Paper Menu >>
Journal Menu >>
 Open Journal of Psychiatry, 2012, 2, 262-268 OJPsych http://dx.doi.org/10.4236/ojpsych.2012.24036 Published Online October 2012 (http://www.SciRP.org/journal/ojpsych/) Relationships between clozapine and norclozapine plasma concentrations, clozapine dose, and clinical response in Tunisian patients with schizophrenia-treatment resistance Aida Taieb1,2*, Fatma B’chir3, Roland Molinié1, José-Edmundo Nava-Saucedo1, Jaafer Nakhli4, Marc-André Fliniaux1, Bechir Ben Hadj Ali4, Saad Saguem2 1Unit of Plant Biology and Insect Pests, University of Picardy Jules Verne, Amiens, France 2Metabolic Biophysics and Applied Pharmacology Laboratory, Department of Biophysics, Medicine Faculty of Sousse, Sousse, Tunisia 3Laboratory of Natural Substances, National Institute of Research and Physical Chemical Analysis, Technopole Sidi Thabet, Tunis, Tunisia 4Psychiatry Department of Central Hospital University (CHU), Sousse , Tunisia Email: *aida.taieb@u-picardie.fr, bchirfatma@hotmail.fr Received 20 April 2012; revised 26 May 2012; accepted 7 June 2012 ABSTRACT The present study investigated relationships between clozapine dose, clozapine and norclozapine plasma concentrations, and clinical responses to clozapine treatment in Tunisian schizophrenics. Fourteen schi- zophrenia-treatment resistant patients, recruited for this study, were treated with clozapine for 45 days. Patient health improvement was assessed before and after each cycle of two weeks of clozapine therapy, using the Brief Psychi atric Rating Scale (BPRS). Pl asma clozapine and norclozapine concentrations were deter- mined by high-performance liquid chromatography (HPLC). No significant correlations between plasma clozapine and norclozapine concentrations and clini- cal health improvement among our schizophrenic patients were found. However, a significant correla- tion was observed between clinical health improve- ment given by BPRS scores and norclozapine plasma concentration to daily clozapine dose ratio (NCZ/D). Despite the small sample size of our study, our find- ings suggest that the clozapine therapy response va- riations observed in our patients may be, in part, ex- plained by the interindividual differences in plasma norclozapine concentration to clozapine dose ratio (NCZ/D). So the NCZ/D parameter could be used as a good indicator for adjusting the clozapine dose-adap- tation strategy and consequently for improving the clinical psychopathological state of schizophrenia-treat- ment resistant patients. Keywords: Clozapine; Norclozapine; HPLC; Tunisian Schizophrenic; BPRS 1. INTRODUCTION Clozapine (CZ) is a second-generation antipsychotic drug . It is primarily used in the treatment of schizophrenia- treatment resistant psychopathology. However, treat- ment with clozapine is associated with several side ef- fects that complicate the use of the drug and necessitate therapeutic monitoring [1,2]. This monitoring allows the optimization of the therapeutic dose-response efficacy and the prevention of excessive toxicity of CZ, reducing in this way the risk of relapse [3-5]. Despite the effec- tiveness of clozapine as a standard drug for schizophre- nia-treatment resistance, important individual variations in the response to clozapine therapy were reported [6,7]. The mechanism underlying the differences in clozap- ine treatment response is not well known. Some studies attribute t hese differences to patient s’ sex, age, body weight, and physiology [8]. Other studies suggest that these vari- ations are related to the plasma concentrations of cloza- pine and its active metabolite, norclozapine [9], with the explanation that monitoring plasma clozapine concentra- tions may play a useful role in the management of pa- tients with schizophrenia. Moreover, it may be possible that the metabolic polymorphism of clozapine elimina- tion, attributed to genetic and/or environmental origins, modifies clozapine plasma concentration and elimin ation and thus its psychotherapeutic effects. Therefore, this study has been undertaken to explore possible correlations between clinical responses to cloza- pine and parameters such as clozapine and norclozapine plasma concentrations, clozapine clearance, clozapine metabolic index, and norclozapine plasma concentration to daily clozapine dose ratio. Our interest is in offering some explanations for the interindividual variability of *Corresponding a uthor. OPEN ACCESS 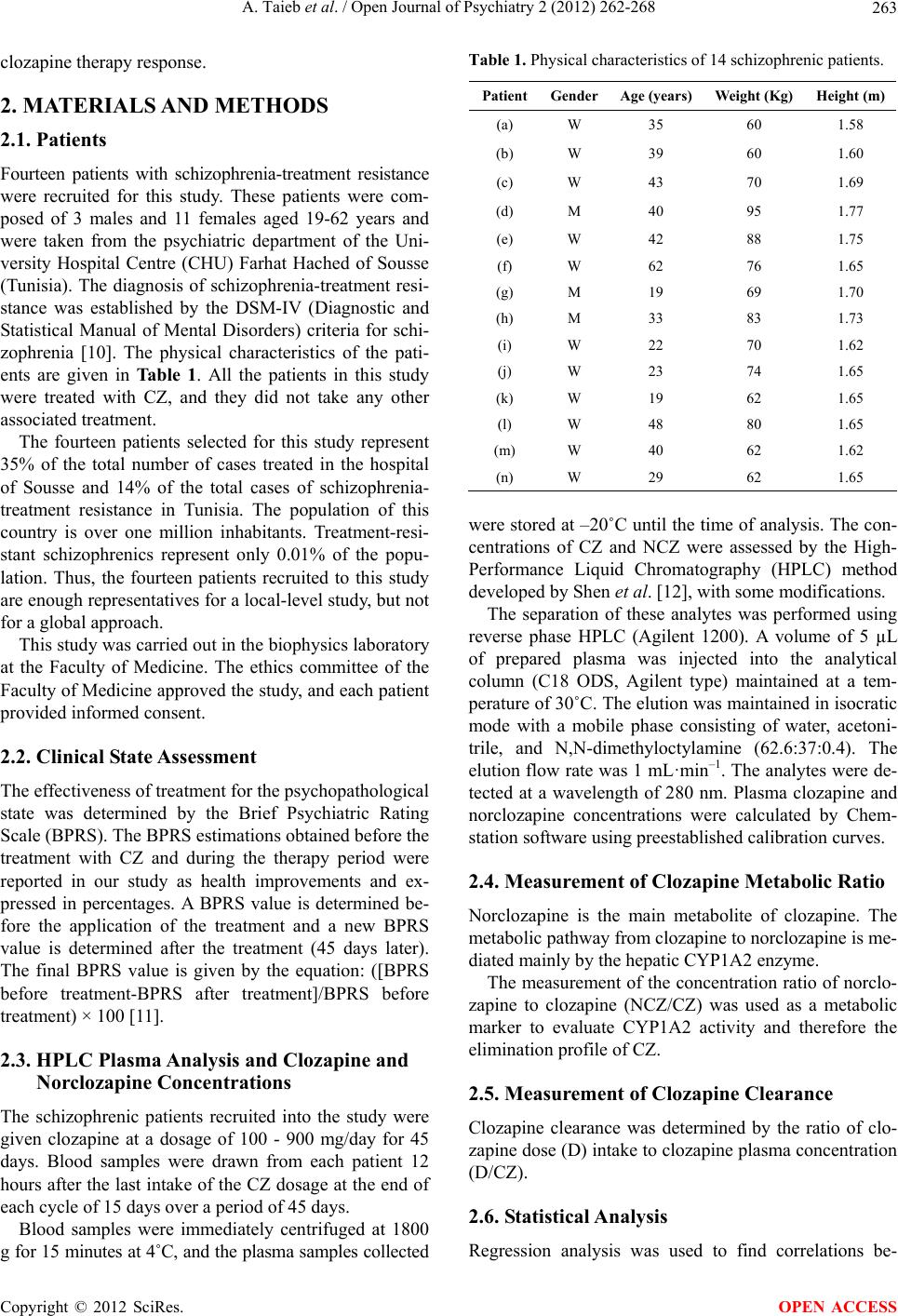 A. Taieb et al. / Open Journal of Psychiatry 2 (2012) 262-268 263 clozapine therapy response. 2. MATERIALS AND METHODS 2.1. Patients Fourteen patients with schizophrenia-treatment resistance were recruited for this study. These patients were com- posed of 3 males and 11 females aged 19-62 years and were taken from the psychiatric department of the Uni- versity Hospital Centre (CHU) Farhat Hached of Sousse (Tunisia). The diagnosis of schizophrenia-treatment resi- stance was established by the DSM-IV (Diagnostic and Statistical Manual of Mental Disorders) criteria for schi- zophrenia [10]. The physical characteristics of the pati- ents are given in Tab le 1. All the patients in this study were treated with CZ, and they did not take any other associated treatment. The fourteen patients selected for this study represent 35% of the total number of cases treated in the hospital of Sousse and 14% of the total cases of schizophrenia- treatment resistance in Tunisia. The population of this country is over one million inhabitants. Treatment-resi- stant schizophrenics represent only 0.01% of the popu- lation. Thus, the fourteen patients recruited to this study are enough representatives for a local-level study, but not for a global approach. This study was carried out in the biophysics laboratory at the Faculty of Medicine. The ethics committee of the Faculty of Medicine approved the study, and each patient provided informed consent. 2.2. Clinical State Assessment The effectiveness of treatment for the psychopathological state was determined by the Brief Psychiatric Rating Scale (BPRS). The BPRS estimations obtained before the treatment with CZ and during the therapy period were reported in our study as health improvements and ex- pressed in percentages. A BPRS value is determined be- fore the application of the treatment and a new BPRS value is determined after the treatment (45 days later). The final BPRS value is given by the equation: ([BPRS before treatment-BPRS after treatment]/BPRS before treatment) × 100 [11]. 2.3. HPLC Plasma Analysis and Clozapine and Norclozapine Concentrations The schizophrenic patients recruited into the study were given clozapine at a dosage of 100 - 900 mg/day for 45 days. Blood samples were drawn from each patient 12 hours after the last intake of the CZ dosage at the end of each cycle of 15 days over a period of 45 days. Blood samples were immediately centrifuged at 1800 g for 15 minutes at 4˚C, and the plasma samples collected Table 1. Physical characteristics of 14 schizophrenic patients. PatientGenderAge (years) Weight (Kg) Height (m) (a) W 35 60 1.58 (b) W 39 60 1.60 (c) W 43 70 1.69 (d) M 40 95 1.77 (e) W 42 88 1.75 (f) W 62 76 1.65 (g) M 19 69 1.70 (h) M 33 83 1.73 (i) W 22 70 1.62 (j) W 23 74 1.65 (k) W 19 62 1.65 (l) W 48 80 1.65 (m) W 40 62 1.62 (n) W 29 62 1.65 were stored at –20˚C until the time of an alysis. The con- centrations of CZ and NCZ were assessed by the High- Performance Liquid Chromatography (HPLC) method develope d by Shen et al. [12], with some modifications. The separation of these analytes was performed using reverse phase HPLC (Agilent 1200). A volume of 5 µL of prepared plasma was injected into the analytical column (C18 ODS, Agilent type) maintained at a tem- perature of 30˚C. The elution was maintained in isocratic mode with a mobile phase consisting of water, acetoni- trile, and N,N-dimethyloctylamine (62.6:37:0.4). The elution flow rate was 1 mL·min–1. The analytes were de- tected at a wavelength of 280 nm. Plasma clozapine and norclozapine concentrations were calculated by Chem- station software using preestablished calibration curves. 2.4. Measurement of Clozapine Metabolic Ratio Norclozapine is the main metabolite of clozapine. The metabolic pathway from clozapine to norclozapine is me- diated mainly by the hepatic CYP1A2 enzyme. The measurement of the concentration ratio of no rclo- zapine to clozapine (NCZ/CZ) was used as a metabolic marker to evaluate CYP1A2 activity and therefore the elimination profile of CZ. 2.5. Measurement of Clozapine Clearance Clozapine clearance was determined by the ratio of clo- zapine dose (D) intake to clozapine plasma concentration (D/CZ). 2.6. Statistical Analysis Regression analysis was used to find correlations be- Copyright © 2012 SciRes. OPEN ACCESS 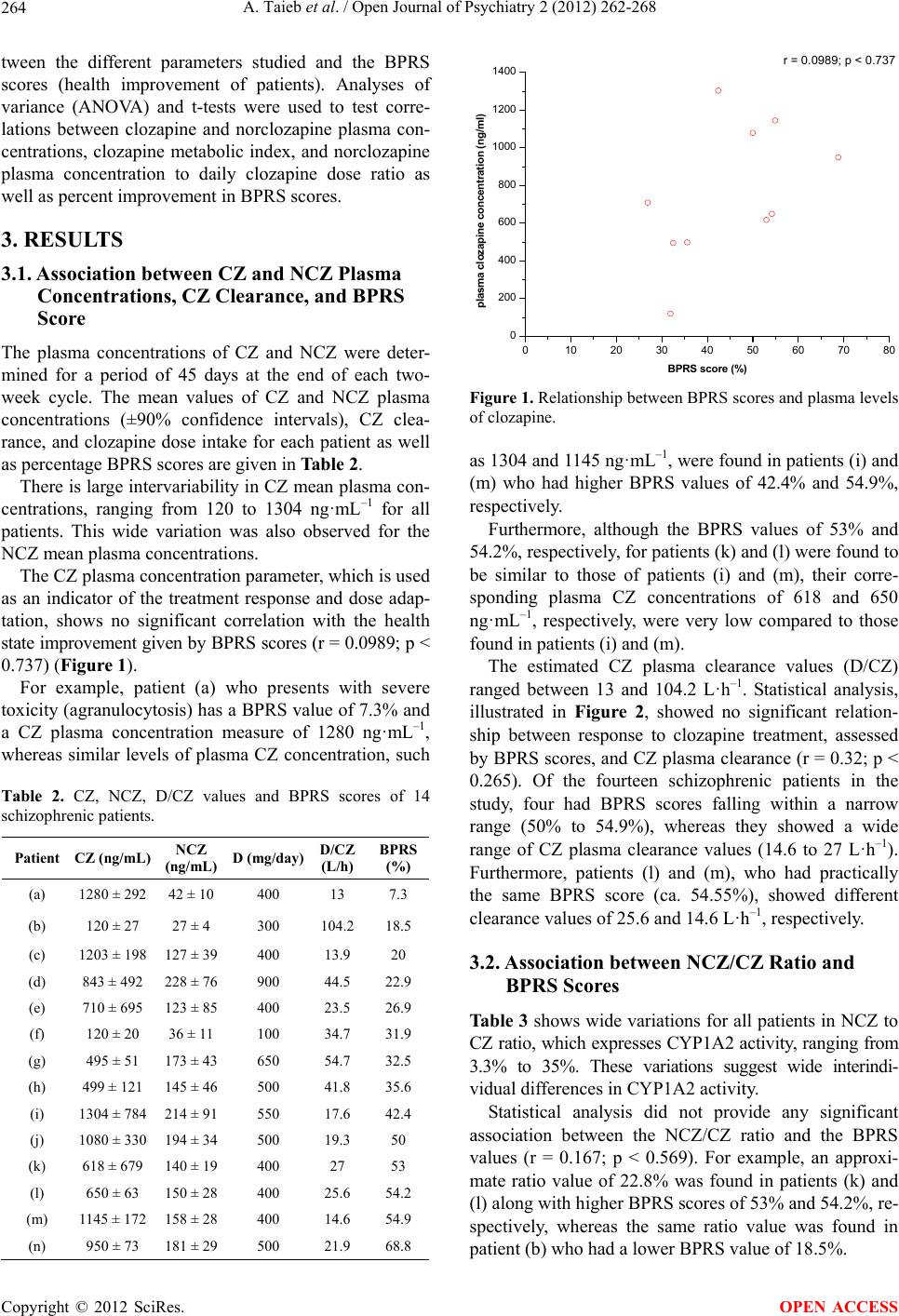 A. Taieb et al. / Open Journal of Psychiatry 2 (2012) 262-268 264 tween the different parameters studied and the BPRS scores (health improvement of patients). Analyses of variance (ANOVA) and t-tests were used to test corre- lations between clozapine and norclozapine plasma con- centrations, clozapine metabolic index, and norclozapine plasma concentration to daily clozapine dose ratio as well as percent improvement in BPRS scores. 3. RESULTS 3.1. Association between CZ and NCZ Plasma Concentrations, CZ Clearance, and BPRS Score The plasma concentrations of CZ and NCZ were deter- mined for a period of 45 days at the end of each two- week cycle. The mean values of CZ and NCZ plasma concentrations (±90% confidence intervals), CZ clea- rance, and clozapine dose intake for each patient as well as percentage BPR S scores are given in Table 2. There is large intervariability in CZ mean plasma con- centrations, ranging from 120 to 1304 ng·mL–1 for all patients. This wide variation was also observed for the NCZ mean plasma concentrations. The CZ plasma concentration parameter, which is used as an indicator of the treatment response and dose adap- tation, shows no significant correlation with the health state improvement given by BPRS scores (r = 0.0989; p < 0.737) (Figure 1). For example, patient (a) who presents with severe toxicity (agranulocytosis) has a BPRS value of 7.3% and a CZ plasma concentration measure of 1280 ng·mL–1, whereas similar levels of plasma CZ concentration, such Table 2. CZ, NCZ, D/CZ values and BPRS scores of 14 schizophrenic patients. Patient CZ (ng/mL) NCZ (ng/mL) D (mg/day) D/CZ (L/h) BPRS (%) (a) 1280 ± 292 42 ± 10 400 13 7.3 (b) 120 ± 27 27 ± 4 300 104.218.5 (c) 1203 ± 198 127 ± 39 400 13.9 20 (d) 843 ± 492 228 ± 76 900 44.5 22.9 (e) 710 ± 695 123 ± 85 400 23.5 26.9 (f) 120 ± 20 36 ± 11 100 34.7 31.9 (g) 495 ± 51 173 ± 43 650 54.7 32.5 (h) 499 ± 121 145 ± 46 500 41.8 35.6 (i) 1304 ± 784 214 ± 91 550 17.6 42.4 (j) 1080 ± 330 194 ± 34 500 19.3 50 (k) 618 ± 679 140 ± 19 400 27 53 (l) 650 ± 63 150 ± 28 400 25.6 54.2 (m) 1145 ± 172 158 ± 28 400 14.6 54.9 (n) 950 ± 73 181 ± 29 500 21.9 68.8 0 102030405060708 0 200 400 600 800 1000 1200 1400 0 plasma clozapine concentration (ng/ml) BPRS score (%) r = 0.0989; p < 0.737 Figure 1. Relationship between BPRS scores and plasma levels of clozapine. as 1304 and 1145 ng·mL–1, were found in patients (i) and (m) who had higher BPRS values of 42.4% and 54.9%, respectively. Furthermore, although the BPRS values of 53% and 54.2%, respectively, for patients (k) and (l) were found to be similar to those of patients (i) and (m), their corre- sponding plasma CZ concentrations of 618 and 650 ng·mL–1, respectively, were very low compared to those found in patients (i) and (m). The estimated CZ plasma clearance values (D/CZ) ranged between 13 and 104.2 L·h–1. Statistical analysis, illustrated in Figure 2, showed no significant relation- ship between response to clozapine treatment, assessed by BPRS scores, and CZ plasma clearance (r = 0.32; p < 0.265). Of the fourteen schizophrenic patients in the study, four had BPRS scores falling within a narrow range (50% to 54.9%), whereas they showed a wide range of CZ plasma clearance values (14.6 to 27 L·h–1). Furthermore, patients (l) and (m), who had practically the same BPRS score (ca. 54.55%), showed different clearance values of 25.6 and 14.6 L·h–1, respectively. 3.2. Association between NCZ/CZ Ratio and BPRS Scores Table 3 shows wide variations for all patients in NCZ to CZ ratio, which expresses CYP1A2 activity, ranging from 3.3% to 35%. These variations suggest wide interindi- vidual differences in CYP1A2 activity. Statistical analysis did not provide any significant association between the NCZ/CZ ratio and the BPRS values (r = 0.167; p < 0.569). For example, an approxi- mate ratio value of 22.8% was found in patients (k) and (l) along with higher BPRS scores of 53% and 54.2%, re- spectively, whereas the same ratio value was found in patient (b) who had a lower BPRS value of 18.5%. Copyright © 2012 SciRes. OPEN ACCESS 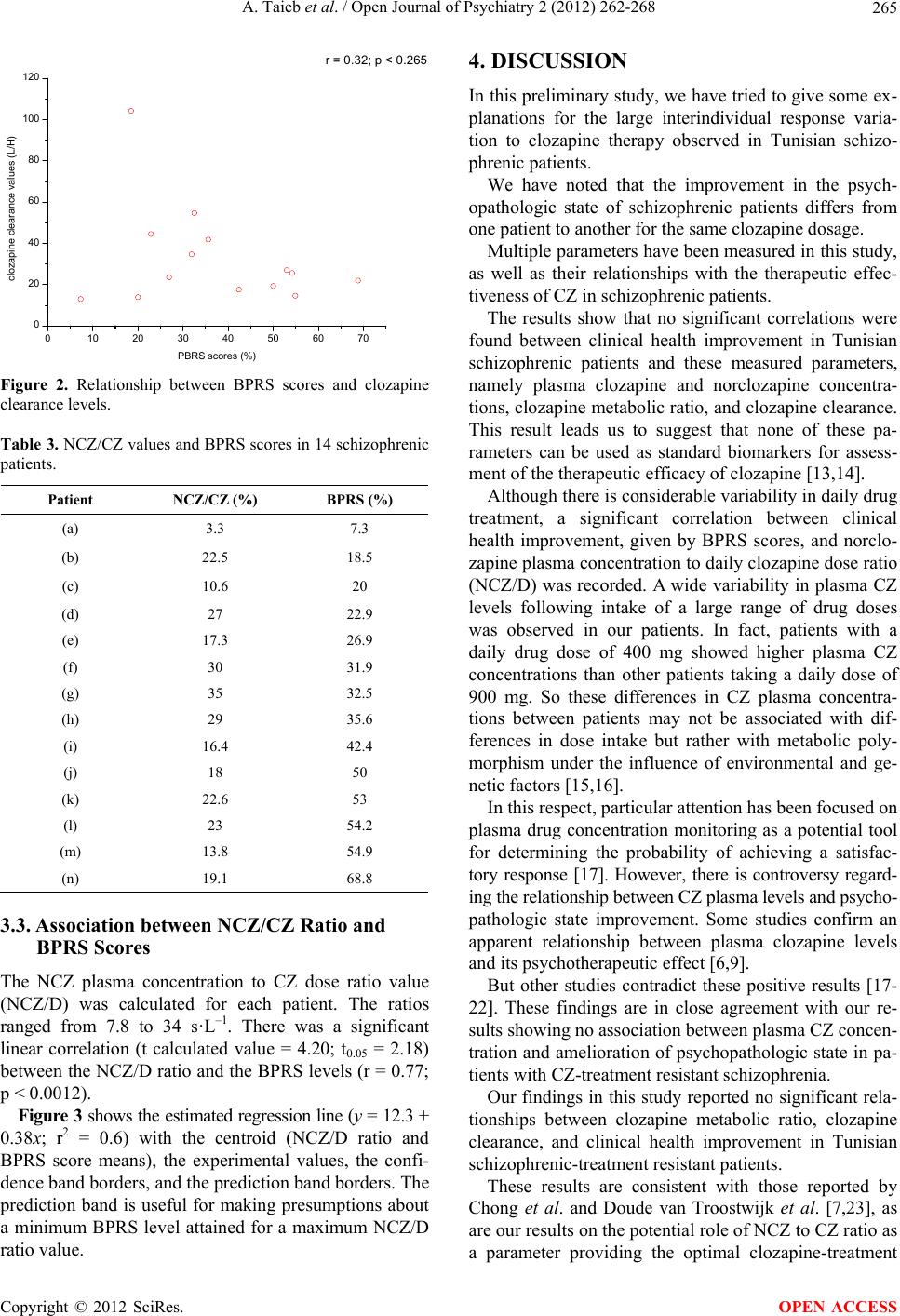 A. Taieb et al. / Open Journal of Psychiatry 2 (2012) 262-268 265 0 10203040506070 0 20 40 60 80 100 120 clozapine clearance values (L/H) PBRS scores (%) r = 0.32; p < 0.265 Figure 2. Relationship between BPRS scores and clozapine clearance levels. Table 3. NCZ/CZ values and BPRS scores in 14 schizophrenic patients. Patient NCZ/CZ (%) BPRS (%) (a) 3.3 7.3 (b) 22.5 18.5 (c) 10.6 20 (d) 27 22.9 (e) 17.3 26.9 (f) 30 31.9 (g) 35 32.5 (h) 29 35.6 (i) 16.4 42.4 (j) 18 50 (k) 22.6 53 (l) 23 54.2 (m) 13.8 54.9 (n) 19.1 68.8 3.3. Association between NCZ/CZ Ratio and BPRS Scores The NCZ plasma concentration to CZ dose ratio value (NCZ/D) was calculated for each patient. The ratios ranged from 7.8 to 34 s·L–1. There was a significant linear correlation (t calculated value = 4.20; t0.05 = 2.18) between the NCZ/D ratio and the BPRS levels (r = 0.77; p < 0.0012). Figure 3 shows the estimated regression line (y = 12.3 + 0.38x; r2 = 0.6) with the centroid (NCZ/D ratio and BPRS score means), the experimental values, the confi- dence band borders, and the prediction band borders. The prediction band is useful for making presumptions about a minimum B PRS level attained for a max imum NCZ/D ratio value. 4. DISCUSSION In this preliminary study, we have tried to give some ex- planations for the large interindividual response varia- tion to clozapine therapy observed in Tunisian schizo- phrenic patients. We have noted that the improvement in the psych- opathologic state of schizophrenic patients differs from one patient to another for the same clozapine dosage. Multiple parameters have been measured in th is study, as well as their relationships with the therapeutic effec- tiveness of CZ in schizophrenic patients. The results show that no significant correlations were found between clinical health improvement in Tunisian schizophrenic patients and these measured parameters, namely plasma clozapine and norclozapine concentra- tions, clozapine metabolic ratio, and clozapine clearance. This result leads us to suggest that none of these pa- rameters can be used as standard biomarkers for assess- ment of the therapeutic efficacy of clozapine [13,14]. Although there is cons iderable variability in daily drug treatment, a significant correlation between clinical health improvement, given by BPRS scores, and norclo- zapine plasma concentration to daily clozapine dose ratio (NCZ/D) was recorded. A wide variability in plasma CZ levels following intake of a large range of drug doses was observed in our patients. In fact, patients with a daily drug dose of 400 mg showed higher plasma CZ concentrations than other patients taking a daily dose of 900 mg. So these differences in CZ plasma concentra- tions between patients may not be associated with dif- ferences in dose intake but rather with metabolic poly- morphism under the influence of environmental and ge- netic factors [15,16]. In this respect, particular attention has been focused on plasma drug concentration monitoring as a potential tool for determining the probability of achieving a satisfac- tory response [17]. However, there is controversy regard- ing the relationship between CZ pl asm a level s and psycho- pathologic state improvement. Some studies confirm an apparent relationship between plasma clozapine levels and its psychotherapeutic effect [6,9]. But other studies contradict these positive results [17- 22]. These findings are in close agreement with our re- sults showing no association between plasma CZ concen- tration and amelioration of psychopathologic state in pa- tients with CZ-treatment resistant schizophrenia. Our findings in this study reported no significant rela- tionships between clozapine metabolic ratio, clozapine clearance, and clinical health improvement in Tunisian schizophrenic-treatment resistant patients. These results are consistent with those reported by Chong et al. and Doude van Troostwijk et al. [7,23], as are our results on the potential role o f NCZ to CZ ratio as a parameter providing the optimal clozapine-treatment Copyright © 2012 SciRes. OPEN ACCESS 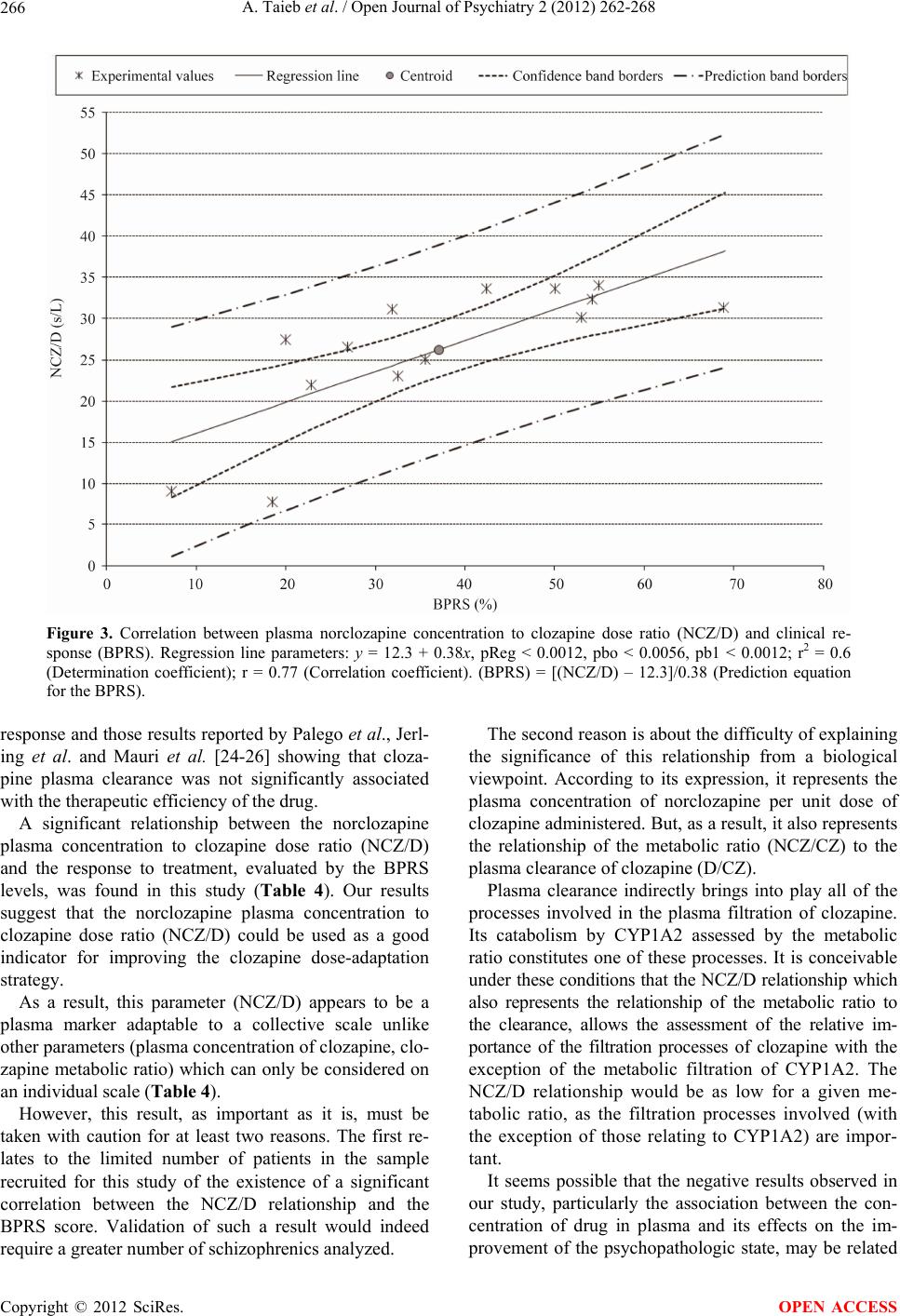 A. Taieb et al. / Open Journal of Psychiatry 2 (2012) 262-268 Copyright © 2012 SciRes. 266 Figure 3. Correlation between plasma norclozapine concentration to clozapine dose ratio (NCZ/D) and clinical re- sponse (BPRS). Regression line parameters: y = 12.3 + 0.38x, pReg < 0.0012, pbo < 0.0056, pb1 < 0.0012; r2 = 0.6 (Determination coefficient); r = 0.77 (Correlation coefficient). (BPRS) = [(NCZ/D) – 12.3]/0.38 (Prediction equation for the BPRS). response and those results reported by Palego et al., Jerl- ing et al. and Mauri et al. [24-26] showing that cloza- pine plasma clearance was not significantly associated with the therapeutic efficien cy of the drug. The second reason is about the difficulty of explaining the significance of this relationship from a biological viewpoint. According to its expression, it represents the plasma concentration of norclozapine per unit dose of clozapine administered. But, as a result, it also represents the relationship of the metabolic ratio (NCZ/CZ) to the plasma clearance of clozapine (D/CZ). A significant relationship between the norclozapine plasma concentration to clozapine dose ratio (NCZ/D) and the response to treatment, evaluated by the BPRS levels, was found in this study (Table 4). Our results suggest that the norclozapine plasma concentration to clozapine dose ratio (NCZ/D) could be used as a good indicator for improving the clozapine dose-adaptation strategy. Plasma clearance indirectly brings into play all of the processes involved in the plasma filtration of clozapine. Its catabolism by CYP1A2 assessed by the metabolic ratio constitutes one of these processes. It is conceivable und er these conditions that the NCZ/D relationship which also represents the relationship of the metabolic ratio to the clearance, allows the assessment of the relative im- portance of the filtration processes of clozapine with the exception of the metabolic filtration of CYP1A2. The NCZ/D relationship would be as low for a given me- tabolic ratio, as the filtration processes involved (with the exception of those relating to CYP1A2) are impor- tant. As a result, this parameter (NCZ/D) appears to be a plasma marker adaptable to a collective scale unlike other parameters (plasma concentration of clozapine, clo- zapine metabolic ratio) which can only be considered on an individual scale (Table 4). However, this result, as important as it is, must be taken with caution for at least two reasons. The first re- lates to the limited number of patients in the sample recruited for this study of the existence of a significant correlation between the NCZ/D relationship and the BPRS score. Validation of such a result would indeed require a greater number of schizophre nics analyzed. It seems possible that the negative results observed in our study, particularly the association between the con- centration of drug in plasma and its effects on the im- provement of the psychopathologic state, may be related OPEN ACCESS  A. Taieb et al. / Open Journal of Psychiatry 2 (2012) 262-268 267 Table 4. Association between measured parameters and clinical health improvement in 14 schizophrenic patients. Measured parameters Clinical health improvement Comments/recommendations Clozapine plasma concentration BPRS There is no correlation between these two parameters. However, plasma concentration of clozapine appears to be an important parameter, both to individually assess the therapeutic interval thresholds at the start of treatment and also to ensure medical follow-up relative to the regularity of taking medications. Clozapine metabolic ratio (NCZ/CZ) BPRS There is no correlation between the metabolic ratio values and the BPRS scores. It is noted, however, that patient (a) is the only one whose ratio value plummeted, showing CYP1A2 enzyme activity of almost zero. It must be noted that not only is the BPRS score of this patient the lowest (7.3%), but it is also the only patient who developed severe toxicity expressed by the appearance of agranulocytosis leading to the termination of clozapine treatment. Overall, if our results show that the metabolic ratio value of clozapine is not a marker assessing therapeutic efficacy, they nevertheless suggest that a ratio of almost zero related to very low CYP1A2 activity may constitute a risk factor of developing severe toxicities during clozapine treatment. Clozapine clearance (D/CZ) BPRS Our results indicate that plasma clozapine clearance cannot constitute a standard marker for assessment of the therapeutic ef ficacy of clozapine. NCZ/D BPRS There was a significant correlation between clinical health improvement, given by BPRS scores, and norclozapine plasma concentration to daily clozapine dose ratio (NCZ/D). Such a result is important insofar as it constitutes an attempt at establishing therapeutic standards based on clozapine. It is in fact the relationship of the metabolic ratio to the clozapine clearance. Our results show that the therapeutic efficacy of the drug would be as great as the value of this parameter is high and no matter which patient is being considered. to the small sample size of the study and the heterogen- eity of the recruited patients—the majority of our popu- lation consists of women. It was reported that sex may have a great influence on health psychotherapeutic im- provement [8]. Moreover, it has been proposed that there is a close association between the genetic polymorphism of various genes involved in the metabo lism and receptor regulatio n of anti-psychotic drugs. So in future investigations, a large and homogeneous sample is needed to characterize in more detail the re- lationship between clozapine pharmacokinetic parame- ters and their antipsychotic effects, in order to explain the wide variation in clozapine-treatment responses ob- served in schizophrenic patients. 5. ACKNOWLEDGEMENTS The authors would like to thank all the individuals who participated in this study. REFERENCES [1] Preskorn, S.H. (2005) Comments on the role of therapeu- tic drug monitoring for clozapine. Journal of Psychiatric Practice, 11, 340-343. doi:10.1097/00131746-200509000-00006 [2] Kronig, M.H., Munne, R.A., Szymanski, S., Safferman, A.Z., Pollack, S., Cooper, T., Kane, J.M. and Lieberman, J.A. (1995) Plasma clozapine levels and clinical response for treatment-refractory schizophrenic patients. American Journal of Psychiatry, 152, 179-182. [3] Schulte, P.F.J. (2003) What is an adequate trial with clozapine? Therapeutic drug monitoring and time to re- sponse in treatement- refractory schizophr enia. Clinical Phar- macokinetics, 42, 607-618. doi:10.2165/00003088-200342070-00001 [4] Chung, C. and Remington, G. (2005) Predictors and mar- kers of clozapi ne response. Psychopharmacology, 179, 317- 335. doi:10.1007/s00213-005-2174-x [5] Liu, H.C., Chang, W.H., Wei, F.C., Lin, S.K. and Jann M.W. (1996) Monitoring of plasma clozapine levels and its metabolites in refractory schizophrenic patients. Thera- peutic Drug Monitoring, 18, 200-207. doi:10.1097/00007691-199604000-00015 [6] Perry, P.J., Miller, D.D., Arndt, S.V. and Cadoret, R.J. (1991) Clozapine and norclozapine plasma concentrations and cli- nical response of treatment-refractory schizophrenic patients. American Journa l of Psychiatr y, 148, 231-23 5. [7] Chong, S.A., Tan, C.H., Khoo, Y.M., Lee, H.S., Wong, K.E., Ngui, F. and Winslow, M. (1997) Clinical evalua- tion and plasma clozapine concentrations in Chinese pa- tients with schizophrenia. Therapeutic Drug Monitoring, 19, 219-223. doi:10.1097/00007691-199704000-00018 [8] Yang, F.D., Wang, X.Q. , Liu, X.P., Zhao , K.X., Fu, W.H., Hao, X.R., Zhang, X.L., Huang, G.S., Qu, S.C., Bai, J.S., Huang, X.F., Kosten, T.R. and Zhang, X.Y. (2001) Sex difference in QTc prolongation in chronic institutional- ized patients with schizophrenia on long-term treatment with typical and atypical antipsychotics. Psychopharma- cology, 216, 9-16. doi:10.1007/s00213-011-2188-5 [9] Spina, E., Avenoso, A., Facciolà, G., Scordo, M.G., An- cione, M., Madia, A.G., Ventimiglia, A. and Perucca, E. (2000) Relationship between plasma concentrations of clozapine and norclozapine and therapeutic response in patients with schizophrenia resist ant to conventiona l neuro- leptics. Psychopharmacology, 148, 83-89. Copyright © 2012 SciRes. OPEN ACCESS  A. Taieb et al. / Open Journal of Psychiatry 2 (2012) 262-268 268 doi:10.1007/s002130050028 [10] American Psychiatric Association (1994) Diagnostic and statistical manual for mental disorders. 4th Edition, Ame- rican Psychiatric Press, Washington DC. [11] Vanelle, J.M. and Amalric, I. (1994) Schizophrénies résis- tantes. In: EMC Psychiatrie, Elsevier, Paris. [12] Shen, Y.L., Wu, H.L., Ko, W. K. and Wu, S.M. (2002) Simul - taneous determination of clozapine, clozapine N-oxide, N-desmethylclozapine, risperidone, and 9-hydroxyrisperi- done in plasma by high performance liquid chromatog- raphy with ultraviolet detection. Analytica Chimica Acta, 460, 201-208. doi:10.1016/S0003-2670(02)00239-8 [13] Guitton, C., Abbar, M., Kinowski, J.M., Chabra nd, P. an d Bressolle, F. (1998) Multiple-dose pharmacokinetics of clozapine in patients with chronic schizophrenia. Journal of Clinical Psychopharmacology, 18, 470-476. doi:10.1097/00004714-199812000-00010 [14] Miller, D.D. (1996) The clinical use of clozapine plasma concentrations in the management of treatment-refractory schizophrenia. Annual Clinical Psychiatry, 8, 99-109. doi:10.3109/10401239609148808 [15] Haring, C., Fleishacker, W.W., Schett, P., Humpel, C., Barnas, C. and Saria, A. (1990) Influence of patient-re- lated variables on clozapine plasma concentrations. Ame- rican Journal of Psychiatry, 147, 1471-1475. [16] Edge, S.C., Markovitz, J.S. and DeVane, C.L. (1997) Clozapine drug-drug interactions: A review of the liter- ature. Human Psychopharmacology, 12, 5-20. doi:10.1002/(SICI)1099-1077(199701/02)12:1<5::AID-H UP831>3.0.CO;2-4 [17] Freeman, D.J. and Oyewumi, L.K. (1997) Will routine therapeutic drug monitoring has a place in clozapine the- rapy? Clinical Pharmacokinetics, 32, 93-100. doi:10.2165/00003088-199732020-00001 [18] Papetti, F., Morel-Pingault, V., Buisse, V., Maziere, L., Ba- nayan, M., Thauby, S., Besnard, T., Darcourt, G. and Pri n- guey, D. (2007) Clozapine-resistant schizophrenia related to an increased metabolism and benefit of fluvoxamine: Four case reports. Encephale, 33, 811-818. doi:10.1016/j.encep.2007.01.005 [19] Olesen, O.V., Thomsen, K., Jensen, P.N., Rosenberg, R., Wulff, C.H., Rasmussen, N.A., Refshammer, C., Bysted, M., Sørensen, J. and Christensen, J. (1995) Clozapine se- rum levels and side effects during steady state treatment of schizophrenic patients: A cross-sectional study. Psy- chopharmacology, 117, 371-378. doi:10.1007/BF02246112 [20] Dettling, M., Sachse, C., Brockmölle r, J., Schley, J., Müller- Oerlinghausen, B., Pickersgill, I., Rolfs, A., Schaub, R.T. and Schmider, J. (2000) Long-ter m therapeutic drug moni- toring of clozapine and metabolites in psychiatric in and out patients. Psychopharmacology, 152, 80-86. doi:10.1007/s002130000503 [21] Llorca, P.M., Lancon, C., Disdier, B., Farisee, J., Sapin, C. and Auquier, P. (2002) Effectiveness of clozapine in neuroleptic-resistant schizophrenia: Clinical response and plasma concentrations. Journal of Psychiatry Neurosci- ence, 27, 30-37. [22] Wong, J.O., Leung, S.P., Mak, T., Ng, R.M., Chan, K.T., Cheung, H.H.K., Choi, W.K., Lai, J. and Tsang, A.W.K. (2006) Plasma clozapine levels and clinical response in treatment-refractory Chinese schizophrenic patients. Pro- gress in Neuro-psychopharmacology and Biological Psy- chiatry, 30, 251-264. doi:10.1016/j.pnpbp.2005.10.008 [23] Doude van Troostwijk, L.J.A.E., Koopmans, R.P., Ver- meulen, H.D.B. and Guchelaar, H.J. (2003) CYP1A2 ac- tivity is an important determinant of clozapine dosage in schizophrenic patients. European Journal of Pharmacol- ogy Science, 20, 451-457. doi:10.1016/j.ejps.2003.09.010 [24] Palego, L., Biondi, L., Giannaccini, G., Sarno, N., Elmi, S., Ciapparelli, A., Cassano, G.B., Lucacchini, A., Mar- tini, C. and Dell’Osso, L. (2002) Clozapine, norclozapine plasma levels, their sum and ratio in 50 psychotic patients: Influence of patient-related variables. Progress in Neuro- psychopharmacology and Biological Psychiatry, 26, 473- 480. doi:10.1016/S0278-5846(01)00291-3 [25] Jerling, M., Merlé, Y., Mentré, F. and Mallet, A. (1997) Population pharmacokinetics of clozapine evaluated with the nonparametric maximum likelihood method. British Journal of Clinical Pharmacology, 44, 447-453. doi:10.1046/j.1365-2125.1997.t01-1-00606.x [26] Mauri, M.C., Volonteri, L.S., Colasanti, A., Fiorentini, A., De Gaspari, I.F. and Bareggi, S.R. (2007) Clinical phar- macokinetics of atypical antipsychotics: A critical review of the relationship between plasma concentrations and clinical response. Clinical Pharmacokinetics, 46, 359-388. doi:10.2165/00003088-200746050-00001 Copyright © 2012 SciRes. OPEN ACCESS |

