Paper Menu >>
Journal Menu >>
 Psychology, 2010, 1: 209-219 doi:10.4236/psych.2010.13028 Published Online August 2010 (http://www.SciRP.org/journal/psych) Copyright © 2010 SciRes. PSYCH 209 Is a Divergent Central Serotonergic Activity Responsible for Either Despair or Learning Behavior in Intact Wistar or Sprague-Dawley CD Rats, Respectively? A Concomitant Behavioral and Electrochemical Analysis Francesco Crespi Biology Deparment, Neurosciences CEDD GlaxoSmithKline, Medicines Research Centre, Verona, Italy. Email: francesco.m.crespi@gsk.com Received May 11th, 2010; revised June 28th, 2010; accepted June 30th, 2010. ABSTRACT Behavioral observations combined with electrochemical analysis have been performed in Wistar or Sprague-Dawley CD rats in the attempt to clarify earlier controversial behavioral reports. In particular, these rats were submitted to FST and to repeated Forced Swimming (rFS, during 4 days). In parallel, voltammetric in vivo analysis of serotonin (5-HT) levels in platelet-rich plasma (PRP) collected daily from these animals was also performed as it is known that peripheral 5-HT levels monitored in rat PRP mirror cerebral 5-HT contents. Thus, combined behavioral-voltammetric studies allow deducing changes of central 5-HT levels that could be correlated to FST or rFS, with the advantage of non invasive analysis of central neurotransmitter activities in intact behaving animals. In particular, combined behav- ioral-voltammetric results suggest that “behavioral despair” is the process interesting Wistar rats when submitted to FST or rFS while “learning to be immobile” is the process involving Sprague-Dawley CD rats. Keywords: Rat Strains, Behavior, Electrochemistry, Fluoxetine, Serotonin, Platelets 1. Introduction Many studies employing the forced swimming test (FST) which is a behavioral test that predicts the clinical effi- cacy of many types of antidepressant drugs [1] have been done using Sprague-Dawley CD rats. A minor number of such experiments have been done in Wistar rats. In the Sprague-Dawley CD strain of rodents submitted to FST in 15 cm water depth [2-5] or 30 cm water depth [6-10] an antidepressant drug such as the selective serotonin reuptake inhibitor (SSRI) fluoxetine produced dose-de- pendent reduction of immobility at doses ranged between 5 or 80 mg/kg. An antidepressant drug such as the selective serotonin reuptake inhibitor (SSRI) fluoxetine at doses ranged be- tween 5 or 80 mg/kg produced dose-dependent reduction of immobility in Sprague-Dawley CD rats submitted to FST performed in 15 cm water depth [2-5] or in 30 cm water depth [6-10]. In contrast, treatment with fluoxetine increased immobility in Wistar rats tested in deep water (30 cm) [11,12] while a decrease or no modification of immobility was monitored when FST was performed in 15 cm water depth [13,14]. Thus, following treatment with fluoxetine, differences in immobility behavior be- tween these two strains of rodents appear when tested in lower water (15 cm) and become evident when tested in deeper water (30 cm). Again, in Sprague-Dawley CD rats peripheral fluoxetine (from 5 to 20 mg/kg) significantly and dose-dependently increased swimming behavior [6,7, 11] while in Wistar rats only the highest dose of fluoxet- ine did so [11]. Conversely, fluoxetine did not alter climbing at any dose tested (5-80 mg/kg) in any of the two rat species [4,6,8-11]. It is known that the swimming behavior is closely re- lated to the activity of cerebral 5-HT [4,6]. Thus, one can propose that the different sensitivity to fluoxetine behav- iorally displayed by the two strains may be related to dissimilarity within their serotonergic system. The current study has been undertaken to analyse this hypothesis. Sprague-Dawley and Wistar rats have been  Is a Divergent Central Serotonergic Activity Responsible for Either Despair or Learning Behavior in Intact 210 Wistar or Sprague-Dawley CD Rats, Respectively? A Concomitant Behavioral and Electrochemical Analysis submitted to FST in deep water (30 cm) after intraperi- toneal treatment with saline (vehicle) or with fluoxetine 20 mg/kg as this appears to be the dose significantly af- fecting the swimming behavior within both types of ro- dents [11]. Deep water has been selected as in such con- ditions rats cannot support themselves by touching the ground and they are obliged to swim and/or climb more actively [4,6]. Furthermore, the duration of the swim- ming test was prolonged up to a total of 4 days [15]. This has been done since previous reports have observed de- velopment of habituation in Wistar rats submitted to re- peated FST [16-18] and that pharmacological blockade of serotonergic activity enhances learning and memory skills [19,20]. Those reports were followed by other studies with the subsequent suggestion that the process involved in FST could be “learning to be immobile” [21,22] more than “behavioral despair” [1,3,4]. In the present work, application of rFS to Sprague-Dawley and Wistar rats together with electrochemical analysis of in vivo serotonergic activities would investigate this hy- pothesis. In particular, voltammetric measurements of 5-HT have been performed in PRP obtained from blood collected from the tail vein of naive or “treated” con- scious Sprague-Dawley or Wistar rats, daily. Many clues in the literature suggest that “peripheral” 5-HT monitored in PRP is directly related to the levels of cerebral 5-HT [23-27]. The feasibility of selective moni- toring of 5-HT by means of voltammetry together with specifically treated carbon fibre micro-electrodes (mCFE) has been demonstrated either in brain as well as in blood [28-32]. Furthermore, we have demonstrated that periph- eral 5-HT levels monitored by means of differential pulse voltammetry (DPV) together with mCFE in rat PRP mirror cerebral 5-HT contents [28]. Therefore, in the present work, this electrochemical methodology has been applied to analyze daily the influence of behav- ioral-pharmacological tests upon in vivo serotonergic levels in the PRP of conscious rats. Substantially, moni- toring “peripheral” 5-HT in PRP in alternative to the analysis of cerebral 5-HT avoids submitting the rats to: 1) Surgery performed under major reversible anesthesia i.e. chloral hydrate [33,34] for implantation of the mCFE within the brain; ii) Daily substitution of the exhausted mCFE, performed under halotane anesthesia, when cen- tral 5-HT is monitored chronically [35]. 2. Material and Methods 2.1 Animals Male Sprague-Dawley CD rats and male Wistar rats (200-250g) were supplied by Charles-River (Italy) and were kept in temperature- and humidity-controlled rooms (22°C, 50%) with lights on from 0700 to 1900 hours with water and food available ad libitum. All procedures were carried out in accordance with the Italian law (Legisla- tive Decree no.116, 27 January 1992), which acknowl- edges the European Directive 86/609/EEC, and were fully compliant with GlaxoSmithKline policy on the care and use of laboratory animal and codes of practice. Fur- thermore, all efforts were made to minimize the number of animals used and their suffering. 2.2 Behaviour Studies Rats were exposed to FST according to Porsolt et al. [1] except that the water was deeper so that rats cannot mod- ify the effects of the forced swim by developing behav- ioral adaptations, such as when they touch the bottom or sides of the tank. Briefly, the animals were placed indi- vidually into a cylinder glass tank [40 cm height; 20 cm diameter] containing water 30 cm deep and at 23-25°C according to Detke et al. [6] and Detke & Lucki [4]. Two swim sessions were conducted i.e. a 15-min pre-test fol- lowed 24 h later by a 5-min test. Separate groups of rats received intraperitoneally ei- ther saline (Sprague-Dawley CD rats n = 5; Wistar rats n = 5) or fluoxetine 20 mg/kg (Sprague-Dawley CD rats n = 6; Wistar rats n = 6) in a volume of 2 ml/kg. Each treatment was administered 23 h, 5 h and 1 h prior to the test as described earlier [4,6]. The 20 mg/kg dose of fluoxetine was also chosen because it has been demon- strated that in some animal models of anxiety, acute fluoxetine treatment may elicit a bell-shaped dose-re- sponse curve with a maximum effect at 20 mg/kg [36]. Other 5 Sprague-Dawley rats and 5 Wistar rats were exposed to a modified FST on four consecutive days. The modified test was called rFS as described earlier [15-17]. On the first day of rFS, rats swam for 15 min. subsequently they were removed from the water tank and dried under warm air. Other three swimming sessions of 5 min each were then conducted (spaced 24 h apart) on the following three days. A time-sampling method was used as described pre- viously [6] in order to score several behaviors during a single viewing. This method has been selected as it has shown to be reliable and valid for detecting the effect of different antidepressant drugs. In particular, immobility, swimming and climbing was monitored in 5-sec period. Briefly, immobility was scored when the animal was making the minimum movements necessary to keep its head above water and stay afloat. Swimming was scored when the animal actively swam around the tank, making movements greater than that necessary to stay afloat. Climbing was scored when the animal made vigorous thrashing movements with its forepaws in and out of wa- ter. It was usually directed against the wall of the tank. All the watching and scoring were performed by the Copyright © 2010 SciRes. PSYCH 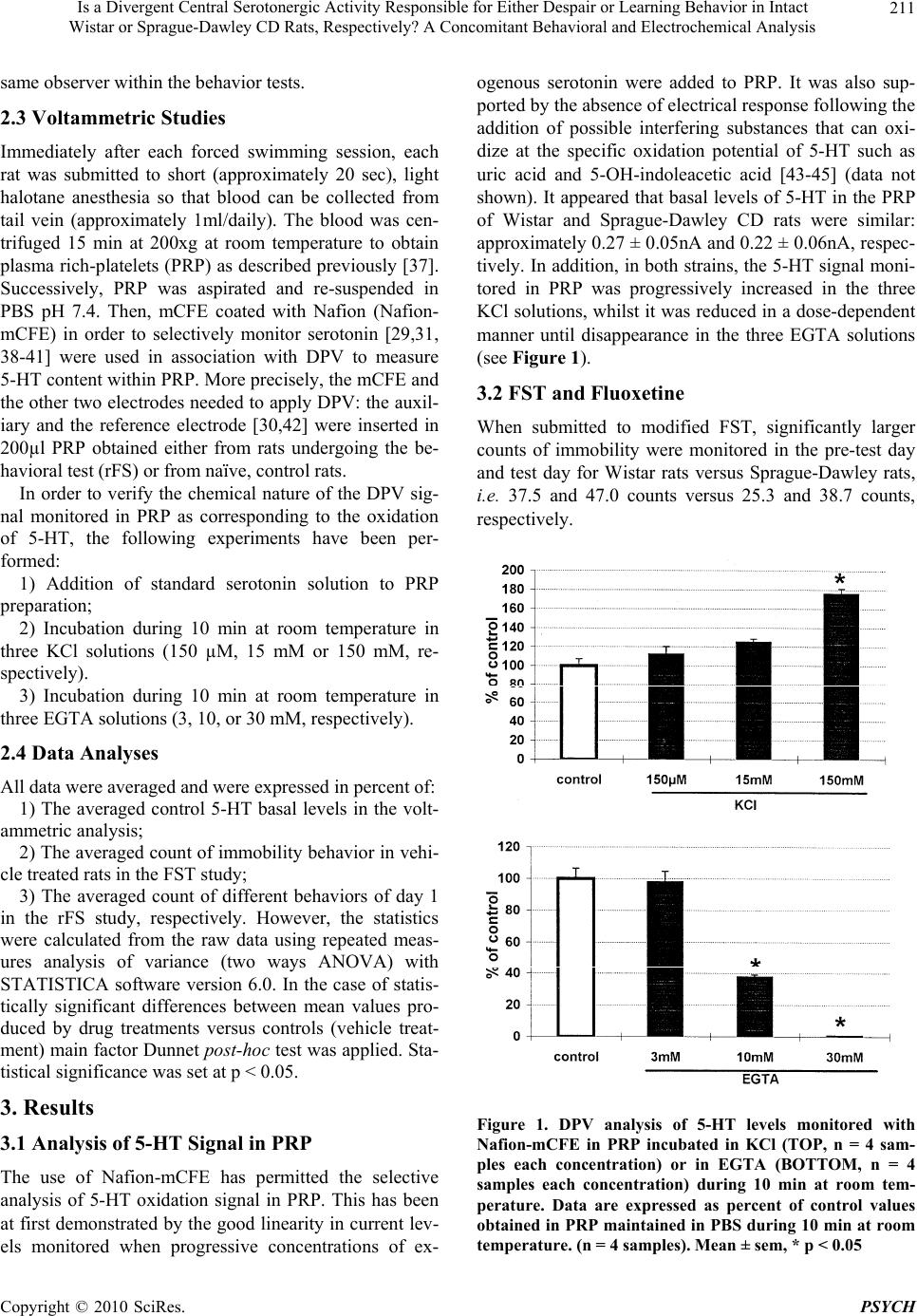 Is a Divergent Central Serotonergic Activity Responsible for Either Despair or Learning Behavior in Intact 211 W istar or Sprague-Dawley CD Rats, Respectively? A Concomitant Behavioral and Electrochemical Analysis same observer within the behavior tests. 2.3 Voltammetric Studies Immediately after each forced swimming session, each rat was submitted to short (approximately 20 sec), light halotane anesthesia so that blood can be collected from tail vein (approximately 1ml/daily). The blood was cen- trifuged 15 min at 200xg at room temperature to obtain plasma rich-platelets (PRP) as described previously [37]. Successively, PRP was aspirated and re-suspended in PBS pH 7.4. Then, mCFE coated with Nafion (Nafion- mCFE) in order to selectively monitor serotonin [29,31, 38-41] were used in association with DPV to measure 5-HT content within PRP. More precisely, the mCFE and the other two electrodes needed to apply DPV: the auxil- iary and the reference electrode [30,42] were inserted in 200µl PRP obtained either from rats undergoing the be- havioral test (rFS) or from naïve, control rats. In order to verify the chemical nature of the DPV sig- nal monitored in PRP as corresponding to the oxidation of 5-HT, the following experiments have been per- formed: 1) Addition of standard serotonin solution to PRP preparation; 2) Incubation during 10 min at room temperature in three KCl solutions (150 µM, 15 mM or 150 mM, re- spectively). 3) Incubation during 10 min at room temperature in three EGTA solutions (3, 10, or 30 mM, respectively). 2.4 Data Analyses All data were averaged and were expressed in percent of: 1) The averaged control 5-HT basal levels in the volt- ammetric analysis; 2) The averaged count of immobility behavior in vehi- cle treated rats in the FST study; 3) The averaged count of different behaviors of day 1 in the rFS study, respectively. However, the statistics were calculated from the raw data using repeated meas- ures analysis of variance (two ways ANOVA) with STATISTICA software version 6.0. In the case of statis- tically significant differences between mean values pro- duced by drug treatments versus controls (vehicle treat- ment) main factor Dunnet post-hoc test was applied. Sta- tistical significance was set at p < 0.05. 3. Results 3.1 Analysis of 5-HT Signal in PRP The use of Nafion-mCFE has permitted the selective analysis of 5-HT oxidation signal in PRP. This has been at first demonstrated by the good linearity in current lev- els monitored when progressive concentrations of ex- ogenous serotonin were added to PRP. It was also sup- ported by the absence of electrical response following the addition of possible interfering substances that can oxi- dize at the specific oxidation potential of 5-HT such as uric acid and 5-OH-indoleacetic acid [43-45] (data not shown). It appeared that basal levels of 5-HT in the PRP of Wistar and Sprague-Dawley CD rats were similar: approximately 0.27 ± 0.05nA and 0.22 ± 0.06nA, respec- tively. In addition, in both strains, the 5-HT signal moni- tored in PRP was progressively increased in the three KCl solutions, whilst it was reduced in a dose-dependent manner until disappearance in the three EGTA solutions (see Figure 1). 3.2 FST and Fluoxetine When submitted to modified FST, significantly larger counts of immobility were monitored in the pre-test day and test day for Wistar rats versus Sprague-Dawley rats, i.e. 37.5 and 47.0 counts versus 25.3 and 38.7 counts, respectively. Figure 1. DPV analysis of 5-HT levels monitored with Nafion-mCFE in PRP incubated in KCl (TOP, n = 4 sam- ples each concentration) or in EGTA (BOTTOM, n = 4 samples each concentration) during 10 min at room tem- perature. Data are expressed as percent of control values obtained in PRP maintained in PBS during 10 min at room temperature. (n = 4 samples). Mean ± sem, * p < 0.05 Copyright © 2010 SciRes. PSYCH 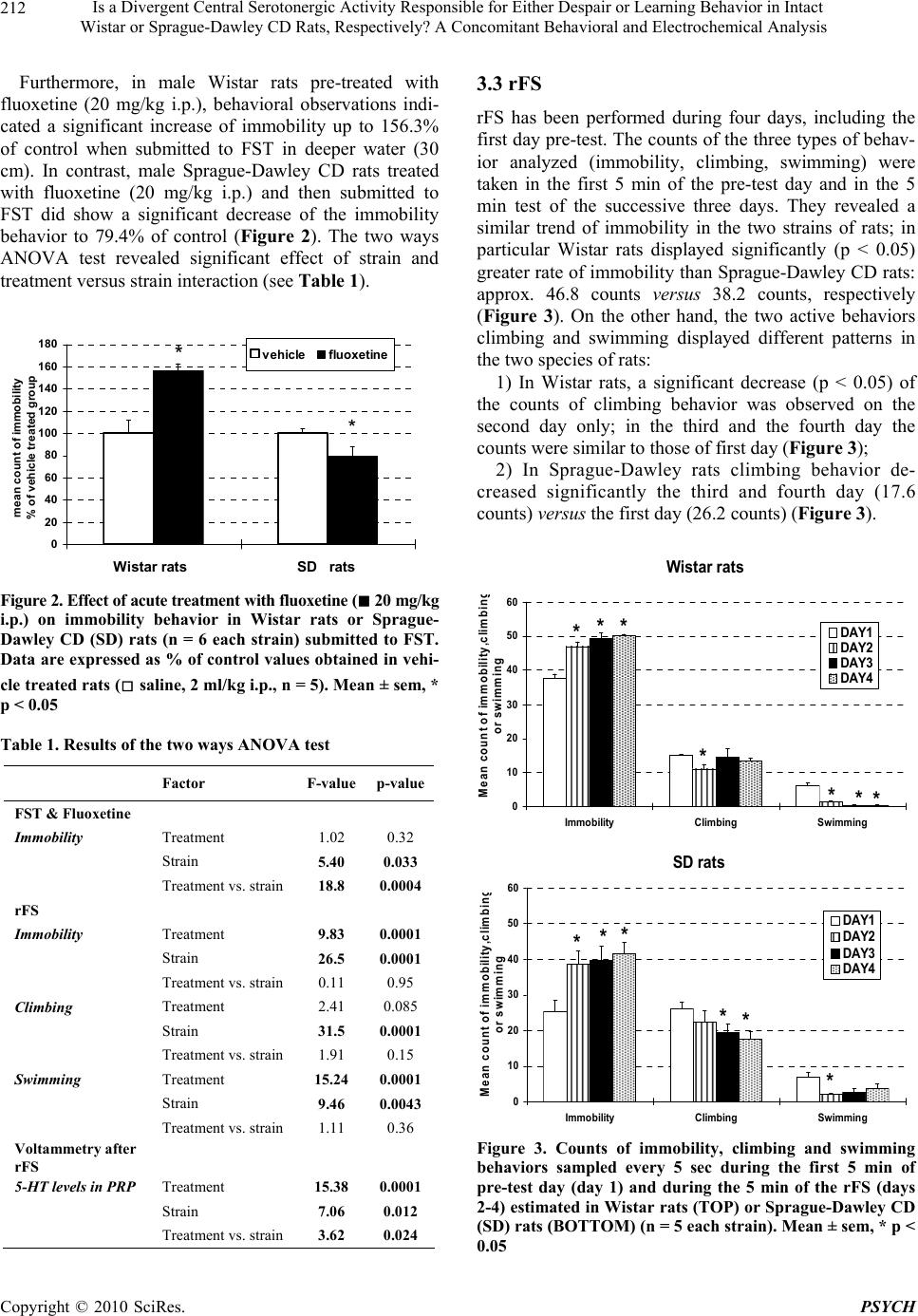 Is a Divergent Central Serotonergic Activity Responsible for Either Despair or Learning Behavior in Intact 212 Wistar or Sprague-Dawley CD Rats, Respectively? A Concomitant Behavioral and Electrochemical Analysis Furthermore, in male Wistar rats pre-treated with fluoxetine (20 mg/kg i.p.), behavioral observations indi- cated a significant increase of immobility up to 156.3% of control when submitted to FST in deeper water (30 cm). In contrast, male Sprague-Dawley CD rats treated with fluoxetine (20 mg/kg i.p.) and then submitted to FST did show a significant decrease of the immobility behavior to 79.4% of control (Figure 2). The two ways ANOVA test revealed significant effect of strain and treatment versus strain interaction (see Table 1). 0 20 40 60 80 100 120 140 160 180 Wistar rats SD rats mean count of im mobili ty % of vehicle t reated group v ehicle fluoxetine * * Figure 2. Effect of acute treatment with fluoxetine (■ 20 mg/kg i.p.) on immobility behavior in Wistar rats or Sprague- Dawley CD (SD) rats (n = 6 each strain) submitted to FST. Data are expressed as % of control values obtained in vehi- cle treated rats (□ saline, 2 ml/kg i.p., n = 5). Mean ± sem, * p < 0.05 Table 1. Results of the two ways ANOVA test Factor F-value p-value FST & Fluoxetine Immobility Treatment 1.02 0.32 Strain 5.40 0.033 Treatment vs. strain 18.8 0.0004 rFS Immobility Treatment 9.83 0.0001 Strain 26.5 0.0001 Treatment vs. strain 0.11 0.95 Climbing Treatment 2.41 0.085 Strain 31.5 0.0001 Treatment vs. strain 1.91 0.15 Swimming Treatment 15.24 0.0001 Strain 9.46 0.0043 Treatment vs. strain 1.11 0.36 Voltammetry after rFS 5-HT levels in PRP Treatment 15.38 0.0001 Strain 7.06 0.012 Treatment vs. strain 3.62 0.024 3.3 rFS rFS has been performed during four days, including the first day pre-test. The counts of the three types of behav- ior analyzed (immobility, climbing, swimming) were taken in the first 5 min of the pre-test day and in the 5 min test of the successive three days. They revealed a similar trend of immobility in the two strains of rats; in particular Wistar rats displayed significantly (p < 0.05) greater rate of immobility than Sprague-Dawley CD rats: approx. 46.8 counts versus 38.2 counts, respectively (Figure 3). On the other hand, the two active behaviors climbing and swimming displayed different patterns in the two species of rats: 1) In Wistar rats, a significant decrease (p < 0.05) of the counts of climbing behavior was observed on the second day only; in the third and the fourth day the counts were similar to those of first day (Figure 3); 2) In Sprague-Dawley rats climbing behavior de- creased significantly the third and fourth day (17.6 counts) versus the first day (26.2 counts) (Figure 3 ). Wistar rats 0 10 20 30 40 50 60 Immobility Climbing Swimming Mean count of imm obility,climbin g or swimm ing DAY1 DAY2 DAY3 DAY4 ** * ** * * SD rats 0 10 20 30 40 50 60 Immobility Climbing Swimming Me a n c o u n t o f immo b ility ,c limb in g o r s w immin g DAY1 DAY2 DAY3 DAY4 * * * * * * Figure 3. Counts of immobility, climbing and swimming behaviors sampled every 5 sec during the first 5 min of pre-test day (day 1) and during the 5 min of the rFS (days 2-4) estimated in Wistar rats (TOP) or Sprague-Dawley CD (SD) rats (BOTTOM) (n = 5 each strain). Mean ± sem, * p < 0.05 Copyright © 2010 SciRes. PSYCH 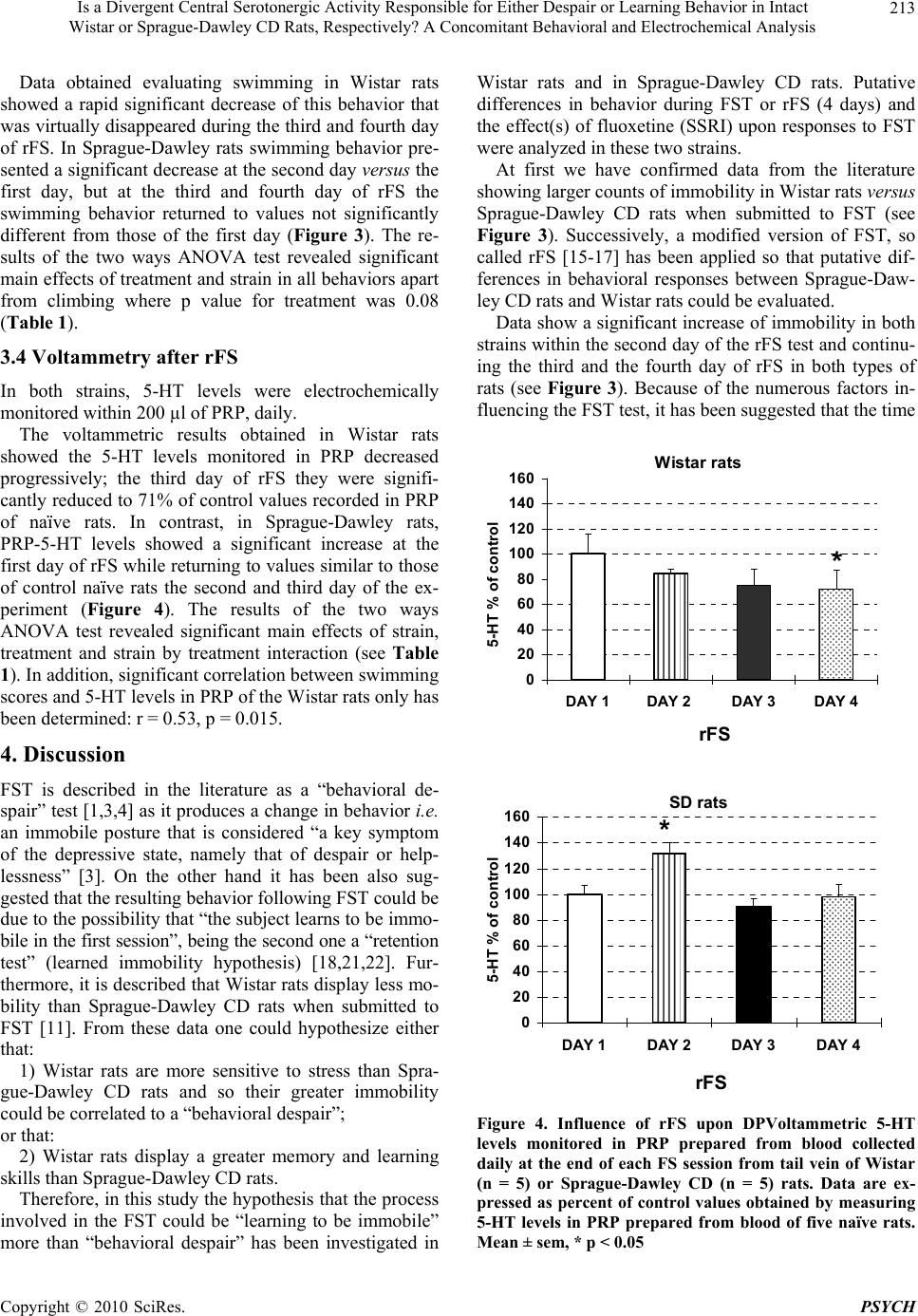 Is a Divergent Central Serotonergic Activity Responsible for Either Despair or Learning Behavior in Intact 213 W istar or Sprague-Dawley CD Rats, Respectively? A Concomitant Behavioral and Electrochemical Analysis Data obtained evaluating swimming in Wistar rats showed a rapid significant decrease of this behavior that was virtually disappeared during the third and fourth day of rFS. In Sprague-Dawley rats swimming behavior pre- sented a significant decrease at the second day versus the first day, but at the third and fourth day of rFS the swimming behavior returned to values not significantly different from those of the first day (Figure 3). The re- sults of the two ways ANOVA test revealed significant main effects of treatment and strain in all behaviors apart from climbing where p value for treatment was 0.08 (Table 1). 3.4 Voltammetry after rFS In both strains, 5-HT levels were electrochemically monitored within 200 µl of PRP, daily. The voltammetric results obtained in Wistar rats showed the 5-HT levels monitored in PRP decreased progressively; the third day of rFS they were signifi- cantly reduced to 71% of control values recorded in PRP of naïve rats. In contrast, in Sprague-Dawley rats, PRP-5-HT levels showed a significant increase at the first day of rFS while returning to values similar to those of control naïve rats the second and third day of the ex- periment (Figure 4). The results of the two ways ANOVA test revealed significant main effects of strain, treatment and strain by treatment interaction (see Table 1). In addition, significant correlation between swimming scores and 5-HT levels in PRP of the Wistar rats only has been determined: r = 0.53, p = 0.015. 4. Discussion FST is described in the literature as a “behavioral de- spair” test [1,3,4] as it produces a change in behavior i.e. an immobile posture that is considered “a key symptom of the depressive state, namely that of despair or help- lessness” [3]. On the other hand it has been also sug- gested that the resulting behavior following FST could be due to the possibility that “the subject learns to be immo- bile in the first session”, being the second one a “retention test” (learned immobility hypothesis) [18,21,22]. Fur- thermore, it is described that Wistar rats display less mo- bility than Sprague-Dawley CD rats when submitted to FST [11]. From these data one could hypothesize either that: 1) Wistar rats are more sensitive to stress than Spra- gue-Dawley CD rats and so their greater immobility could be correlated to a “behavioral despair”; or that: 2) Wistar rats display a greater memory and learning skills than Sprague-Dawley CD rats. Therefore, in this study the hypothesis that the process involved in the FST could be “learning to be immobile” more than “behavioral despair” has been investigated in Wistar rats and in Sprague-Dawley CD rats. Putative differences in behavior during FST or rFS (4 days) and the effect(s) of fluoxetine (SSRI) upon responses to FST were analyzed in these two strains. At first we have confirmed data from the literature showing larger counts of immobility in Wistar rats versus Sprague-Dawley CD rats when submitted to FST (see Figure 3). Successively, a modified version of FST, so called rFS [15-17] has been applied so that putative dif- ferences in behavioral responses between Sprague-Daw- ley CD rats and Wistar rats could be evaluated. Data show a significant increase of immobility in both strains within the second day of the rFS test and continu- ing the third and the fourth day of rFS in both types of rats (see Figure 3). Because of the numerous factors in- fluencing the FST test, it has been suggested that the time Wistar rats 0 20 40 60 80 100 120 140 160 DAY 1DAY 2 DAY 3DAY 4 5- HT % of control rFS * SD rats 0 20 40 60 80 100 120 140 160 DAY 1DAY 2DAY 3DAY 4 5-HT % of co ntrol rFS * Figure 4. Influence of rFS upon DPVoltammetric 5-HT levels monitored in PRP prepared from blood collected daily at the end of each FS session from tail vein of Wistar (n = 5) or Sprague-Dawley CD (n = 5) rats. Data are ex- pressed as percent of control values obtained by measuring 5-HT levels in PRP prepared from blood of five naïve rats. Mean ± sem, * p < 0.05 Copyright © 2010 SciRes. PSYCH  Is a Divergent Central Serotonergic Activity Responsible for Either Despair or Learning Behavior in Intact 214 Wistar or Sprague-Dawley CD Rats, Respectively? A Concomitant Behavioral and Electrochemical Analysis of immobility on the second day of rFS might be consid- ered a nonspecific parameter referred to as “learning to be immobile” rather than “behavioral despair” [21]. The present data also show that treatment with fluoxetine actually increases immobility as well. Since fluoxetine is a compound that inhibits serotonin reuptake, thus making it more available at its receptors, this result could appears like a contradiction, however in the Porsolt’ test the SSRIs have been found to be ineffective or even pro- longing the time of immobility [6,46]. Similarly, while in the literature, the results concerning the ineffectiveness of the SSRIs in rats are still controversial, it has been constantly reported that the SSRIs extend the time of immobility in mice [46]. It is also well known that acute treatment with SSRIs may produce anxiogenic-like ef- fects in humans [47] as well as in animals [48]. Further- more, Borsini et al. [49] have indirectly illustrated that the emotional state of an animal might be important for the outcome of the test. Accordingly, our data show that Wistar rats display greater immobility and that is in agreement to the reported observation that such an inbred rat strain is inclined to higher behavioral and physiologi- cal responses to stress across a variety of situations in comparison to other strains [15]. This would also explain the significant decrease of swimming mainly observable in Wistar rats. 4.1 Voltammetry in PRP Concomitant in vivo DPVoltammetric analysis per- formed accordingly with Zen et al. [32] has shown that the signal monitored with Nafion-mCFE in PRP at the oxidation potential of 5-HT was selectively sensitive to addition of exogenous 5-HT (data not shown). Further- more, experiments performed in PRP accordingly with Barja-Fidalgo et al. [50] have shown that incubation in KCl stimulates increase of the 5-HT related DPV signal. Finally, accordingly with Lyons and Shaw [51] treatment of PRP with the calcium-chelating agent EGTA has demonstrated a dose dependent reduction of the 5-HT related signal (see Figure 1). Thus, these DPVoltammet- ric data support the chemical nature of the signal moni- tored in PRP as corresponding to the oxidation of basal, endogenous 5-HT level in PRP. Successively, DPV measurements performed during the behavioral tests showed that 5-HT level in PRP decreased progressively in Wistar rats submitted to rFS, while they increased at day 1 of rFS in Sprague-Dawley rats and returned to control levels the next days. The fact that “peripheral” 5-HT levels [in PRP] mirror central 5-HT levels has been already documented [23-27]. Therefore the present data on 5-HT level in PRP of Wistar rats taken together with the observation that pharmacological blockade of sero- tonergic activity enhance learning and memory skills [19,20] may suggest that the increased immobility fol- lowing rFS monitored in Wistar rats could be due to “learning to be immobile” more than “behavioral de- spair”. Thus, the concomitant decrease of 5-HT levels monitored in PRP and considered as peripheral marker of modifications of central 5-HT system may be correlated to the enhancement of learning skill; i.e. it could lead Wistar rats to develop habituation to be immobile when submitted to rFS. Conversely, other authors have re- ported that serotonin receptor agonists deteriorate such skills [52,53], therefore the hypothesis proposed above is still matter of discussion. When related to the same ob- servation reported above [19,20] the significant increase of 5-HT level in PRP of Sprague-Dawley CD rats leads one to consider that in this strain of rats the significant increase of immobility following rFS could be unrelated to “learning to be immobile” but may be interpreted in the light of a “behavioral despair” hypothesis. However, this conclusion is in disaccord with the finding that (diet) tryptophan restriction, and therefore brain serotonin re- duction, could impair normal cholinergic activity in some brain areas such as the hippocampus and the cerebral cortex that are involved in learning and memory proc- esses [54]. These observations lead to the comment that work remains to be done to further elucidate the con- trasting data present in literature on this matter. De- creased levels of central 5-HT has been recently reported in Wistar rats submitted to rFS [55]. Our voltammetric data on 5-HT levels monitored in PRP of Wistar rats match these recent findings which therefore support the proposal that peripheral 5-HT levels monitored by means of DPV together with mCFE in rat PRP mirror cerebral 5-HT contents. Furthermore and on the basis of the 5-HT hypothesis of depression suggesting a relationship between vulner- ability to this illness and a deficit in the brain serotoner- gic activity [56] the depletion of 5-HT caused by forced swimming may be one of the reasons for the develop- ment of depressive-like behavior in Wistar rats. On the other hand, the observation of an increased level of 5-HT on day 2 returning to ‘control” values on days 3 and 4 in the PRP of Sprague-Dawley CD rats lead one to propose that there is not such a central 5-HT defi- cit in this second strain of rats. This is supporting the idea that rFS may display learning behavior in Spra- gue-Dawley CD rats as the present results suggest that experience and learning may be the principal processes at the basis of the significant increase of immobility in Sprague-Dawley CD. Indeed, in these rats, immobility levels were continuously increasing during the 3 days of rSF following the pre-test day (day 1, see Figure 3), without reaching a plateau while showing higher vari- ability on their response when compared to that of Wistar rats (see Figure 3). In contrast, the Wistar rats submitted to rFS present less mobility than the Sprague-Dawley CD Copyright © 2010 SciRes. PSYCH  Is a Divergent Central Serotonergic Activity Responsible for Either Despair or Learning Behavior in Intact 215 W istar or Sprague-Dawley CD Rats, Respectively? A Concomitant Behavioral and Electrochemical Analysis rats; i.e. they show less variability on their response (see Figure 3 top) and reach a stable (plateau) level of immo- bility the third and fourth day of the test. Thus, “behav- ioral despair” seems to be the cause of the increased im- mobility in Wistar rats as their “answer” to the recurring stress produced by the repetition of the test is the ‘sud- den’ increased immobility to a plateau as indeed found at the third and fourth day of rFS. Accordingly with previ- ous studies indicating that various stressors alter 5-HT synthesis / turnover [57,58], the different behavior within these two strains may therefore also be related to the dif- ferent biochemical “reaction” of their 5-HT central sys- tem when submitted to FST: i.e. progressive decrease of 5-HT levels in Wistar rats versus a significant increase in day 1 followed by return to control values days 2 and 3 in Sprague-Dawley CD rats (Figure 4). The present study also shows a rapid decrease of swimming until virtually its disappearance during the third-fourth day of rFS in Wistar rats. In contrast, in Sprague-Dawley rats swimming behavior presented a significant decrease at the second day versus the first testing day, but at the third and fourth day it returned to values not significantly different from those recorded in the first day of rFS. Again, this difference in swimming behavior between the two strains of rats could be related to the difference in 5-HT levels monitored after each swimming session in PRP of both species of rats. In Wistar rats 5-HT levels monitored in PRP decreased progressively; reaching the minimum significant value at the third day of rFS. In Sprague-Dawley rats, 5-HT levels in PRP showed a significant increase at the first day of rFS, returning to values similar to those of control rats the following days of experiment. This is in accord with the relationship described in literature between seroton- ergic system activities and swimming behavior during FST: in particular an enhancement of 5-HT neurotrans- mission mediates positively the swimming behavior of Sprague-Dawley CD rats [4,6]. Moreover, it is interesting to note the different climb- ing behavior displayed by the two rat strains when sub- mitted to rFS: 1) Progressive decrease of climbing in Sprague-Daw- ley CD rats; 2) Initial decrease followed by a return to the values of day 1 in Wistar rats. Therefore, when submitted to rFS, these two strains of rats display opposite swimming and climbing behaviors (see Figure 3). Briefly: 1) In Wistar rats climbing at first decreased [day 2] then restored the levels of day1 while swimming de- creased progressively; 2) In Sprague-Dawley CD rats climbing decreased progressively while swimming at first decreased [day 2] then tended to return to the initial levels. It is reported that climbing is a behavior mediated by noradrenergic neurotransmission and swimming a be- havior mediated by serotonergic neurotransmission [4,6]. Thus, one can argue that the behavioral data monitored here, could be an index of distinct activities of the two neurotransmitter systems within the two strains of rats when submitted to FST as well as to rFS. In particular, the dissimilarity observed within the serotonergic system of the two strains i.e. when submitted to rFS may account for the different behavioral response to fluoxetine treat- ment observed here i.e. either increase or reduction of immobility in Wistar rats or in Sprague-Dawley CD rats, respectively (see Figure 2). Accordingly, the data con- cerning swimming counts led one to argue about the ad- ditional presence of a divergent activity of the noradren- ergic system in the two rat strains during FST or rFS. Further studies should be done to analyze this point. Yet, it is interesting to note the parallel, significantly related decrease of swimming counts and 5-HT levels monitored in PRP of the Wistar rats submitted to rFS (see Figure 5). Taken together with the findings that reductions of noradrenaline or 5-HT, which do not by themselves im- pair place learning, aggravate the place-learning deficit produced by reductions of Ach [59] the present data support the idea that in Wistar rats the increased immo- bility following rFS could be due to “behavioral despair” more than “learning to be immobile”. On the contrary, the lack of change on 5-HT levels as well as in swim- ming behavior observed in the Sprague-Dawley CD rats may suggest that the increased immobility following rFS could be due to “learning to be immobile” more than “behavioral despair”. Differential sensitivity to the behavioral effects of fluoxetine by different rat strains has been already re- ported. In particular it has been shown that genetic or constitutive differences may determine the distinct be- havioral profiles for antidepressant compounds with se- lective pharmacological effects in different rat strains such as male Sprague Dawley and Wistar Kyoto rats [11, 60]. The latter is a line derived from Wistar rats, pre- senting significant higher plasma levels of corticosterone and ACTH compared to Wistar rats that are the “control line” of the Wistar Kyoto rats [61]. On the other hand, these authors have also shown that Wistar rats exhibited significantly longer immobility duration in the swim test compared with the Sprague-Dawley CD rats. This is in accord with the present observation of a significantly higher counts of immobility in Wistar rats versus Spra- gue-Dawley CD rats submitted to rFS (see Figure 3 and Table 1). Altogether such results suggest that different rat strains may demonstrate great variability in the be- havioral response to antidepressants according to their genetic or constitutive differences as well as pharmacol- ogical selectivity, differences that should be not ruled out Copyright © 2010 SciRes. PSYCH  Is a Divergent Central Serotonergic Activity Responsible for Either Despair or Learning Behavior in Intact 216 Wistar or Sprague-Dawley CD Rats, Respectively? A Concomitant Behavioral and Electrochemical Analysis WISTAR rats 0 1 2 3 4 5 6 7 8 DAY 1DAY 2DAY 3DAY 4 5- HT [nA x10] swimm in g co unts SD rats 0 1 2 3 4 5 6 7 8 9 DAY 1DAY 2DAY 3DAY 4 5- HT [ nA x10] swim m ing counts Figure 5. Influence of rFS upon swimming and DPVoltam- metric 5-HT levels monitored in PRP prepared from blood collected daily at the end of each FS from tail vein of Wistar rats (n = 5) or Sprague-Dawley CD rats (n = 5). Mean counts (± sem) are shown together with 5-HT levels (i.e. nanoAmperes [nA] multiplied by 10 for graphic purpose). Significant correlation between swimming scores and 5-HT levels in PRP of Wistar rats has been determined: r = 0.53, p = 0.015 when evaluating behavioral and neurochemical changes in response to antidepressants such as fluoxetine. In this sense, it is worth mentioning that it has recently been shown that four 5-HT receptor systems (5-HT1A, 5-HT2A, 5-HT4, 5-HT6) are highlighted as suitable targets for en- hancing cognition and memory (for a review see [62]) with in particular the 5-HT6 receptor playing a role in learning and memory processes in healthy and disease states (see reviews by Mitchell and Neumaier [63] and Schreiber et al. [64]). The putative synergistic interaction of 5-HT6 receptors with other serotonin receptors is also shown to be important for memory processes [65]. Therefore, differences in such aspects within the differ- ent strains of rats may also account for the variability in behavioral responses. 5. Conclusions In conclusion, the present in vivo electrochemical analy- sis support our previous work showing that peripheral 5-HT levels selectively monitored in rat PRP by means of DPV together with Nafion-mCFE mirror cerebral 5-HT contents [28]. In particular it allows deducing changes of central 5-HT levels that could be correlated to behavioral tests such as FST or rFS. Therefore, this in vivo approach displays the clear advantage of non inva- sive behavioral-pharmacological analysis of neurotrans- mitter activities in conscious animals. Finally, while fur- ther work is needed to support the following idea, the combined behavioral-pharmacological data presented suggest that “learning to be immobile” seems to be the process involved in Sprague-Dawley CD rats submitted to rFS while “behavioral despair” seems to be the process involved in Wistar rats submitted to rFS. 6. Acknowledgements For technical support to Dr. E. Vecchiato and Dr. C. Lazzarini. REFERENCES [1] R. D. Porsolt, M. Le Pichon and M. Jalfre, “Depression: A New Animal Model Sensitive to Anti Depressant Treat- ments,” Nature, Vol. 266, No. 5604, 1977, pp. 730-732. [2] F. Borsini and A. Meli, “Is the Forced Swimming Test a Suitable Model for Revealing Antidepressant Activity?” Psychopharmacology, Vol. 94, No. 2, 1988, pp. 147-160. [3] T. J. Connor, P. Kelliher, Y. Shen, A. Harkin, J. P. Kelly and B. E. Leonard, “Effect of Subchronic Antidepressant Treatments on Behavioral, Neurochemical, and Endocrine Changes in the Forced-Swim Test,” Pharmacology Bio- chemistry and Behavior, Vol. 65, No. 4, 2000, pp. 591-597. [4] M. J. Detke and I. Lucki, “Detection of Serotonergic and Noradrenergic Antidepressants in the Rat Forced Swim- ming Test: The Effect of Water Depth,” Behavioural Brain Research, Vol. 73, No. 1-2, 1996, pp. 43-46. [5] R. D. Porsolt, A. Bertin, N. Blavet, M. Deniel and M. Jalfre, “Immobility Induced by Forced Swimming in Rats: Ef- fects of Agents which Modify Central Catecholamine and Serotonin Activity,” European Journal of Pharmacology, Vol. 57, No. 2-3, 1979, pp. 201-210. [6] M. J. Detke, M. Rickels and I. Lucki, “Active Behaviors in the Rat Forced Swimming Test Differentially Produced by Serotonergic and Noradrenergic Antidepressants,” Psy- chopharmacology, Vol. 121, No. 1, 1995, pp. 66-72. [7] S. E. Hemby, I. Lucki, G. Gatto, A. Singh, C. Thornley, J. Matasi, N. Kong, J. E. Smith, H. M. L. Davies and S. I. Dworkin, “Potential Antidepressant Effects of Novel Tro- pane Compounds, Selective for Serotonin or Dopamine Transporters,” Journal of Pharmacology and Experimen- tal Therapeutics, Vol. 282, No. 2, 1997, pp. 727-733. [8] L. G. Kirby and I. Lucki, “Interaction between the Forced Swimming Test and Fluoxetine Treatment on Extracellu- lar 5-Hydroxytryptamine and 5-Hydroxyindoleacetic Acid in the Rat,” Journal of Pharmacology and Experimental Therapeutics, Vol. 282, No. 2, 1997, pp. 967-976. Copyright © 2010 SciRes. PSYCH  Is a Divergent Central Serotonergic Activity Responsible for Either Despair or Learning Behavior in Intact 217 W istar or Sprague-Dawley CD Rats, Respectively? A Concomitant Behavioral and Electrochemical Analysis [9] M. E. Page, M. J. Detke, G. Kirby and I. Lucki, “Sero- tonergic Mediation of the Effects of Fluoxetine, but not Desipramine, in the Rat Forced Swimming Test,” Psy- chopharmacology, Vol. 147, No. 2, 1999, pp. 162-167. [10] J. P. Reneric and I. Lucki, “Antidepressant Behavioral Effects by Dual Inhibition of Monoamine Reuptake in the Rat Forced Swimming Test,” Psychopharmacology, Vol. 136, No. 2, 1998, pp. 190-197. [11] C. Lόpez-Rubalcava and I. Lucki, “Strain Differences in the Behavioral Effects of Antidepressant Drugs in the Rat Forced Swimming Test,” Neuropsychopharmacology, Vol. 22, No. 2, 2000, pp. 191-199. [12] T. Skrebuhhova, L. Allikmets and V. Matto, “Effect of Anxiogenic Drugs in Rat Forced Swimming Test,” Meth- ods & Findings in Experimental & Clinical Pharmacol- ogy, Vol. 21, No. 3, 1999, pp. 173-178. [13] J. De Vry, S. Maurel, R. Schreiber, R. de Beun and K. R. Jentzsch, “Comparison of Hypericum Extracts with Imi- pramine and Fluoxetine in Animal Models of Depression and Alcoholism,” European Neuropsychopharmacology, Vol. 9, No. 6, 1999, pp. 461-468. [14] G. Griebel, C. Cohen, G. Perrault and D. J. Sanger, “Be- havioral Effects of Acute and Chronic Fluoxetine in Wis- tar-Kyoto Rats,” Physiology & Behavior, Vol. 67, No. 3, 1999, pp. 315-320. [15] J. F. Cryan, M. E. Page and I. Lucki, “Differential Be- havioral Effects of the Antidepressants Reboxetine, Fluo- xetine, and Moclobemide in a Modified Forced Swim Test Following Chronic Treatment,” Psychopharmacol- ogy (Berl), Vol. 182, No. 3, 2005, pp. 335-339. [16] S. Dal-Zotto, O. Marti and A. Armario, “Influence of Single or Repeated Experience of Rats with Forced Swim- ming on Behavioral and Physiological Responses to the Stressor,” Behavioural Brain R esearch, Vol. 114, No. 1-2, 2000, pp. 175-181. [17] L. G. Kirby and I. Lucki, “The Effect of Repeated Expo- sure to Forced Swimming on Extracellular Levels of 5-Hydroxytryptamine in the Rat,” Stress, Vol. 2, No. 4, 1998, pp. 251-263. [18] A. Parra, C. Vinader-Caerols, S. Monleόn and V. M. Simόn, “Learned Immobility is also Involved in Forced Swim- ming Test in Mice,” Psicothema, Vol. 11, No. 2, 1999, pp. 239-246. [19] Y. Lamberty and A. J. Gower, “Cholinergic Modulation of Spatial Learning in Mice in a Morris-Type Water Maze,” Archives Internationales de Pharmacodynamie et de Therapie, Vol. 309, 1991, pp. 5-19. [20] G. Richter-Levin and M. Segal, “The Effect of Serotonin Depletion and Raphe Grafts on Hippocampal Electro- physiology and Behavior,” Journal of Neuroscience, Vol. 11, No. 6, 1991, pp. 1585-1596. [21] J. M. De Pablo, A. Parra, S. Segovia and A. Guillamon, “Learned Immobility Explains the Behavior of Rats in the Forced Swimming Test,” Physiology & Behavior, Vol. 46, No. 2, 1989, pp. 229-237. [22] A. J. Martos, C. Vinader-Caerols, S. Monleόn, M. C. Are- nas and A. Parra, “Effect of Physostigmine and Nicotine on Learned Immobility in the Forced Swimming Test,” Psicothema, Vol. 11, No. 3, 1999, 631-639. [23] E. H. Cook, K. E. Fletcher, M. Wainwright, N. Marks, S. Y. Yan and B. L. Leventhal, “Primary Structure of the Human Platelet Serotonin 5-HT2A Receptor: Identity with Frontal Cortex Serotonin 5-HT2A Receptor,” Journal of Neurochemistry, Vol. 63, No. 2, 1994, pp. 465-469. [24] C. R. Pfeffer, P. A. McBride, G. M. Anderson, T. Ka- kuma, L. Fensterheim and V. Khait, “Peripheral Sero- tonin Measures in Prepubertal Psychiatric Inpatients and Normal Children: Association with Suicidal Behavior and its Risk Factors,” Biological Psychiatry, Vol. 44, No. 7, 1988, pp. 568-577. [25] S. D. Mendelson, “The Current Status of the Platelet 5-HT2A Receptor in Depression,” Journal of Affective Disorders, Vol. 57, No. 1, 2000, pp. 13-24. [26] J. M. Sneddon, “Blood Platelets as a Model for Mono- amine Containing Neurones,” Progress in Neurobiology, Vol. 1, No. 2, 1973, pp. 151-198. [27] S. M. Stahl, “The Human Blood Platelet: A Diagnostic and Research Tool for the Study of Biogenic Amines in Psychiatric and Neurologic Disorders,” Archives of Gen- eral Psychiatry, Vol. 34, No. 5, 1977, pp. 509-516. [28] M. Bianchi, C. Moser, C. Lazzarini, E. Vecchiato and F. Crespi, “Forced Swimming Test and Fluoxetine Treat- ment: In Vivo Evidence that Peripheral 5-HT in Rat Platelet-Rich Plasma Mirrors Cerebral Extracellular 5-HT Levels, whilst 5-HT in Isolated Platelets Mirrors Neu- ronal 5-HT Changes,” Experimental Brain Research, Vol. 143, No. 2, 2002, pp. 191-197. [29] F. Congestri, F. Formenti, V. Sonntag and F. Crespi, “The Selective D3 Receptor Antagonist SB-277011-A Potenti- ates the Effect of Cocaine on Extracellular Dopamine in the Nucleus Accumbens: A Dual Core-Shell Voltammetry Study in Anesthetized Rats,” Sensors, Vol. 8, No. 11, 2008, pp. 6936-6951. [30] F. Crespi, “In Vivo Voltammetry with Micro-Biosensors for Analysis of Neurotransmitter Release and Metabo- lism,” Journal of Neuroscience Methods, Vol. 34, No. 1-3, 1990, pp. 53-65. [31] F. Crespi, K. F. Martin and C. A. Marsden, “Measurement of Extracellular Basal Levels of Serotonin in Vivo Using Nafion-Coated Carbon Fibre Electrodes Combined with Differential Pulse Voltammetry,” Neuroscience, Vol. 27, No. 3, 1988, pp. 885-896. [32] J.-M. Zen, I.-L. Chen and Y. Shih, “Voltammetric Deter- mination of Serotonin in Human Blood Using a Chemi- cally Modified Electrode,” Analytica Chimica Acta, Vol. 369, No. 1-2, 1998, pp. 103-108. [33] F. Crespi, “In Vivo Voltammetry and Concomitant Elec- trophysiology at a Single Biosensor to Analyse Ischaemia, Depression and Drug Dependence,” Journal of Neuro- science Methods, Vol. 119, No. 2, 2002, pp. 173-184. [34] F. Crespi and M. Jouvet, “Differential Pulse Voltammetry: Parallel Peak 3 Changes with Vigilance States in Raphe Copyright © 2010 SciRes. PSYCH  Is a Divergent Central Serotonergic Activity Responsible for Either Despair or Learning Behavior in Intact 218 Wistar or Sprague-Dawley CD Rats, Respectively? A Concomitant Behavioral and Electrochemical Analysis Dorsalis and Raphe Magnus of Chronic Freely Moving Rats and Evidence for 5HT Contribution to this Peak after Monoamine Oxidase Inhibitors,” Brain Research, Vol. 272, No. 2, 1983, pp. 263-268. [35] A. Louilot, A. Serrano and M. D’Angio, “A Novel Car- bon Fiber Implantation Assembly for Cerebral Voltam- metric Measurements in Freely Moving Rats,” Physiology & Behavior, Vol. 41, No. 3, 1987, pp. 227-231. [36] S. L. Handley and J. W. McBlane, “Opposite Effects of Fluoxetine in Two Animal Models of Anxiety,” British Journal of Pharmacology, Vol. 107S, 1997, p. 446. [37] M. L. Rao, B. Hawellek, A. Papassotiropoulos, A. Deister and C. Frahnert, “Upregulation of the Platelet Serotonin2A Receptor and Low Blood Serotonin in Suicidal Psychiat- ric Patients,” Neuropsychobiology, Vol. 38, No. 2, 1998, pp. 84-89. [38] F. Crespi, “Apamin Increases 5-HT Cell Firing in Raphe Dorsalis and Extracellular 5-HT Levels in Amygdala: A Concomitant in Vivo Study in Anesthetized Rats,” Brain Research, Vol. 1281, 2009, pp. 35-46. [39] K. F. Martin, C. A. Marsden and F. Crespi. “In Vivo Electrochemistry with Carbon Fibre Electrodes: Princi- ples and Application to Neuropharmacology,” Trends in Analytical Chemistry, Vol. 7, No. 9, 1988, pp. 334-339. [40] J. A. Stamford, F. Crespi and C. A. Marsden, “In Vivo Voltammetric Methods for Monitoring Monoamine Re- lease and Metabolism,” Monitoring Neuronal Activity, a Practical Approach, Oxford University Press, Oxford, 1992, pp. 113-145. [41] T Self and F. Crespi, “Electron Microscopic and Volt- ammetric Analysis of Carbon Fibre Electrode Pretreat- ments,” Journal of Materials Science: Mate rials in Medi- cine, Vol. 3, No. 6, 1992, pp. 418-425. [42] F. Crespi and Z. L. Rossetti, “Pulse of Nitric Oxide Re- lease in Response to Activation of N-Methyl-D-Aspartate Receptors in the Rat Striatum: Rapid Desensitisation, In- hibition by Receptor Antagonists and Potentiation by Glycine,” Journal of Pharmacology and Experimental Therapeutics, Vol. 309, No. 2, 2004, pp. 462-468. [43] F. Crespi, T. Sharp, N. Maidment and C. A. Marsden, “Differential Pulse Voltammetry in Vivo–Evidence that Uric Acid Contributes to the Indole Oxidation Peak,” Neuroscience Letters, Vol. 43, No. 2-3, 1983, pp. 203-207. [44] F. Crespi, T. Sharp, N. Maidment and C. A. Marsden, “Dif- ferential Pulse Voltammetry: Simultaneous in Vivo Meas- urement of Ascorbic Acid, Catechols and 5-Hydroxyindoles in the Rat Striatum Using a Single Carbon Fibre Elec- trode,” Brain Research, Vol. 322, No. 1, 1984, pp. 135- 138. [45] F. Crespi, P. Keane and M. Morre, “Does Concomitant Analysis of Extracellular DOPAC and 5HIAA with a Sin- gle Carbon Fibre Electrode Enable the Detection of Stri- atal Dopamine-Serotonin Interaction?” Journal of Neu- rochemistry, 1985, Vol. 44, pp. 109-112. [46] F. Borsini, “Role of the Serotonergic System in the Forced Swimming Test,” Neuroscience & Biobehavioral Reviews, Vol. 19, No. 3, 1995, pp. 377-395. [47] W. F. Boyer and J. P. Feighner, “Side Effects of the Se- lective Serotonin Re-Uptake Inhibitors,” In: J. P. Feigh- ner and W. F. Boyer, Ed., Selective Serotonin Re-Uptake Inhibitors. Perspectives in Psychiatry 1, Wiley Press, New York, 1991, pp. 133-152. [48] P. Chopin and M. Briley, “Animal Models of Anxiety: The Effect of Compounds that Modify 5-HT Neurotrans- mission,” Trends in Pharmacological Sciences, Vol. 8, No. 10, 1987, pp. 383-388. [49] F. Borsini, A. Lecci, A. Sessarego, R. Frassine and A. Meli, “Discovery of Antidepressant Activity by Forced Swimming Test may Depend on Pre-Exposure of Rats to a Stressful Situation,” Psychopharmacology, Vol. 97, No. 2, 1989, pp. 183-188. [50] C. Barja-Fidalgo, J. A. Guimaraes and C. R. Carlini, “The Secretory Effect of Canatoxin on Rat Brain Synaptosomes Involves A Lipoxygenase-Mediated Pathway,” Brazilian Journal of Medical and Biological Research, Vol. 21, No. 3, 1988, pp. 549-552. [51] R. M. Lyons and J. O. Shaw, “Interaction of Ca2+ and Protein Phosphorylation in the Rabbit Platelet Release Reaction,” Journal of Clinical Investigation, Vol. 65, No. 2, 1980, pp. 242-255. [52] H. C. Buhot, S. Martin and L. Segu, “Role of Serotonin in Memory Impairment,” Annals of Medicine, Vol. 32, No. 3, 2000, pp. 210-221. [53] W. J. McEntee and T. H. Crook, “Serotonin, Memory, and the Aging Brain,” Psychopharmacology, Vol. 103, No. 2, 1991, pp. 143-149. [54] I. Gonzalez-Burgos, M. I. Perez-Vega, A. R. Del Angel- Meza and A. Feria-Velasco, “Effect of Tryptophan Re- striction on Short-Term Memory,” Physiology & Behav- ior, Vol. 63, No. 2, 1998, pp. 165-169. [55] G. T. Shishkina, T. S. Kalinina and N. N. Dygalo, “Sero- tonergic Changes Produced by Repeated Exposure to Forced Swimming: Correlation with Behavior,” Annals of the New York Academy of Sciences, Vol. 1148, 2008, pp. 148-153. [56] M. H. Maes and Y. Meltzer, “The Serotonin Hypothesis of Major Depression,” In: F. E. Bloom and D. J. Kupfer, Ed., Psychopharmacology: The Fourth Generation of Pro- gress, Raven Press, New York, 1995, pp. 933-944. [57] F. Chaouloff, “Physiopharmacological Interactions between Stress Hormones and Central Serotonergic Systems,” Brain Research Reviews, Vol. 18, No. 1, 1993, pp. 1-32. [58] L. E. Rueter, C. A. Fornal and B. L. Jacobs, “A Critical Review of 5-HT Brain Microdialysis and Behavior,” Re- views in th e Neuros cien ces, Vol. 8, No. 2, 1997, pp. 117-137. [59] R. K. McNamara and R. W. Skelton, “The Neurophar- macological and Neurochemical Basis of Place Learning in the Morris Water Maze,” Brain Research Reviews, Vol. 18, No. 1, 1993, pp. 33-49. [60] S. Tejani-Butt, J. Kluczynski and W. P. Paré, “Strain- Dependent Modification of Behavior Following Antide- Copyright © 2010 SciRes. PSYCH  Is a Divergent Central Serotonergic Activity Responsible for Either Despair or Learning Behavior in Intact Wistar or Sprague-Dawley CD Rats, Respectively? A Concomitant Behavioral and Electrochemical Analysis Copyright © 2010 SciRes. PSYCH 219 pressant Treatment,” Progress in Neuro-Psychopharma- cology & Biological Psychiatry, Vol. 27, No. 1, 2003, pp. 7-14. [61] O. Malkesman, Y. Braw, R. Maayan, A. Weizman, D. H. Overstreet, M. Shabat-Simon, Y. Kesner, et al., “Two Different Putative Genetic Animal Models of Childhood Depression,” Biological Psychiatry, Vol. 59, No. 1, 2006, pp. 17-23. [62] B. L. Roth, S. M. Hanizavareh and A. E. Blum, “Sero- tonin Receptors Represent Highly Favorable Molecular Targets for Cognitive Enhancement in Schizophrenia and Other Disorders,” Psychopharmacology, Vol. 174, No. 1, 2004, pp. 17-24. [63] E. S. Mitchell and J. F. Neumaier, “5-HT6 Receptors: A Novel Target for Cognitive Enhancement,” Pharmacol- ogy & Therapeutics, Vol. 108, No. 3, 2005, pp. 320-333. [64] R. Schreiber, A. J. Sleight and M. L. Woolley, “5-HT 6 Receptors as Targets for the Treatment of Cognitive Deficits in Schizophrenia,” In: B. R. Roth, Ed., Serotonin Receptors: F rom Molecular Pharmacol ogy to Human The r- apeutics, Humana Press, Totowa, 2006, pp. 495-515. [65] E. S. Mitchell, B. J. Hoplight, S. P. Lear and J. F. Neu- maier, “BGC20-761, a Novel Tryptamine Analog, En- hances Memory Consolidation and Reverses Scopola- mine-Induced Memory Deficit in Social and Visuospatial Memory Tasks through a 5-HT6 Receptor-Mediated Me- chanism,” Neuropharmacology, Vol. 50, No. 4, 2006, pp. 412-420. |

