp 32 2.66 980.4 Dipterocarpus sp. 2 D. sp2 2.61 - - - terocarpus turbinatD. tur 0.74 - 1.69 - Elaeocarp us robusus E. rob 0.79 - - - Eurycoma longifolia E. lon 2.12 6.4 1.4521 Fagraea fragrans F. fra - .49 - - Ficus callosa F. cal 15.86 1 0.7 - Gi G 2 7.9. 2.09 9.5113.1 Firmiana colorata F. col - - arcinia hanburyG. han 8.1 12.95 6.1111.54 arcinia nigrolineataG. nig 3.29 .018812 Garcinia specio sa G. spe - 7.25 2.661.5 Heritiera javanica H. jav - 2.33 - 0.46 Copyright © 2013 SciRes. 44  N. PUMIJUMNONG, P. PAYOMRAT Continue IVI Species Sp. code Type 1 Type 2 Type 3Type 4 Hoperei H.iea pier p23.9 530.55 1144.61.36 Horsfieldia irya Ira 1 L Lits H. iry2.28 - - 3.39 avingia malayanI. mal5.17 - - 2.13 Ixora cibdela I. cib 0.7 .17- - Knema linifolia K. lin2.48 - - - itchi chinensis L. chi1.47 - - - hocarpus ceriferuL. cer16.11 6.35 1.3. 1.6. Lophnum 0.62 Mara 02 56 Lithocarpus sp. L. sp 2.85 - - - Lithocarpus sp. 2 L. sp24.4 - - - Litsea pierrei L. pie12.14 2.928396 opetalum duperreaL. dup- - - dhuca grandifloM. g ra10.75 10.94 8.228.74 Madhuca pier rei M. pie217 7. 111 M Ms .4046.93.2 Mangifera caloneura M. cal- - 1.3 2.02 aytenus marcaniiM. mar0.7 1.12 0.64- Melanorrhoe a usitata. M. usi- - 6.339.54 emecylon garci nioideM. gar5.26 12.46 12.611 6 2. 5.7 Mesua ferrea M. fer013 Oncria 0.96 Sc 2 4.7. 6. 2 2. S. Sy 4.7 6.09 1.58 Ochna intege rrima O. int4.25 - - - osperma tigillaO. tig- - - 2.69 Paeinari anamense P. ana1.5 - - - Phyllanthus e mblica P. emb- - - aphium macropodumS. mac7.39 .394191 Semecarpus Sp. S. sp 82.01 5.26- Shorea hyp ochra S. hyp8.62 9.31 6.42 8.23 Sloanea sigum S. sig- 4.67 2.7867 Spondias bipinnata S. bip2.22 - - - terculia foetida LS. foe- - 1.22- zygium diospyrifoliumS. dio0.7 - - - Tetrameles nudiflora T. nud246 - 1. 1 6.5. Xerosum 5. 1 2. 0. .90.7 94 Vatica odorata V. ode- 2.879168 Walsura angul at a W. ang3.57 - 1.952.69 permum noronhianX. nor82 1.01 1.46- Unknow 1 Uk1 - - - 0.47 Unknow 2 Uk2 1.39 - - - Unknow 3 Uk3 .2- - - Unknow 4 Uk4 0.7 - - - Unknow 5 Uk5 - 32- - Unknow 6 Uk6 0.78 - 64- Note: *The for some IVI values indicate I5 (5%or each community. ter analyall islands rl(a result ofhe CCA ordination graph shown in Figure 3), it clearly showed a d. The eigen- alysis represent the relative contribu- tio gray shading VI values ≥ 1) f sis on a sm tendto oveap t difference in the abundance of dominant species. Hopea pierrei Heim (Dipterocapus) was found in all the communities, includ- ing the Hopea pierrei Heim community, secondary succession community, Calophyllum thorelii Pierre community, and low- land multi-aged stands, but with different IVIs, which were 38.15%, 20.54%, 10.18%, and 7.86%, respectively. A similar pattern was obtained when a cluster analysis was performed on the literal evergreen forest of Samesan Island (Payomrat, 2011). Ordination diagram and species composition of five sub-com- munity types showed an overlap of Memecylon plebejum, which is a dominant species between four communities, but different IVI ratios were observed. However, some studies of small islands have identified distinct vegetation communities using cluster analysis. Phra Thong Island has a large alluvial deposit sand plain with sinking seashore and productive mud- beaches on estuaries of many long rivers, which is different than the terrain of Chang Island. This island covers the Cajuput forest, Cajuput swamp, Peat swamp forest, Beach forest, grass- lands, and mangroves (Pumijumnong, 2005). Cluster analysis of the sub-forest community (Thaisatuen, 2010) and Poaceae and Cyperaceae (Boutrat, 2009) on Phra Thong Island were successfully classified as communities with 60% - 95% re- maining information, which is similar to the present study, with the exception of the grassland type. Relationshi p be t w e en C ommunities and Topographic Factors Elevation and TWI were similarly important factors control- ling the community distribution of Chang Islan values in an ordination an n of each axis to the explanation of the total variation in the data. The CCA eigenvalues of the 3 two ordination axes were 0.303, 0.253, and 0.172, respectively (Table 3). A Monte Carlo test confirmed the statistical significance of the axes (P = 0.05). The first three axes explained 12.5% of the variance in the spe- cies data. From the Table 4, the highest intraset correlations with axis 1 was TWI (0.819) and axis 2 was the elevation (−0.858). Figure 3. CCA plot ordination with the community types derived from cluster analysis. Type 1 = lowland pe 2 = Calophyllum tho - multi-aged stands; Ty relii Pierre community; Type 3 = Hopea pierrei Heim community; and Type 4 = secondary succession community. Copyright © 2013 SciRes. 45 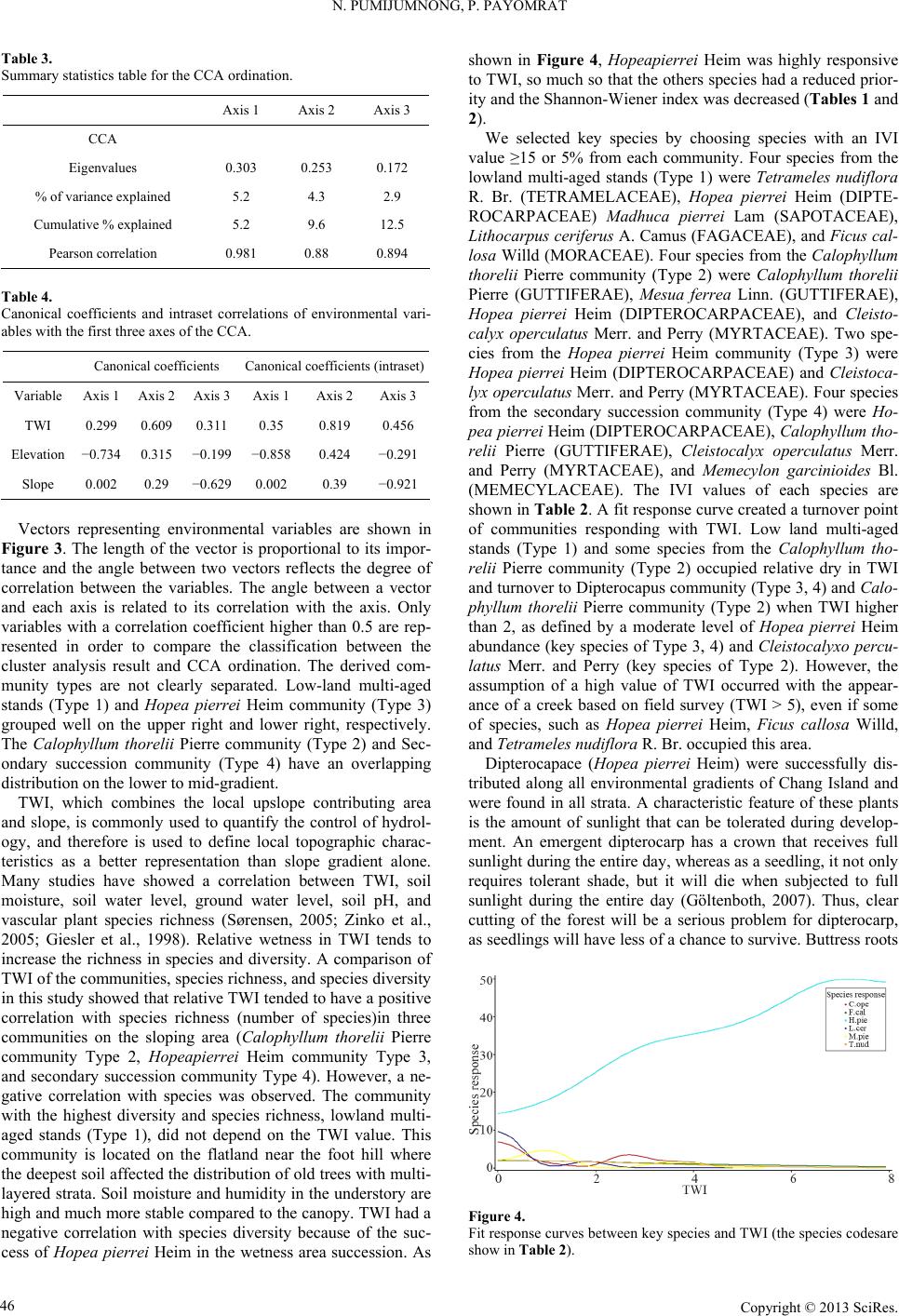 N. PUMIJUMNONG, P. PAYOMRAT Table 3. Summary s Axis 1 Axis 2 Axis 3 tatistics table for the CCA ordination. CCA Eigenvalues % of variaxplained 5.4.2. Cumud 0. 0.303 0.253 0.172 nce e2 3 9 lative % explaine5.2 9.6 12.5 Pearson correlation 981 0.88 0.894 Tabl Canoniintraset cions oronmental vari- bles ws of the C Canonical coefficients Canonical coefficients (intraset) e 4. cal coefficients and ith the first three axe orrelat CA. f envi a Variable Axis 1 Axis 2 Axis 3Axis 1 Axis 2Axis 3 TWI 0.299 0.609 0.3110.35 0.819 0.456 Elevation −0.734 0.315 −0.199 −0.858 0.424 −0.291 Slope 0.002 0.29 −0.6290.002 0.39 −0.921 vnb Fi . Tgthe is proportional to it- nce and the angle between two vectors reflects the degree of o r. (TETRAMELACEAE), Hopea pierrei Heim (DIPTE- R g develop- m Vectors representing enironmetal variales are shown in gure 3he len of th vectors impor ta crrelation between the variables. The angle between a vector and each axis is related to its correlation with the axis. Only variables with a correlation coefficient higher than 0.5 are rep- resented in order to compare the classification between the cluster analysis result and CCA ordination. The derived com- munity types are not clearly separated. Low-land multi-aged stands (Type 1) and Hopea pierrei Heim community (Type 3) grouped well on the upper right and lower right, respectively. The Calophyllum thorelii Pierre community (Type 2) and Sec- ondary succession community (Type 4) have an overlapping distribution on the lower to mid-gradient. TWI, which combines the local upslope contributing area and slope, is commonly used to quantify the control of hydrol- ogy, and therefore is used to define local topographic charac- teristics as a better representation than slope gradient alone. Many studies have showed a correlation between TWI, soil moisture, soil water level, ground water level, soil pH, and vascular plant species richness (Sørensen, 2005; Zinko et al., 2005; Giesler et al., 1998). Relative wetness in TWI tends to increase the richness in species and diversity. A comparison of TWI of the communities, species richness, and species diversity in this study showed that relative TWI tended to have a positive correlation with species richness (number of species)in three communities on the sloping area (Calophyllum thorelii Pierre community Type 2, Hopeapierrei Heim community Type 3, and secondary succession community Type 4). However, a ne- gative correlation with species was observed. The community with the highest diversity and species richness, lowland multi- aged stands (Type 1), did not depend on the TWI value. This community is located on the flatland near the foot hill where the deepest soil affected the distribution of old trees with multi- layered strata. Soil moisture and humidity in the understory are high and much more stable compared to the canopy. TWI had a negative correlation with species diversity because of the suc- cess of Hopea pierrei Heim in the wetness area succession. As shown in Figure 4, Hopeapierrei Heim was highly responsive to TWI, so much so that the others species had a reduced prior- ity and the Shannon-Wiener index was decreased (Tables 1 and 2). We selected key species by choosing species with an IVI value ≥15 or 5% from each community. Four species from the lowland multi-aged stands (Type 1) were Tetrameles nudiflora R. B OCARPACEAE) Madhuca pierrei Lam (SAPOTACEAE), Lithocarpus ceriferus A. Camus (FAGACEAE), and Ficus cal- losa Willd (MORACEAE). Four species from the Calophyllum thorelii Pierre community (Type 2) were Calophyllum thorelii Pierre (GUTTIFERAE), Mesua ferrea Linn. (GUTTIFERAE), Hopea pierrei Heim (DIPTEROCARPACEAE), and Cleisto- calyx operculatus Merr. and Perry (MYRTACEAE). Two spe- cies from the Hopea pierrei Heim community (Type 3) were Hopea pierrei Heim (DIPTEROCARPACEAE) and Cleistoca- lyx operculatus Merr. and Perry (MYRTACEAE). Four species from the secondary succession community (Type 4) were Ho- pea pierrei Heim (DIPTEROCARPACEAE), Calophyllum tho- relii Pierre (GUTTIFERAE), Cleistocalyx operculatus Merr. and Perry (MYRTACEAE), and Memecylon garcinioides Bl. (MEMECYLACEAE). The IVI values of each species are shown in Table 2. A fit response curve created a turnover point of communities responding with TWI. Low land multi-aged stands (Type 1) and some species from the Calophyllum tho- relii Pierre community (Type 2) occupied relative dry in TWI and turnover to Dipterocapus community (Type 3, 4) and Calo- phyllum thorelii Pierre community (Type 2) when TWI higher than 2, as defined by a moderate level of Hopea pierrei Heim abundance (key species of Type 3, 4) and Cleistocalyxo percu- latus Merr. and Perry (key species of Type 2). However, the assumption of a high value of TWI occurred with the appear- ance of a creek based on field survey (TWI > 5), even if some of species, such as Hopea pierrei Heim, Ficus callosa Willd, and Tetrameles nud i f l or a R. Br. occupied this area. Dipterocapace (Hopea pierrei Heim) were successfully dis- tributed along all environmental gradients of Chang Island and were found in all strata. A characteristic feature of these plants is the amount of sunlight that can be tolerated durin ent. An emergent dipterocarp has a crown that receives full sunlight during the entire day, whereas as a seedling, it not only requires tolerant shade, but it will die when subjected to full sunlight during the entire day (Göltenboth, 2007). Thus, clear cutting of the forest will be a serious problem for dipterocarp, as seedlings will have less of a chance to survive. Buttress roots Figure 4. Fit response curves between key species and TWI (the species codesare show in Table 2). Copyright © 2013 SciRes. 46  N. PUMIJUMNONG, P. PAYOMRAT are another characteristic feature of dipterocarp (common name of Hopea pierrei Heim is TaKhianRak; “Rak” means root in Thai). It occurs when trees with a shallow root system prevail in areas where nutrient concentrations are largest near the soil surface or stand against a steep slope. Why these structure are formed is still not fully understood, as research has shown that buttresses do not increase the trees’ resistance to mechanical stress, such as trunk snapping, or alleviate the pulling strain on the roots (Richard, 1996). Elevation was positively correlated with tree density (r = 0.979). The Calophyllum thorelii Pierre community (Type 2), which was distributed along the high land until the peak, had t tree density (2225 tree·ha−1), whereas the tree den- unity (Type 4), and low land multi-aged 2), and low land multi-aged stands (T u- nity Type 2 (5300.04 ha or 30.41%), Hopea pierrei Heim com- a or 13.53%), secondary succession community the highes sity of the Hopea pierrei Heim community (Type 3), secondary succession comm stands (Type 1) were 1807, 1782, and 1287 tree ha−1, respec- tively. This relation pattern is seen in the tropical rainforest of Phukhet Island as well (Kiratiprayoon, 1986). Normally, high density leads to a high percentage of basal area, but the basal area of Chang Island was not increased. TWI showed a posi- tive correlation with the basal area in this study (r = 0.928). The average TWI of Hopea pierrei Heim community (Type 3), se- condary succession community (Type 4), Calophyllum thorelii Pierre community (Type ype 1) was 4.13 ± 2.36, 1.70 ± 1.95, 1.29 ± 1.16, and 0.5 ± 0.55, respectively, while the percentage of basal area was 0.5886%, 0.4056%, 0.3396%, and 0.3845%, respectively. Many studies have shown the same results as our study, where mois- ture is a factor that can potentially control the above ground biomass in 6 forest types of Thailand: tropical evergreen rain forest, seasonal rain forest, lower mountain coniferous forest, upper mountain rain forest, mixed deciduous forest, and de- ciduous dipterocarp forest (Working group I: Scientific Basis of Climate Change, 2011; Rueangruea, 2009; Sunthisuk, 2006; Kiratiprayoon, 1986). Species response curve with regard to elevation showed six key species responding in three patterns (Figure 5). The Hopea pierrei Heim curve primarily showed a bell-shape curve (abundant at mid-elevation), which intersected the Calophyllum thorelii Pierre curve at an elevation at 550 m asl. It can be concluded that the Dipterocarpus (Hopea pierrei Heim) community tends to turnover to the Calophyllum thorelii Pierre community at elevations greater than 550 m asl, and turnover to the secondary succession community (Type 4) at low elevation with an increase of Memecylon garcinioides Bl., which is the other key species of this community. The response curve of Madhuca pierrei Lam and Tetrameles nudiflora R. Br., which are key species of lowland multi-aged stands (Type1), showed a negative correlation with elevation. A positive corre- lation of the Mesua ferrea Linn curve (key species of Calo- phyllum thorelii Pierre community, Type 2) intersected the Madhuca pierrei Lam curve at an elevation of 250 m asl. Moreover, the decrease of Hopea pierrei Heim at a low eleva- tion suggests that the limit of distribution of lowland multi-aged stands (Type1) is 250 m asl. Lithocarpus ceriferus A. Camus, Cleistocalyx operculatus Merr. and Perry, and Ficus callosa Willd were excluded because these species did not respond to a change in elevation (the curves ran parallel with the x-axis). Area of Community Based on the fit respond curve for elevation and TWI, we conclude that the environmental conditions of each community are as follows: Lowland multi-aged stands (Type 1): Elevation < 250 m asl and TWI < 2, Calophyllum thorelii Pierre commu- nity (Type 2): Elevation > 250 m asl and TWI < 5, Hopea pi- errei Heim community (Type 3): 250 < Elevation < 550 m asl and 2 < TWI < 5, Secondary succession community (Type 4): Elevation < 550 asl and 2 < TWI < 5 and Creek representation: All elevation gradient and TWI > 5. From the Figure 6, the total forest area was 17428.45 ha, which was comprised of low land multi-aged stands Type 1 (4807.99 ha or 27.59%), Calophyllum thorelii Pierre comm munity Type 3 (2358.54 h Type 4 (3731.24 ha or 21.41%), and creek area (1230.65 ha or 7.06%). Some areas of the secondary succession community were complicated. These areas were composed of Figure 5. Fit response curves between key species and elevation (the species Table 2c odes are shown in ). Figure 6. Area of four community types (Type 1-4) and creek. Copyright © 2013 SciRes. 47  N. PUMIJUMNONG, P. PAYOMRAT Copyright © 2013 SciRes. 48 two probable communities: the natural Dipterocarpus commu- nity and secondary succession community, because one of the factors controlling the secondary succession community was human disturbance. Therefore, it was not included in this study. Conclusion Chang Island has a particularly high evenness and richness of species. We investigated an area of 2.48 ha and recorde total of 78 species from a total of 32 families. Two-way cluster analysis classified the tropical rain forest into four sub-communities: lowland multi-aged stands (Type 1), Calophyllum thorelii Pi- erre community (Type 2), Hopea pierrei Heim community (Type 3), and secondary succession community (Type 4). Ele- vation and the topographic wetness index (TWI) affected the distribution of each community. Lowland multi-age stands, which had the highest diversity and richness, correlated with low elevation and relatively low TWI or dry conditions. The Calophyllum thorelii Pierre community had the highest tree density and correlated with high elevation and relative moder- ate to dry TWI conditions. The Hopea pierrei Heim community had the highest percentage of basal area and the lowest diver- sity, and was extensively covered by the Hopea pierrei Heim species. It correlated with a mid-elevation near creeks and had relatively wet TWI conditions. Finally, the secondary succes- sion community (Type 4) was related to mid to low elevation and relatively moderate to wet TWI conditions. REFERENCES Bird, M. I., Taylor, D., & Hunt, C. (2005). Palaeoenvironments of insular southeast Asia during the last glacial period: A savanna cor- ridor in Sundaland? Qua te rn ary Science Reviews, 24, 2228-2242. doi:10.1016/j.quascirev.2005.04.004 Boutrat, W. (2009). Relationship between soil properties with Poaceae and Cyperaceae on the grassland at Phra Thong island, Kuraburi district, Phangnga provs, Nakron Pratom: Mahi- dol University C ince. Master Thesi hamchumroon, V., & Puff, C. (2003) The Rubiaceae of Ko Chang, southeastern Thailand. Thai Forest Bulletin (Botany), 31, 13-26. Curtis, J. T. (1959). The vegetation of Wisconsin. Madison: University of Wisconsin Press. Environmental Research Institute (2007). Study area and the index of environmental quality to sustainable tourism and the island group project (phase 2). Bangkok: Chulalongkorn University. Esselstyn, J. A. & Brown, R. M. (2009). The role of repeated sea-level fluctuations in the generation of shrew (Soricidae: Crocidura) diver- sity in the Philippine Archipelago. Molecular Phylogenetics and Evolution, 53, 171-181 doi:10.1016/j.ympev.2009.05.034 Finch, H. (2005). Comparison of distance measures in cluster analysis with dichotomous data. Journal of Data Science, 3, 85-100. Giesler, R., Högberg, M., & Högberg, P. (1998). Soil chemistry and plant in Fennoscandian boreal forest as exemplified by a local gradi- ent. Ecology, 79, 119-137. doi:10.1890/0012-9658(1998)079[0119:SCAPIF]2.0.CO;2 Ge K(1986) Comparative study on the structure of the rat- tware Design. Pu Thong is- Ral study. R The land and forest resources. Conser- Rontane forests in Thailand. Mas- S Wildlife and Plant Conservation. /bhl.title.55188 öltenboth, F., Langenberger, G., & Widmann, P. (2007). Tropical lo- land evergreen rainforest. Ecology of Insular Southeast Asia: Th Indonesian Archipelago, 297-384. Hill, D., Fasham, M., Tucker, G., Shewry, M., & Shaw, P. (2007). Handbook of biodiversity methods. Cambridge: Cambridge Univer- sity Press. iratiprayoon, S. tan bearing tropical rain forests. Master Thesis, Bangkok: Kasetsart University McCune, B., & Grace, J. B. (2002). Analysis of ecological communities. Glenden Beach: MJM Sof Office of Science for Land Development (2005). Manual analysis of soil, water, fertilizers, soil and plant analysis for certification (2nd ed.). Bangkok: Land Development Department. Payomrat, P. (2011). Effects of ecological factors on vegetation and carbon stocks at Samesan island, Chonburi, Thailand. Master Thesis, Nakron Pratom: Mahidol University mijumnong, N. (2005). Effects of the tsunami on the Pra land ecosystem. Nakron Pratom: Mahidol University. ichards, P. W. (1996). The tropical rainforest: An ecologic Cambridge: Cambridge University Press. oyal Forest Department (1997). Master plan report Koh Chang marine national park, Trad province. vation of natural resources. Royal Forest Department. (2005). Master plan report Ton Nga Chang wildlife sanctuary, Songkhla province. ueangrues, S. (2009). Structure of M ter Thesis, Bangkok: Kasetsart University anthisuk, T. (2006). Forest of Thailand. Bangkok: Department of Na- tional Parks, Schmidt, J. (1900). Flora of Koh Chang: Contributions to the knowl- edge of the vegetation in the Gulf of Siam. Copenhagen: B. Luno. doi:10.5962 different methods based on T of geology, Chang ter M. (1995). Canonical corre- 877430 Sørensen, R., Zinko, U., & Seibert, J. (2005). On the calculation of the topographic wetness index: Evaluation of field observation. hydrology and earth system sciences discussions, 1807-1834. ansuwan, V., & Kitisarom, N. (2007). Study report island, Trat Province. Bureau of Geology, Department of Mineral Resources. Braak, C. J. F., & Verdonschot, P. F. spondence analysis and related multivariate methods in aquatic eco- logy. Aquatic Science, 57, 255-289. doi:10.1007/BF00 at province. ince. Master Thesis, Nakron Pratom: Tration routes of large mam- m Thailand. Palaeo, 168, Whange (2011). Thai- Z, M., & Nilsson, C. (2005) Plant species Thai Meteorological Department (2010). Data of climate between the years 1990-2009 at Khlong Yai station, Tr Thaisatuen, T. (2010). Multivariate analysis of relationship between plant communities and soil properties on Phra Thong island, Kura- buri district, Phangnga prov Mahidol University. ougard, C. (2001). Biogeography and mig mal faunas in South-East Asia during the late middle pleistocene: Focus on the fossil and extant faunas fro 337-358. orking Group I: Scientific Basis of Climate C land’s first assessment report on climate change 2011. Bangkok: The Thailand Research Fund. inko, U., Seibert, J., Dynesius numbers predicted by a topographhy based groundwater-flow index. Ecosystem, 8, 430-441. doi:10.1007/s10021-003-0125-0 |

