Paper Menu >>
Journal Menu >>
 Journal of Environmental Protec tion, 2013, 4, 5-9 doi:10.4236/jep.2013.41b002 Published Online January 2013 (http://www.SciRP.org/journal/jep) Copyright © 2013 SciRes. JEP 5 Recycle of Wastewater from Lead-Zinc Sulfide Ore Flotation Process by Ozone/BAC Techonlogy Liu Xingyu1, Chen Bowei1, Li Wenjuan1, Song Yongsheng1, Wen Jiankang1, Wang Dianzuo2 1National Engineering Laboratory of Biohydrometallurgy, General Research Institute for Nonferrous Metals, Beijing, China; 2General R esearch In s titute for Nonferrous Metals, Beijing, China. Email: wellwoodliu@gmail.com Received 2013 ABSTRACT Lead-zinc sulphide ore contains lead sulphide (galena), and zinc sulphide (sphalerite). I n the first flotatio n stage, galena is rendered hydrophobic with an organic collector such as xanthate, while sphalerite is kept from floating by depressants, and in the second flotation stage, activator was used to activated zinc flotation. Since the organic regent used are differ- ent in the two flotation stage, wastewater from the second zinc flotation stage can’t be directly recycled to the first lead flotation stage. Wastewater from flotation process for concentrating lead-zinc sulphide ore often containing organic compounds such as diethyldithiocarbamate(DDTC), xanthate, terpenic oil(2# oil) and thionocarbamate esters (Z-200), are environmentally hazardous. Their removal from contaminated water and the reuse of the water is one of the main challenges facing lead-zinc sulphide ore processing plants. In this study, synthetic wastewater containing DDTC, xan- thate, 2# o il and Z-200 at concentrations ranging from 21 to 42 mg/L was fed into an Ozone/Biological activated carbon (BAC) reactor. Analyses of the effluent indicated a chemical oxygen demand (COD) removal over 86.21% and Total organic carbon (TOC) removal over 90.00% were achieved under Hydraulic retention time (HRT) of 4h and O3 feeding concentration of 33.3 mg/L. The effluent was further recycled to the lab scale lead concentrating process and no sig- nificant difference was found in compare with fresh water. Furthermore, lead-zinc sulphid e mineral co ncentra ting process was carried out at lab scale. The produced wastewater was treated by Ozone/BAC reactor at O3 feeding concentration of 16.7 mg/L and HRT o f 4h. The e fflue nt anal ysis showe d th at TO C remova l was 74. 58%. T his e ffluent was r ecycled to the lab scale lead-zinc sulphide mineral concentrating process and the recovery of lead was not affected. The results showed that b y u sing Ozone/BAC technolo gy, the lead-zinc sulphide mineral processing wastewater could be recycled. Keywords: Lead-Zinc Sulphide Ore; Ozone/BAC; Flotation Wastewater; Recycle 1. Introduction Flotation is in fact the most common process in metallic mineral separation and is the main way for recovering valuable metals as lead (Pb) and zinc (Zn) (Barbaro, 2000). For Lead-zinc sulphide ore, the most widely used method is that of two stage selective flotation, where the zinc and iron minerals are depressed, allowing the galena to float, follo wed by the activatio n of the zinc minerals i n the lead tailings to allow a zinc float (Wills and Napier- Munn, 2005; Ki nal et a l., 2009). Wastewater from flotatio n process for concentrating lead-zinc sulphide ore often containing organic compounds such as DDTC, xanthate, 2# oil and Z-200, are environmentally hazardous. Since the organic regent used are different in the two flotation stage, wastewater from the second zinc flotation stage can’t be directly recycled to the first lead flotation stage. Thus such organic regents removal from contaminated water and the reuse of the water is one of the main challenges facing lead-zin c sulphi de ore pr ocess ing pl ants . Ozonation of water is a well-known technology and the strong oxidative properties of ozone have been well documented (Glaze et al., 1987). Ozone alone has the ability to genera te hydro xyl radic als (•OH) when applied to wastewater due to side reactions with electron-rich moieties, such as amines, phenols, and alkoxylated aromatics (Nöthe et al., 2009; Pocostales et al. 2010). In this way, the aromatic or hydrophobic organic compounds can be converted to more hydrophilic, biodegradable organic compounds, such as aldehydes, carboxylic acids, ketones and other organic acids (Huang et al., 2005; Hammes e t al ., 2006). With respect to oxidation byproducts, recent studies suggest that post-ozone biological filtra- tion (BAC) is sufficient to eliminate the toxicity that has been attributed to ozonation of effluent organic matter (EfOM) (Chiang et al., 20 02 ; Xu et a l. , 20 07 ; S talt er et al. , 2010). Since Oone /BAC t echno logy ha s man y adva ntage s such as low cost, high e fficie ncy, short HRT , it might be  Recycle of Wastewat er from Lea d-Zinc Sulfide Ore Flotation Process by Ozone/BAC Techonlogy Copyright © 2013 SciRes. JEP 6 a suitable treatment for wastewater effluent from lead- zinc sulphide ore flotation. The purpose of this study was to determine whether ozone/BAC technology could be applied in lead-zinc sulphide or e flotation waste water treat ment. That include investigate the characteristics of degradation/conversion of organic regents used in lead-zinc flotation process in ozone/BAC process and whether the treated water could be reused back to lead circuit. The following sections summarize the methodology and results from synthetic flotation wastewater and real lab produced flotation wastewater treatment over the course of five months of continuous o peration and the evaluation o f recycle of the treated water. 2. Materials and Methods 2.1. Synthetic Wastewater and Real Lab Produ ced Waste water Synthetic wastewater and real lab produced wastewater were fed into ozonation and BAC treatment system, re- spectively. Synthetic wastewater composed of DDTC, xanthate, 2# oil and Z-200, with concentration adjusted to concentrations ranging from 21 to 42 mg/L. The above regents were provided by Chehe lead-zinc mining Crop. (Guanxi, China). The lead-zinc sulphide ore used in this research were provided by Zhangbeizhuan lead-zinc mine (Heibei, China). Table 1 were the chemical composition of the t wo Z ha ngb ei z hua n l ea d -zinc sulphide ore samples used in this research. The lab produced wastewater was from lab scale Zhangbeizhuan lead-zinc sulphide ore flota- tion process via a flow sheet showed in Figure 1. Table 1 . Che mical c o mposit i on o f t he tw o Zhanbeizhuan l e ad- zinc sulphide or e sample s used in this res earch. Elements Composition (%) Sample A Sample B Cu - 0.071 Pb 3.47 2.86 Zn 1.87 2.01 Fe - 9.41 Cd - 0.014 Ca - 0.29 Mg - 0.46 As - 1.87 Al2O3 - 10.03 SiO2 - 57.88 Ag/g/t - 93.5 Au/g/t - 0.4 Mo - 0.001 C - 0.98 Figure 1. F low sheet of the Zhangbeizhuan lead-zinc sulphide ore flotation process (time used were minute, concentration showed were mg/l).  Recycle of Wastewat er from Lea d-Zinc Sulfide Ore Flotation Process by Ozone/BAC Techonlogy Copyright © 2013 SciRes. JEP 7 2.2. Reactor Set-Up and Reuse of the Treated Wastewater Ozone was gener ated f r om oxygen by means of a PL A SMA ozone generator with maximum capacity of 20 mg/min. The ozone reaction chamber is a 10 × 20 × 20 (cm) rec- tangular plexiglas tank and had working volume of 2 L. There was a cover o n the top of the tank that prevented the volatilizatio n of ozone. The tank was fitted at the b ottom with a stone diffuser connected to the ozone generator. Because of this configuration, ozone concentration in the gas phase could not be independently adjusted and the dose of ozone was controlled by changing the gas flow rate. Effluent was drawn from a port at 10 cm above the base and entered another similar plexiglas tank filled with activated carbon for BAC treatment. During the start-up stage, activated sludge were inoculated in to the BAC reactor which would allow the growth of bacteria on the surface of the activated carbon. During the whole experiments, the HRT of ozone re- actor and BAC reactor were maintained at 2 h, 2 h respect- tively. T he reuse of the treated water to the lead flotation circuit was conducted via a flow sheet showed in Figure 2. 2.3. Analytical Methods TOC was measured with a TOC analyzer (SHIMADZU, TOC-5000). COD were determined by the standard photo- metric method (DWAF, 1992) using the Spectroquant Nova 60 Photometer supplied by Merck NT PTY Ltd. Samples for COD analyses were digested with Merck Thermo reactor Model TR 300 and then analysed by the Merck Nova 60 photometer. 3. Results and Discussion 3.1. Ozone/BAC Reactor Performance Treating Synthetic Wastewater Synthetic wastewater composed of DDTC, xanthate, 2# oil and Z-200, with concentration adjusted to 42 mg/l, 28 mg/l, 21 mg/l and 21 mg/l respectively was feeding into the ozone/BAC reactor. As mentioned before, the HRT was maintained at 4 h. Different ozone feeding concentration was applied to the ozone reacto r. Under each feeding co n- centration, the reactor was operated for one week, and then the influent and effluent were sampled for TOC and COD a nal ysis. Tab le 2 sho wed TOC and COD remo va l of the Ozone/BAC reactor treating synthetic wastewater unde r d iffe rent fee di ng ozo ne conc entration. Ozo ne feeding concentration could significa ntly affect the TOC and COD rem oval, at r elat ive l ow fe e d in g c oncentration of 13.2 mg/ L, the TOC removal were only 58.33% while at ozone feed- ing concentration of 33.3 mg/L, the TOC removal ex- ceeded 90 %. The re is ve r y stro ng co r re la tio n o f T O C and COD concentration according to the four organic regents which adde d into the synthetic wastewater (Table 2). Figure 2. Flow sheet of the lead flotation circ uit which use the t reated wastewater, untreated wastewater and fresh water. Table 2. TOC and COD removal of the O zone/B AC reactor treating synthetic wastewater. Ozone conce ntration ( mg / L ) Influent TOC ( mg / L ) Effluent TOC ( mg / L ) TOC removal (%) Infl uent COD ( mg / L ) Effluent COD ( mg / L ) CO D removal (%) 13.3 18 7.5 58.33 85 42 50.59 16.7 18 5.4 70 87 50 42.53 22.2 18 1.8 90 116 16 86.21 33.3 18 1.8 90 98 12 87.76 66.6 18 - - 82 22 73.17  Recycle of Wastewat er from Lea d-Zinc Sulfide Ore Flotation Process by Ozone/BAC Techonlogy Copyright © 2013 SciRes. JEP 8 3.2. Reuse o f the Treated Synth etic Wastewater to the Lead Circuit Unde r t he o zone fe ed i n g co nc entr a t ion of 3 3. 3 mg/L, 10 L effluent was collected and recycled to the lead circuit. Zhangbeizhuang ore sample A was used for this part of work. Table 3 showed the comparison of lead recovery in the lead circuit using treated synthetic wastewater and fresh water. Compared with fresh water, by using th e treated synthetic wastewater, the production rate was increased from 10.31% to 11.81% while the lead grade in the lead concentrate was decreased from 29.92% to 26.30%, thus the lead recovery has no significant change(treated syn- thetic wastewater: 88.95%, fresh water: 88.89%). By using the treated synthetic wastewater, zinc grade have no significant change but the zinc recovery increased from 15.5 to 18.61%, which indicate that using treated synthetic waste water, small amount of zinc may entering lead circuit. This effect is now under further investigation. 3.3. Ozone/BAC Reactor Performance Treating Real Lab Produced Wastewater Lab scale Zhangbeizhuang ore sample B flotation was carried out for the production of real lab flotation waste- water. Tabl e 4 showed T OC removal of the Ozone/ BAC reactor treating real lab produced flotation wastewater under different ozone feeding concentration. Unlike syn- thetic wastewater, there is no strong correlation between ozone feeding concentration and the TOC removal. Un- der feeding ozone concentration of 16.7 mg/l, the highest TOC removal of 74.58% was achieved. It is worth to note that TOC of the lab produced wastewater is lower than the synthetic wastewater. The removal rate for the produced wastewater is lower than synthetic wastewater, this may due to more complex organic within the pro- duced wastewater. 3.4. Reuse o f the Treated R eal Flot a tion Wastewater to the Lead Circuit Under the ozone feeding concentration of 16.7 mg/L, 10 L treated lab produced wastewater effluent was collected. Together with another 10 L untreated lab produced waste- water and fresh water were applied to the lead flotation process respectively. Zhangbeizhuang ore sample B was used for this part of work. Table 5 showed the compare- son of lead recovery in the lead circuit using treated real lab produced wastewater, untreated real lab produced wastewater and fresh water. Compared with fresh water, by using the untreated lab produced wastewater, the production rate was increased from 10.10% to 15.35%, the lead recovery increased from 83.40% to 87.83%, and the lead grade in the lead concentrate was significantly decreased from 23.57% to 16.71%. Meanwhile, the zinc grade in the lead concentrate increased from 2.62% to 8.84% and the zinc recovery in the lead concentrate in- creased from 12.05% to 65.34%. The above results indi- cated that directly recycle of zinc flotation wastewater to the lead circuit may have negative effect on lead flotation, large amount o f zinc will en tering le ad circ uit. Whe n the treated lab produced wastewater was used, all the indices were nearly similar if compared with fresh water (Table 5). This indicated that by using ozone/BAC technology, the treated flotation wastewater could recycle to lead circuit, and the lea d flotation wa s not affected. 4. Conclusions This study investigates the performance of ozone/BAC treatment processes for s ynthetic lead -zinc flotat ion wa ste- water and real lab produced lead-zinc flotation wastewa- ter, and evaluates the reuse of treated water back to lead circuit. The conclusions obtained from this study are as follows: Table 3. Co mpa rison of lead recov ery in the le ad circuit using treated synthetic wastew ater and fresh water. Production rate (%) Lead gr ad e(%) Zinc grade (%) Lead recovery(%) Zinc recovery(%) A* B* A* B* A* B* A* B* A* B* Lea d concentrate 11.87 10.31 26.3 29.92 2.87 2.84 89 88.89 18.6 15.5 Tailing 88.13 89.69 0.44 0.43 1.69 1.78 11.1 11.11 81.4 84.5 Raw ore 100 100 3.51 3.47 1.83 1.9 1 00 100 100 100 *A: treated synthetic wastewater; *B: fresh wate r. Table 4. TOC removal of the Ozo ne/BAC reactor treating real lab produced flotation wastewater. Ozone concentration (mg/l) Influent TOC (mg/l) Effluent TOC (mg/l) TOC removal (%) 13.3 14.4 5.06 64.86 16.7 14.4 3.66 74.58 22.2 14.4 4.04 71.94 33.3 14.4 5.31 63.13 66.6 14.4 6.49 54.93 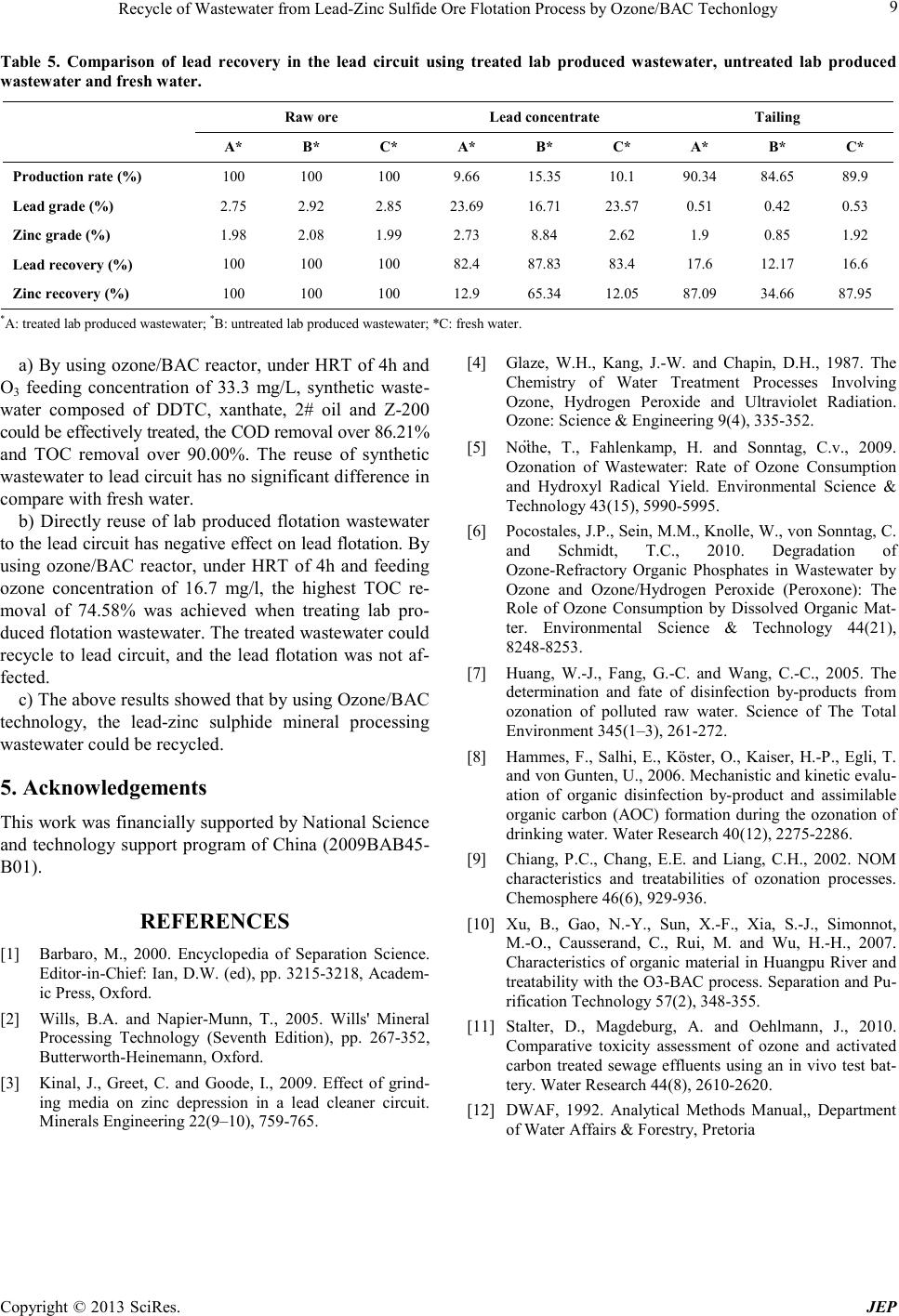 Recycle of Wastewat er from Lea d-Zinc Sulfide Ore Flotation Process by Ozone/BAC Techonlogy Copyright © 2013 SciRes. JEP 9 Table 5. Comparison of lead recovery in the lead circuit using treated lab produced wastewater, untreated lab produced w ast ewater and fresh water. Raw ore L ead concentrate Tailing A* B* C* A* B* C* A* B* C* Production rate (%) 100 100 100 9.66 15.35 10.1 90.34 84.65 89.9 Lead grade (%) 2.75 2.92 2.85 23.69 16.71 23.57 0.51 0.42 0.53 Zinc grade (%) 1.98 2.08 1.99 2.73 8.84 2.62 1.9 0.85 1.92 Lea d recovery (%) 100 100 100 82.4 87.83 83.4 17.6 1 2.17 16.6 Zinc recovery (%) 100 100 100 12.9 65.34 12.05 87.09 34.66 87.95 *A: treated lab produced wastewater; *B: untreated lab produced wastewater; *C: fresh water. a) By usi ng ozone /BAC r eactor , under HRT o f 4h and O3 feeding concentration of 33.3 mg/L, synthetic waste- water composed of DDTC, xanthate, 2# oil and Z-200 could be effectively treated, the COD removal over 86.21% and TOC removal over 90.00%. The reuse of synthetic wastewater to le ad circui t has no si gnificant difference in compare with fresh water. b) Directly reuse of lab produced flotation wastewater to the l ead circ uit has ne gative effect o n lead flot ation. B y using ozone/BAC reactor, under HRT of 4h and feeding ozone concentration of 16.7 mg/l, the highest TOC re- moval of 74.58% was achieved when treating lab pro- duced flotation wastewater. The treated wastewater could recycle to lead circuit, and the lead flotation was not af- fected. c) The a bo ve r e sults s ho wed t ha t by usi ng O zo ne /BAC technology, the lead-zinc sulphide mineral processing wastewater could be recycled. 5. Acknowledgements Thi s work was fina nci all y sup po rted by Nat iona l Sc ienc e and technology support program of China (2009BAB45- B01). REFERENCES [1] Barbaro, M., 2000. Encyclopedia of Separation Science. Editor-in-Chief: Ian, D.W. (ed), pp. 3215-3218, Academ- ic Press, Oxford . [2] Wills, B.A. and Napier-Munn, T., 2005. Wills' Mineral Processing Technology (Seventh Edition), pp. 267-352, Butterworth-Heinemann, Oxford. [3] Kinal, J., Greet, C. and Goode, I., 2009. Effect of grind- ing media on zinc depression in a lead cleaner circuit. Minerals Engineering 22(9–10), 759-765. [4] Glaze, W.H., Kang, J.-W. and Chapin, D.H., 1987. The Chemistry of Water Treatment Processes Involving Ozone, Hydrogen Peroxide and Ultraviolet Radiation. Ozone: Science & E ngineering 9(4), 335 -352. [5] Nöthe, T., Fahlenkamp, H. and Sonntag, C.v., 2009. Ozonation of Wastewater: Rate of Ozone Consumption and Hydroxyl Radical Yield. Environmental Science & Technology 43(15), 5990-5995. [6] Pocostales, J.P., Sein, M.M., Knolle, W., von Sonntag, C. and Schmidt, T.C., 2010. Degradation of Ozone-Refractory Organic Phosphates in Wastewater by Ozone and Ozone/Hydrogen Peroxide (Peroxone): The Role of Ozone Consumption by Dissolved Organic Mat- ter. Environmental Science & Technology 44(21), 8248-8253. [7] Huang, W.-J., Fang, G.-C. and Wang, C.-C., 2005. The determination and fate of disinfection by-products from ozonation of polluted raw water. Science of The Total Environment 345(1–3), 26 1-272. [8] Hammes, F., Salhi, E., Köster, O., Kaiser, H.-P., Egli, T. and von Gunten, U., 2006. Mechanis tic and kin etic evalu- ation of organic disinfection by-product and assimilable organic carbon (AOC) formation during the ozonation of drinking water. Water Research 40(12), 2275-2286. [9] Chiang, P.C., Chang, E.E. and Liang, C.H., 2002. NOM characteristics and treatabilities of ozonation processes. Chemosphere 46(6), 929-936. [10] Xu, B., Gao, N.-Y., Sun, X.-F., Xia, S.-J., Simonnot, M.-O., Causserand, C., Rui, M. and Wu, H.-H., 2007. Characteristics o f organic material in Huangpu River and treatability with the O3-BAC process. Separat ion and Pu- rification Technology 57(2), 348-355. [11] Stalter, D., Magdeburg, A. and Oehlmann, J., 2010. Comparative toxicity assessment of ozone and activated carbon treated sewage effluents using an in vivo test bat- tery. Water Resear c h 44(8), 2610-2620. [12] DWAF, 1992. Analytical Methods Manual,, Department of Water Affairs & Forestry, Pretoria |

